Abstract
Flecainide acetate is a class IC antiarrhythmic agent and its clinical efficacy has been confirmed by the results of several clinical trials. Nowadays, flecainide is recommended as one of the first line therapies for pharmacological conversion as well as maintenance of sinus rhythm in patients with atrial fibrillation and/or supraventricular tachycardias. Based on the Cardiac Arrhythmia Suppression Trial study results, flecainide is not recommended in patients with structural heart disease due to high proarrhythmic risk. Recent data support the role of flecainide in preventing ventricular tachyarrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia associated both with ryanodine receptor and calsequestrin mutations. We herein review the current clinical data related to flecainide use in clinical practice and some concerns about its role in the management of patients with coronary artery disease.
Keywords: Flecainide, Class IC antiarrhythmic drugs, Atrial fibrillation, Ventricular tachycardia, Proarrhythmia
Core tip: Flecainide acetate is recommended as one of the first line antiarrhythmic drugs in patients with atrial fibrillation and/or supraventricular tachycardias for the restoration and maintance of sinus rhythm. Based on the Cardiac Arrhythmia Suppression Trial study results, flecainide is contraindicated for patients with structural heart disease due to high proarrhythmic risk. Recent data support the role of flecainide in preventing ventricular tachyarrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia associated both with ryanodine receptor and calsequestrin mutations.
INTRODUCTION
Flecainide acetate is a class IC antiarrhythmic agent that was first synthesized in 1972. Its development began in 1966 in an attempt to generate new fluorinated anesthetic organic agents with the substitution of a trifluoroethoxy group on the aromatic ring in the place of the amine group (Figure 1)[1]. The clinical efficacy of flecainide was confirmed by the results of several clinical trials, both in animals and humans[2-6]. Oral use of flecainide was approved in 1984 from the Food and Drug Administration for the suppression of sustained ventricular tachycardia since study results showed about 90% efficacy without significant adverse events[7]. However, safety data from 1330 patients between 1980 and 1985 demonstrated that flecainide use was associated with increased proarrhythmic events in patients with severe cardiac disease primarily in those who were starting therapy with high-dose[8].
Figure 1.
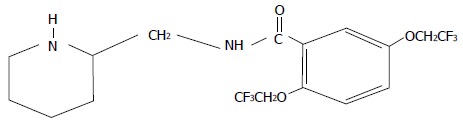
Chemical structure of flecainide acetate.
The publication of the Cardiac Arrhythmia Suppression Trial (CAST) study in 1989, which was designed to investigate the efficacy of class I antiarrhythmic agents moricizine, encainide or flecainide in patients after myocardial infarction with reduced ejection fraction and frequent ventricular ectopic beats, resulted in a major revision of the role of these antiarrhythmic drugs[9]. Thus, while flecainide suppressed ventricular ectopy in those patients, a threefold increase of arrhythmic death was recorded compared to placebo[9]. Based on CAST results, flecainide nowadays is not recommended for patients with structural heart disease and coronary artery disease. However, it is recommended as one of the first line therapies for pharmacological conversion as well as maintenance of sinus rhythm in patients with atrial fibrillation and/or supraventricular tachycardias without structural heart disease[10]. This review aims to present the existing data regarding the use, effectiveness and safety of flecainide in current clinical practice of arrhythmia management.
CLINICAL PHARMACOLOGY OF FLECAINIDE
Pharmacodynamics
Orally administrated flecainide is administrated twice daily and is absorbed rapidly without any significant interactions with food or antacid. Its bioavailability is around 90%, indicating no significant first pass effect through the liver. In normal subjects, plasma peak levels are reached after 2-3 h and steady state levels within 3-5 d. The half-life of flecainide ranges from 7 to 23 h and seems to be unaffected by dose[11,12]. Patients with ventricular ectopic beats have a longer half-life (mean 20 h) compared with normal subjects, mainly due to reduced renal function[13]. Flecainide levels are higher in cardiac tissues compared to plasma[14]. Two major metabolites, the active meta-O-dealkylated flecainide and its inactive lactam, are produced by hepatic oxidative metabolism via cytochrome CYP2D6 and CYP1A2. Both flecainide and metabolites are excreted mostly in urine, so patients with impaired renal function require close monitoring and dose reduction. Approximately 30% of flecainide is excreted unchanged into the urine[13,14]. It should be pointed that the antiarrhythmic efficacy of flecainide is closely correlated to the QRS duration. Therapeutic plasma levels range between 0.2 and 1.0 mg/mL and higher values are associated with toxic cardiac effects, such as bradycardia or conduction abnormalities[15]. The recommended starting dose in patients without renal insufficiency and paroxysmal supraventricular tachycardia or paroxysmal atrial fibrillation is 50 mg bid and may be increased in increments of 50 mg bid until efficacy is achieved (maximum recommended dose 300 mg/d). In patients with ventricular tachycardia and no contraindications for flecainide administration, the starting dose is 100 mg bid and the maximum recommended dose is 400 mg/d. Attention should be paid in patients concomitantly receiving amiodarone, although rare to encounter, who may require a dose reduction about 50%[11]. Additionally, caution is needed prior to but also following drug initiation, to exclude concomitant electrolyte disturbances, especially hypokalemia. In patients with severe hepatic insufficiency, flecainide administration should be carefully considered and monitoring of drug levels in plasma may be required[12].
Electrophysiological properties
As mentioned above flecainide belongs to the class IC antiarrhythmic agents which produce a potent and selective blockade of the cardiac fast inward sodium (Na+) current resulting in conduction slowing[11]. A high affinity for open-state Na+ channels and the slow unbinding kinetics from these channels during diastole has been described, explaining the slowing of the recovery time during the cardiac diastole and the prolongation of refractoriness[11,12]. Moreover, flecainide inhibits opening of potassium channels, especially the rapid component of the delayed rectifier K+ current (IKr), prolonging the action potential duration (APD) in ventricular and atrial muscle fibers. In opposite, in Purkinje fibers, flecainide causes a shortening in the APD due to the Na+ channel blockade[16-18]. Recent data suggest that flecainide blockades ryanodine receptor opening, thus reducing spontaneous sarcoplasmic reticulum Ca2+ release, which potentially results in afterdepolarization and triggered activity[19,20]. Therefore, flecainide has been used for the therapy of catecholaminergic polymorphic ventricular tachycardia (CPVT), which is an inherited arrhythmogenic disease with mutations of either the cardiac ryanodine receptor or calsequestrin that can cause sudden cardiac death[21].
Regarding the impact of flecainide in the intracardiac intervals, flecainide increases the AH interval (15%-22%) and the HV interval (25%-50%), thus slightly slowing both intra-atrial and atrioventricular nodal conduction[22,23]. In patients with evidence of dual atrioventricular node pathway physiology, flecainide has shown to prolong mainly the retrograde refractoriness of the fast pathway[22]. Also, in patients with accessory atrioventricular pathways, flecainide can cause complete retrograde pathway block especially in patients with refractoriness more than 270 ms at baseline, although potentially decreases both anterograde and retrograde refractory periods[24]. Additionally, flecainide does not seem to affect normal sinus node function but in patients with sinus node dysfunction an increase of the corrected sinus node recovery time and the sinoatrial conduction time have been reported[25,26]. Finally, patients with implanted cardiac rhythm devices (pacemakers or internal cardiac defibrillators) and concomitant flecainide treatment may experience an increase in the pacing thresholds[25].
All the above mentioned electrophysiological properties of flecainide are deflected in the twelve lead surface electrocardiogram with an increase in PR, QRS and QT intervals duration. The QTc interval is not significantly increased since most of QT prolongation is due to the QRS widening[11,22]. During exercise, flecainide usually shortens QTc interval[27].
Proarrhythmic and inotropic effects of flecainide
It is well known that class IC antiarrhythmic drugs may potentially be associated with proarrhythmia, either as atrial flutter with 1:1 antrioventricular conduction or ventricular tachyarrhythmia. Flecainide can convert atrial fibrillation into atrial flutter, potentially resulting in a rapid tachycardia with more than 200 bpm in case of 1:1 atrioventricular conduction[28]. The reported rate of this proarrhythmic effect is 3.5% to 5.0% and has been associated with high adrenergic conditions[29]. Drugs with atrioventricular nodal blockade properties, such as β-blockers, verapamil and diltiazem, should be administered concomitantly in order to lower the risk.
Ventricular tachycardias due to proarrhythmic effect seem to be rare in patients without structural heart disease, electrolyte disturbances and coronary artery disease. Ventricular proarrhythmia manifests either as monomorphic or polymorphic tachycardia not only early but also late after the initiation of therapy according to the results of CAST study[9]. The incidence of ventricular proarrhythmia in patients receiving flecainide for acute cardioversion of atrial fibrillation was reported in a systematic review to be less than 3%[30].
Flecainide exerts a negative inotropic effect and therefore is contraindicated in patients with congestive heart failure, coronary artery disease and reduced ejection fraction. In this population flecainide significantly reduces stroke volume index and left ventricle ejection fraction and increases right atrial and pulmonary capillary wedge pressures[31,32]. Even in patients with normal ejection fraction, oral administration of flecainide can slightly reduce the ejection fraction. Intravenous administration of flecainide (2 mg/kg) in healthy subjects was associated with a reduction in cardiac output and stroke volume during the first 90 min after dosing[33]. These hemodynamic effects are related to the reduced Na+ and Ca2+ entry into the myocardial cells. Moreover, as discussed above, flecainide has proved to blockade the ryanodine receptor opening and its interaction with the Ca2+ diastolic waves[34].
Mechanism of action of flecainide in maintenance of sinus rhythm and cardioversion in patients with atrial fibrillation
It is well known that atrial fibrillation causes both electrical and structural remodeling in atrial myocardium. Flecainide has proven its efficacy in the cardioversion of atrial fibrillation in sinus rhythm in both human and animal trials causing a shortening of the APD and prolongation of atrial refractoriness in a rate-dependent manner. Trial data have shown that the slow conduction properties of flecainide could result in a significant reduction of atrial wavelength so that atrial fibrillation cannot be maintained[34,35].
Atrial fibrillation has been associated with significant structural changes in the atria which subsequently cause remodeling of the myocardial fibers and mitochondrial dysfunction due to oxidative stress[34]. Several inflammatory adhesion molecules associated with oxidative stress and subsequent myocardial ischemia, such as nuclear factor kappa β (NFκB), reactive oxygen species and glycogen, impair the cellular physiology enhancing apoptotic process and cellular protein decomposition[36]. Rapid atrial activation encountered during atrial fibrillation results in intracellular Ca2+ accumulation, thus promoting ischemia and cellular dysfunction. This process is facilitated by the high transient intracellular Na+ concentration during tachycardia, which accentuates the entry of Ca2+ via the Na+/Ca2+ exchanger (Figure 2). Flecainide attenuates the intracellular Ca2+ accumulation by blocking Na+ channels, thus reducing oxidative stress process and further atrial remodeling[37].
Figure 2.
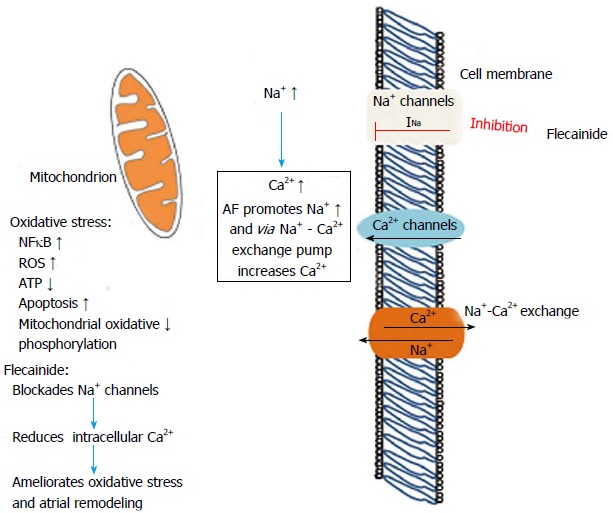
Mechanism of flecainide action during atrial fibrillation by inhibition of Na+ channels which reduces intracellular Ca2+ accumulation and reduces oxidative stress and mitochondrial dysfunction. AF; Atrial fibrillation; INa: Fast inward Na+ current; ROS: Reactive oxygen species; NFκB: Nuclear factor kappa β; ATP: Adenosine triphosphate.
CLINICAL TRIALS
Acute conversion of atrial fibrillation of recent onset
The efficacy of flecainide, both oral and intravenous formulation, in terminating recent-onset atrial fibrillation has been evaluated in several studies (Table 1) and is affected from the study design characteristics and the time intervals from drug initiation to assessment of reversion rate[38-42].
Table 1.
Reversion rate of recent-onset atrial fibrillation to sinus rhythm in clinical trials evaluating the conversion efficacy of oral or intravenous flecainide
| Clinical trial | Patients in flecainide arm | AF duration | Formulation | Reversion rate |
| Capucci et al[38] | 22 patients | ≤ 7 d | Single oral dose (300 mg) | 8 h → 91% |
| 24 h → 95% | ||||
| Donovan et al[39] | 51 patients | ≤ 3 d | iv (2 mg/kg-max 150 mg) | 1 h → 57% |
| 6 h → 67% | ||||
| Donovan et al[40] | 34 patients | ≤ 3 d | iv (2 mg/kg-max 150 mg) | 2 h → 59% |
| 8 h → 68% | ||||
| Boriani et al[41] | 69 patients | < 8 d | Single oral dose (300 mg) | 1 h → 13% |
| 3 h → 57% | ||||
| 8 h → 75% | ||||
| Martínez-Marcos et al[42] | 50 patients | ≤ 2 d | iv (2 mg/kg followed by 1 mg/kg at 8 h if not SR) | 1 h → 58% |
| 8 h → 82% | ||||
| 12 h → 90% | ||||
| Romano et al[43] | 138 patients | ≤ 3 d | Intravenous | 1 h → 73% |
| 3 h → 80% | ||||
| 6 h → 86% | ||||
| 24 h → 90% |
AF: Atrial fibrillation; SR: Sinus rhythm.
Several randomized controlled clinical trials have also compared the efficacy of flecainide to other antiarrhythmic agents in acute conversion of recent-onset atrial fibrillation. Capucci et al[38] found that a single oral loading dose of flecainide was significantly more efficient than intravenous amiodarone within 8 h but not at 24 h. In a randomized, double-blind trial, intravenous flecainide was also shown to result in earlier reversion of recent onset atrial fibrillation to sinus rhythm as compared to intravenous amiodarone[40]. In accordance, Martínez-Marcos et al[42] found that a significantly higher proportion of patients reverted to sinus rhythm when treated with intravenous flecainide as compared to intravenous amiodarone and propafenone, although the difference in reversion rate with intravenous propafenone reached statistical significance at 12 h but not at 8 h following treatment onset[42]. On the other hand, Romano et al[43] found a significantly higher efficacy of intravenous flecainide compared to intravenous propafenone in acute conversion of recent onset atrial fibrillation at 1, 3 and 6 h, although no difference was evident at 24 h. Finally, in a comparative study, Boriani et al[41] evaluated the conversion efficacy of different antiarrhythmic drug protocols and reported that oral flecainide had a similar conversion rate to oral propafenone.
Prevention of atrial fibrillation recurrences
Continuous treatment: The long-term safety and efficacy of continuous treatment with flecainide for prevention of atrial fibrillation recurrences has been studied extensively in comparison to either placebo or other antiarrhythmic agents (Table 2). However, the majority of the trials published in the literature are hampered by methodological limitations, such as open-label design, small sample size, suboptimal follow-up and underestimation of AF burden mainly due to inability to document asymptomatic or short-lasting arrhythmia bouts. In the context of these limitations, oral flecainide has been demonstrated to be superior to placebo[44-48], and similar to quinidine[49,50], sotalol[48] and propafenone[51,52] in preventing AF recurrences. The clinical efficacy of flecainide in the maintenance of sinus rhythm has been proved in a meta-analysis of 60 trials showing that 65% of patients were responders to short term treatment and 49% in the long term treatment[53]. In terms of safety, flecainide is better tolerated than quinidine[46,50], and is associated with a lower rate of adverse events as compared to propafenone[51,52].
Table 2.
Flecainide for prevention of atrial fibrillation recurrences - Randomized controlled clinical trials
| Clinical trial | Patient population | Compared treatments | Endpoint of AF recurrence | Results | Comments |
| Steinbeck et al[44] | 45 patients Paroxysmal AF | Quinidine + digoxin Flecainide + digoxin Digoxin | AF recurrence at 12 mo | Flecainide and digoxin superior to other regimens and safer than quinidine and digoxin | Quinidine is practically not used any more for sinus rhythm maintenance |
| Anderson et al[45] | 64 patients Paroxysmal AF | Flecainide (median daily dose: 300 mg) Placebo | Patients without AF recurrences Time to first AF recurrence Time interval between AF recurrences | Flecainide superior to placebo Five-fold increase in time to first recurrence Four-fold increase in time interval between attacks Significantly increased percentage of patients free of AF recurrences Adverse cardiac events in 11% of patients during flecainide therapy | Transtelephonic monitoring Double-blind randomized crossover trial (8-wk observation period) Daily flecainide dose > 300 mg in 29% of patients |
| van Wijk et al[49] | 26 patients Paroxysmal AF | Flecainide (200-300 mg daily) Quinidine (1.0-1.5 daily) | AF recurrence during 3-mo follow-up period | Flecainide superior to quinidine in the lower dosing regimen Flecainide similar efficacy to quinidine in higher dosing regimen | 20% discontinuation rate with higher quinidine dosing regimen FU with 24-h Holter at the end of each month |
| van Gelder et al[46] | 81 patients Persistent AF/flutter | Flecainide Placebo | AF recurrence at 12 mo | Flecainide superior to placebo in preventing arrhythmia recurrences | Difficult to treat patients (mean AF duration: 12 mo) |
| Pietersen et al[47] | 43 patients Paroxysmal AF/flutter | Flecainide (300 mg/d) Placebo | AF recurrence at 3 mo | Flecainide superior to placebo in preventing arrhythmia recurrences Adverse effects in 74% of patients treated with flecainide | Tolerable adverse events in flecainide group (only 2 withdrawals) One episode of sudden death |
| Carunchio et al[48] | 66 patients Paroxysmal AF | Flecainide Sotalol Placebo | AF recurrence at 1, 3, 6 and 12 mo | Flecainide similar efficacy to sotalol and superior to placebo | |
| Aliot et al[51] | 97 patients Paroxysmal AF/flutter | Flecainide (100-300 mg/d) Propafenone (600-1200 mg/d) | AF recurrence at 12 mo | Flecainide similar efficacy to propafenone Treatment discontinuation rate lower with flecainide (38% vs 53%, P = 0.079) | Multicenter, randomized, open-label study One episode of sudden death in the propafenone group |
| Chimienti et al[52] | 200 patients Paroxysmal AF | Flecainide (200-300 mg/d) Propafenone (450-900 mg/d) | Palpitation recurrence on days 15, 30, 90, 180, 270, and 360 | Flecainide similar efficacy to propafenone Similar rate of adverse cardiac and noncardiac events | Multicenter, open label, randomized, parallel study Suboptimal follow-up of AF recurrence |
| Naccarelli et al[50] | 239 patients Paroxysmal AF | Flecainide (100-300 mg/d) Quinidine | AF recurrence at 12 mo | Flecainide similar efficacy to quinidine Flecainide better tolerated than quinidine | Multicenter, open label, randomized, parallel study Self-reporting of symptomatic AF recurrences (diary recording) |
| Gulizia et al[54] | 176 pacemaker recipients with sinus node disease and paroxysmal AF | Ic AAD (flecainide or propafenone) Amiodarone | Primary endpoint: time to first occurrence of death, atrial cardioversion, cardiovascular hospitalization, or change of AAD | Class Ic AADs non-inferior to amiodarone in terms of the primary endpoint. Similar efficacy in freedom from AT recurrences based on post-hoc analyses | One patient experienced sudden cardiac death in flecainide group Capability of continuous rhythm monitoring by pacemaker AF recurrence and burden not included in primary endpoint |
| Kirchhof et al[56] | 635 patients Persistent AF | Short-term flecainide Long-term flecainide Placebo | Time to first recurrence of persistent atrial fibrillation or death from any cause | Flecainide superior to placebo Short-term flecainide not non-inferior to long-term | Largest, prospective randomized clinical trial Meticulous follow-up |
AF: Atrial fibrillation; AAD: Antiarrhythmic drug; AT: Atrial tachycardia.
Special emphasis should be placed in two trials, the PITAGORA[54,55] and the FLEC-SL trial[56]. The PITAGORA trial was a multicenter, prospective, single-blind randomized trial which aimed to compare amiodarone with class IC antiarrhythmic agents (propafenone and flecainide) when administered as prophylactic treatment for sinus rhythm maintenance among pacemaker recipients with sinus node disease and history of atrial fibrillation[54]. The maintenance daily doses of tested antiarrhythmic agents were 200 mg amiodarone, 450-750 mg propafenone and 200 mg flecainide. The main strength of the study was the ability to quantify the frequency and burden of both symptomatic and asymptomatic atrial fibrillation episodes via pacemaker diagnostics. However, the primary endpoint of the study was not related to AF burden per se but was a composite endpoint defined as time to the first occurrence of one of the following adverse events: death, hospitalization for AF or heart failure, atrial cardioversion or AAD change owing to failure of AF prophylaxis or adverse events[54]. In terms of the incidence of this endpoint, flecainide and propafenone proved to be non-inferior to amiodarone[55]. Furthermore, when flecainide and propafenone, as single agents, were compared with amiodarone, only flecainide satisfied the criterion for noninferiority. Post-hoc analysis of arrhythmia burden data demonstrated that amiodarone and class Ic agents demonstrated similar efficacy in preventing arrhythmic episodes > 10 min, or > 1 d, with a trend in favor of amiofarone for prevention of episodes lasting more than a week[55].
The Flec-SL trial is the largest, prospective randomized clinical trial testing the efficacy of oral antiarrhythmic treatment for prevention of atrial fibrillation recurrence, with a meticulous follow-up and endpoint assessment[56]. The aim of the study was to evaluate whether short-term (4 wk) flecainide treatment is non-inferior to long-term (6 mo) treatment following cardioversion of persistent atrial fibrillation. The tested hypothesis was based on the concept that provision of antiarrhythmic protection n until completion of reverse atrial electrical remodeling might provide a long-term effect with an enhanced safety profile due to reduced drug exposure. In total, 635 patients were randomly assigned in three treatment arms (placebo vs short-term vs long-term oral flecainide treatment). Patients were followed up for 6 mo with daily telemetric electrocardiograph recordings and Holter ECGs when atrial fibrillation was noted in more than two consecutive telemetric recordings. The primary outcome measure of the study was time to first recurrence of persistent atrial fibrillation or death from any cause. Based on the study results, in the per-protocol population 46% of patients receiving short-term treatment presented a recurrence of persistent AF as compared to 39% in the long-term treatment group. Additionally, short-term treatment with flecainide was superior to placebo but failed to demonstrate non-inferiority as compared to long-term treatment. However, short-term treatment demonstrated about 80% of the 6-mo effect of long-term treatment, supporting that the former could be considered a viable treatment option in patients with infrequent AF recurrences or increased risk of proarrhythmia.
Pill-in-the-pocket strategy: The safety and feasibility of treatment with a single oral dose of either flecainide (200-300 mg) or propafenone (450-600 mg) on an outpatient basis, for termination of recent onset atrial fibrillation was validated in a pivotal trial including 268 patients without severe heart disease and a previously successful in-hospital treatment[57]. The tested “pill-in-the-pocket” strategy was successful in 94% of arrhythmic episodes (equally effective for both propafenone and flecainide), while in 84% of patients the treatment was effective during all the arrhythmic episodes. The mean conversion time to sinus rhythm was about 2 h, while only one case (0.6%) of atrial flutter with rapid ventricular rate was reported. Furthermore, the implemented treatment approach resulted in significant reduction of hospitalizations and visits to the emergency rooms.
The pill-in-the-pocket strategy can be used in symptomatic patients with infrequent recurrences of atrial fibrillation. Patients with sinus node dysfunction causing bradycardia and patients with bradycardia or syncope due to atrioventricular conduction defects may not be considered candidates for the pill-in-the-pocket strategy. Prerequisites for the safe implementation of this strategy are the initial in-hospital testing of its efficacy and safety as well as careful screening of candidate patients to rule out underlying structural heart disease. However, it should be noted that in-hospital testing should include only oral formulations of antiarrhythmic agents, since tolerance to intravenous administration of flecainide or propafenone has not been shown to predict adverse events during out-of-hospital self administration of these drugs[57].
RECOMMENDATIONS FOR FLECAINIDE USE IN ATRIAL FIBRILLATION - ESC GUIDELINES
Based on the guidelines of the European Society of Cardiology, iv flecainide (2 mg/kg over 10 min) is recommended for cardioversion of recent onset AF (duration less than 48 h) when pharmacological cardioversion is preferred and there is no structural heart disease (Class IA recommendation). In selected patients without significant structural heart disease, the pill-in-the-pocket strategy (single dose of 200-300 mg) should be considered if previously tested in a medically secure environment (Class IIa recommendation). Flecainide is also recommended for long-term rhythm control in patients without significant underlying heart disease (Class IA recommendation)[10].
Ventricular tachycardias
Ventricular tachycardias in patients with underlying heart disease: The role of flecainide in the treatment of ventricular tachycardias among patients with underlying heart disease has been formulated mainly by the results of the CAST trial[1]. This multicenter, randomized, placebo-controlled trial was conducted to evaluate whether suppression of asymptomatic or mildly symptomatic ventricular arrhythmias after myocardial infarction with antiarrhythmic drugs (flecainide, encainide or moricizine) would result in reduction of arrhythmic mortality. Eligible patients had prior myocardial infarction (6 d to 2 years), ventricular arrhythmias (≥ 6 ventricular extrasystoles per hour or ventricular tachycardia runs less than 15 beats) and impaired ventricular function (ejection fraction ≤ 0.55 if recruited within 90 d of the myocardial infarction, or ≤ 0.40 if 90 d or more after the myocardial infarction). Flecainide was not given to patients with an ejection fraction below 0.30. It should be highlighted that 789 of 1498 patients included in the study had ejection fraction ≥ 0.40[23]. The treatment arms of flecainide and encainide were prematurely discontinued after a mean follow-up of 10 mo due to a significantly increased risk of arrhythmia-related (2.64; 95%CI: 1.60-4.36) and all-cause mortality (2.38; 95%CI: 1.59-3.57)[1].
As regards the role of flecainide in patients with ventricular tachycardia without structural heart disease, type IC antiarrhythmic drugs seem to be useful in patients with right ventricular outflow ventricular tachycardia[58].
Catecholaminergic polymorphic ventricular tachycardia: Accumulating data have verified that flecainide inhibits the cardiac ryanodine receptor open state, thus directly targeting the molecular defect responsible for diastolic calcium release, delayed afterdepolarizations, and triggered arrhythmias in CPVT[33,59]. Case reports and series have reported that flecainide may prove useful in preventing ventricular tachyarrhythmias in patients with CPVT associated both with ryanodine receptor and calsequestrin mutations[21,60]. Van der Werf et al[61] reported the clinical experience from several international centers on the efficacy and safety of flecainide treatment in CPVT. The role of flecainide (median daily dose 150 mg in responders) was evaluated in 33 genotype-positive CPVT patients on optimal tolerated conventional treatment using as primary outcome measure the reduction of ventricular arrhythmias during exercise testing[61]. In total, 76% of patients had either a partial or complete suppression of exercise-induced ventricular arrhythmias by flecainide while no patient experienced worsening of exercise-induced ventricular arrhythmias[61]. Flecainide has also been shown to be effective in reducing ventricular arrhythmias during exercise testing and preventing arrhythmia events during long-term follow-up in patients with genotype-negative CPVT[62]. Marai et al[63] recently reported that the combination of flecainide and β-blockers can completely suppress exercise-induced ventricular arrhythmias and prevent recurrent ICD shocks in patients with calsequestrin-associated CPVT and high-risk features despite treatment with β-blockers.
PRACTICAL ASPECTS OF FLECAINIDE USE
The following practical considerations should be kept in mind in the management of patients under flecainide treatment: (1) performance of exercise stress test before treatment initiation to assess the presence of underlying coronary artery disease; (2) screening of candidates for sinus and atrioventricular node disease; (3) performance of regular ECG monitoring upon treatment initiation and upon dose titration. In case of QRS prolongation more than 25% as compared to baseline value, flecainide dosage should be halved and if QRS is not normalized thereafter it should be discontinued; (4) performance of exercise stress testing under flecainide treatment to assess increased risk of proarrhythmia, especially in the presence of slight QRS prolongation at rest. Flecainide exerts use-dependent properties and a potential minor or modest increase in QRS duration at rest may increase dramatically during exercise-related rapid heart rate; (5) control of pacing threshold in pacemaker recipients, especially if pacemaker-dependent. Flecainide may increase the pacing threshold occasionally to a significant extent; and (6) concomitant use of agents with negative dromotropic effect is recommended to avoid one-to-one atrioventricular conduction and very rapid ventricular rates, if atrial fibrillation is converted to atrial flutter.
IS THERE ANY ROOM FOR FLECAINIDE IN CORONARY ARTERY DISEASE PATIENTS?
The main precaution for flecainide administration is to rule out the presence of “structural” heart disease and/or ischemic cardiomyopathy in order to avoid the associated increased risk of proarrhythmia. This caveat was mainly formulated on the basis of the CAST results which demonstrated that flecainide is associated with increased mortality when administered in patients with prior myocardial infarction. In this trial more than half of the enrolled patients had an ejection fraction > 40% and it is noteworthy that the hazard ratio of AAD therapy versus placebo for arrhythmic death was similar in patients with ejection fraction < 0.40 as compared to those with ejection fraction ≥ 0.40. Furthermore, the presence of a non-Q wave myocardial infarction was the only variable which significantly interacted with encainide or flecainide for prediction of arrhythmic or all cause mortality (hazard ratio 7.9 in non-Q wave myocardial infarction patients as compared to 1.8 in those with Q-wave myocardial infarction)[57]. Therefore, it seems prudent to contraindicate flecainide treatment among patients with prior myocardial infarction (either Q or non-Q wave) even if left ventricular ejection fraction is preserved. Furthermore, flecainide administration should be precluded in the presence of myocardial ischemia since the latter increases the risk of proarrhythmia.
However, the group of coronary artery disease patients, with preserved ejection fraction, no prior myocardial infarction and no evidence of ischemia represents a grey zone where there is absolute paucity of data regarding safety of flecainide treatment. According to the 2012 update of the ESC guidelines for the management of atrial fibrillation[10], flecainide can be used in patients with “minimal structural heart disease”, but it cannot be used in patients with coronary artery disease. Two main issues can be raised from these recommendations. Firstly, there is no commonly accepted and well established definition of “minimal structural heart disease”. Secondly, there are no data supporting an increased proarrhythmic risk of flecainide among coronary artery disease patients in the absence of underlying scar and myocardial ischemia. Besides, even the definition of coronary artery disease may be obscured by the widespread use of advanced imaging techniques which may actually identify patients with coronary lesions but in low arrhythmic risk. Meanwhile, decision making in similar cases is not supported by solid evidence and all available treatment options, including catheter ablation in atrial fibrillation patients, should be taken into account.
Taking into consideration the limited therapeutic modalities that are currently available for the patients with coronary artery disease and atrial fibrillation, it is obvious that new studies need to be undertaken aiming to evaluate the safety of old antiarrhythmics, like flecainide, in the wide spectrum of patients with coronary artery disease, that we treat today. Nowadays, it is quite common to treat patients with stable coronary artery disease who have preserved left ventricular ejection fraction, lack of symptoms and absence of detectable myocardial ischemia. Flecainide and other antiarrhythmics may be useful in the management of these patients, who remain vulnerable to atrial fibrillation and have limited access to invasive management of atrial tachyarrhythmias.
Finally, there is one more unsolved issue in the area of antiarrhythmic therapy in patients with ischemic heart disease. There is lack of evidence for the potential benefit from flecainide treatment in patients with implantable cardioverter defibrillators (ICDs) who have “minimal heart disease” and present symptomatic arrhythmias, refractory to sotalol and/or amiodarone. Although there is no available evidence to support this hypothesis, given that ICDs ameliorate the proarrhythmic risk, flecainide may be an alternative treatment. Unfortunately, due to medical and economic restrains, the aforementioned hypotheses have not been properly addressed in the scheme of a specifically designed randomized clinical trial.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 28, 2014
First decision: August 14, 2014
Article in press: November 19, 2014
P- Reviewer: Bonanno C, Sakabe K, Yang BF S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Hudak JM, Banitt EH, Schmid JR. Discovery and development of flecainide. Am J Cardiol. 1984;53:17B–20B. doi: 10.1016/0002-9149(84)90495-8. [DOI] [PubMed] [Google Scholar]
- 2.Somani P. Antiarrhythmic effects of flecainide. Clin Pharmacol Ther. 1980;27:464–470. doi: 10.1038/clpt.1980.65. [DOI] [PubMed] [Google Scholar]
- 3.Breithardt G, Borggrefe M, Yeh HL, Seipel L. Electrophysiological effects of flecainide on stimulus-inducible ventricular tachycardia. Z Kardiol. 1982;71:278–283. [PubMed] [Google Scholar]
- 4.Borgeat A, Goy JJ, Maendly R, Kaufmann U, Grbic M, Sigwart U. Flecainide versus quinidine for conversion of atrial fibrillation to sinus rhythm. Am J Cardiol. 1986;58:496–498. doi: 10.1016/0002-9149(86)90022-6. [DOI] [PubMed] [Google Scholar]
- 5.Crijns HJ, van Wijk LM, van Gilst WH, Kingma JH, van Gelder IC, Lie KI. Acute conversion of atrial fibrillation to sinus rhythm: clinical efficacy of flecainide acetate. Comparison of two regimens. Eur Heart J. 1988;9:634–638. doi: 10.1093/oxfordjournals.eurheartj.a062553. [DOI] [PubMed] [Google Scholar]
- 6.Cha YM, Zhang AP, Liu L, Sun JP, Huang W. Flecainide acetate in dogs with ischemic tachyarrhythmia. An electrophysiologic study. Chin Med J (Engl) 1988;101:710–714. [PubMed] [Google Scholar]
- 7.Hodges M, Haugland JM, Granrud G, Conard GJ, Asinger RW, Mikell FL, Krejci J. Suppression of ventricular ectopic depolarizations by flecainide acetate, a new antiarrhythmic agent. Circulation. 1982;65:879–885. doi: 10.1161/01.cir.65.5.879. [DOI] [PubMed] [Google Scholar]
- 8.Morganroth J, Anderson JL, Gentzkow GD. Classification by type of ventricular arrhythmia predicts frequency of adverse cardiac events from flecainide. J Am Coll Cardiol. 1986;8:607–615. doi: 10.1016/s0735-1097(86)80190-5. [DOI] [PubMed] [Google Scholar]
- 9.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 10.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 11.Holmes B, Heel RC. Flecainide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1985;29:1–33. doi: 10.2165/00003495-198529010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Roden DM, Woosley RL. Drug therapy. Flecainide. N Engl J Med. 1986;315:36–41. doi: 10.1056/NEJM198607033150106. [DOI] [PubMed] [Google Scholar]
- 13.McQuinn RL, Quarfoth GJ, Johnson JD, Banitt EH, Pathre SV, Chang SF, Ober RE, Conard GJ. Biotransformation and elimination of 14C-flecainide acetate in humans. Drug Metab Dispos. 1984;12:414–420. [PubMed] [Google Scholar]
- 14.Conard GJ, Ober RE. Metabolism of flecainide. Am J Cardiol. 1984;53:41B–51B. doi: 10.1016/0002-9149(84)90501-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Stewart JR, Perry BA, Van Hamersveld DD, Johnson TA, Conard GJ, Chang SF, Kvam DC, Pitt B. Oral flecainide acetate for the treatment of ventricular arrhythmias. N Engl J Med. 1981;305:473–477. doi: 10.1056/NEJM198108273050901. [DOI] [PubMed] [Google Scholar]
- 16.Tamargo J, Caballero R, Gómez R, Valenzuela C, Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Campbell TJ, Vaughan Williams EM. Voltage- and time-dependent depression of maximum rate of depolarisation of guinea-pig ventricular action potentials by two new antiarrhythmic drugs, flecainide and lorcainide. Cardiovasc Res. 1983;17:251–258. doi: 10.1093/cvr/17.5.251. [DOI] [PubMed] [Google Scholar]
- 18.Kvam DC, Banitt EH, Schmid JR. Antiarrhythmic and electrophysiologic actions of flecainide in animal models. Am J Cardiol. 1984;53:22B–25B. doi: 10.1016/0002-9149(84)90497-1. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Denegri M, Ruan Y, Avelino-Cruz JE, Perissi A, Negri S, Napolitano C, Coetzee WA, Boyden PA, Priori SG. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ Res. 2011;109:291–295. doi: 10.1161/CIRCRESAHA.111.247338. [DOI] [PubMed] [Google Scholar]
- 21.Pott C, Dechering DG, Reinke F, Muszynski A, Zellerhoff S, Bittner A, Köbe J, Wasmer K, Schulze-Bahr E, Mönnig G, et al. Successful treatment of catecholaminergic polymorphic ventricular tachycardia with flecainide: a case report and review of the current literature. Europace. 2011;13:897–901. doi: 10.1093/europace/euq517. [DOI] [PubMed] [Google Scholar]
- 22.Hellestrand KJ, Bexton RS, Nathan AW, Spurrell RA, Camm AJ. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J. 1982;48:140–148. doi: 10.1136/hrt.48.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson SB, Edvardsson N. Clinical electrophysiologic study of antiarrhythmic properties of flecainide: acute intraventricular delayed conduction and prolonged repolarization in regular paced and premature beats using intracardiac monophasic action potentials with programmed stimulation. Am Heart J. 1981;102:864–871. doi: 10.1016/0002-8703(81)90037-5. [DOI] [PubMed] [Google Scholar]
- 24.Hellestrand KJ, Nathan AW, Bexton RS, Spurrell RA, Camm AJ. Cardiac electrophysiologic effects of flecainide acetate for paroxysmal reentrant junctional tachycardias. Am J Cardiol. 1983;51:770–776. doi: 10.1016/s0002-9149(83)80131-3. [DOI] [PubMed] [Google Scholar]
- 25.Hellestrand KJ, Nathan AW, Bexton RS, Camm AJ. Electrophysiologic effects of flecainide acetate on sinus node function, anomalous atrioventricular connections, and pacemaker thresholds. Am J Cardiol. 1984;53:30B–38B. doi: 10.1016/0002-9149(84)90499-5. [DOI] [PubMed] [Google Scholar]
- 26.Vik-Mo H, Ohm OJ, Lund-Johansen P. Electrophysiologic effects of flecainide acetate in patients with sinus nodal dysfunction. Am J Cardiol. 1982;50:1090–1094. doi: 10.1016/0002-9149(82)90423-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang JA, Lau CP, Tai YT, Wu BZ. Effects of flecainide on exercise hemodynamics and electrocardiography in patients without structural heart disease. Clin Cardiol. 1995;18:140–144. doi: 10.1002/clc.4960180307. [DOI] [PubMed] [Google Scholar]
- 28.Nabar A, Rodriguez LM, Timmermans C, Smeets JL, Wellens HJ. Radiofrequency ablation of “class IC atrial flutter” in patients with resistant atrial fibrillation. Am J Cardiol. 1999;83:785–787, A10. doi: 10.1016/s0002-9149(98)00992-8. [DOI] [PubMed] [Google Scholar]
- 29.Falk RH. Proarrhythmia in patients treated for atrial fibrillation or flutter. Ann Intern Med. 1992;117:141–150. doi: 10.7326/0003-4819-117-2-141. [DOI] [PubMed] [Google Scholar]
- 30.McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med. 2003;139:1018–1033. doi: 10.7326/0003-4819-139-12-200312160-00012. [DOI] [PubMed] [Google Scholar]
- 31.Josephson MA, Kaul S, Hopkins J, Kvam D, Singh BN. Hemodynamic effects of intravenous flecainide relative to the level of ventricular function in patients with coronary artery disease. Am Heart J. 1985;109:41–45. doi: 10.1016/0002-8703(85)90413-2. [DOI] [PubMed] [Google Scholar]
- 32.de Paola AA, Horowitz LN, Morganroth J, Senior S, Spielman SR, Greenspan AM, Kay HR. Influence of left ventricular dysfunction on flecainide therapy. J Am Coll Cardiol. 1987;9:163–168. doi: 10.1016/s0735-1097(87)80096-7. [DOI] [PubMed] [Google Scholar]
- 33.Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, Piston DW, Huke S, Knollmann BC. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anno T, Hondeghem LM. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage dependence of recovery. Circ Res. 1990;66:789–803. doi: 10.1161/01.res.66.3.789. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Pagé P, Nattel S. Mechanism of flecainide’s antiarrhythmic action in experimental atrial fibrillation. Circ Res. 1992;71:271–287. doi: 10.1161/01.res.71.2.271. [DOI] [PubMed] [Google Scholar]
- 36.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 37.Iwai T, Tanonaka K, Inoue R, Kasahara S, Motegi K, Nagaya S, Takeo S. Sodium accumulation during ischemia induces mitochondrial damage in perfused rat hearts. Cardiovasc Res. 2002;55:141–149. doi: 10.1016/s0008-6363(02)00282-1. [DOI] [PubMed] [Google Scholar]
- 38.Capucci A, Lenzi T, Boriani G, Trisolino G, Binetti N, Cavazza M, Fontana G, Magnani B. Effectiveness of loading oral flecainide for converting recent-onset atrial fibrillation to sinus rhythm in patients without organic heart disease or with only systemic hypertension. Am J Cardiol. 1992;70:69–72. doi: 10.1016/0002-9149(92)91392-h. [DOI] [PubMed] [Google Scholar]
- 39.Donovan KD, Dobb GJ, Coombs LJ, Lee KY, Weekes JN, Murdock CJ, Clarke GM. Efficacy of flecainide for the reversion of acute onset atrial fibrillation. Am J Cardiol. 1992;70:50A–54A; discussion 54A-55A. doi: 10.1016/0002-9149(92)91078-i. [DOI] [PubMed] [Google Scholar]
- 40.Donovan KD, Power BM, Hockings BE, Dobb GJ, Lee KY. Intravenous flecainide versus amiodarone for recent-onset atrial fibrillation. Am J Cardiol. 1995;75:693–697. doi: 10.1016/s0002-9149(99)80655-9. [DOI] [PubMed] [Google Scholar]
- 41.Boriani G, Biffi M, Capucci A, Botto G, Broffoni T, Ongari M, Trisolino G, Rubino I, Sanguinetti M, Branzi A, et al. Conversion of recent-onset atrial fibrillation to sinus rhythm: effects of different drug protocols. Pacing Clin Electrophysiol. 1998;21:2470–2474. doi: 10.1111/j.1540-8159.1998.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Marcos FJ, García-Garmendia JL, Ortega-Carpio A, Fernández-Gómez JM, Santos JM, Camacho C. Comparison of intravenous flecainide, propafenone, and amiodarone for conversion of acute atrial fibrillation to sinus rhythm. Am J Cardiol. 2000;86:950–953. doi: 10.1016/s0002-9149(00)01128-0. [DOI] [PubMed] [Google Scholar]
- 43.Romano S, Fattore L, Toscano G, Corsini F, Coppo A, Catanzaro M, Romano A, Martone A, Caccavale F, Iodice E, et al. Effectiveness and side effects of the treatment with propafenone and flecainide for recent-onset atrial fibrillation. Ital Heart J Suppl. 2001;2:41–45. [PubMed] [Google Scholar]
- 44.Steinbeck G, Doliwa R, Bach P. [Therapy of paroxysmal atrial fibrillation. Cardiac glycosides alone or combined with anti-arrhythmia agents?] Dtsch Med Wochenschr. 1988;113:1867–1871. doi: 10.1055/s-2008-1067903. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JL, Gilbert EM, Alpert BL, Henthorn RW, Waldo AL, Bhandari AK, Hawkinson RW, Pritchett EL. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double-blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circulation. 1989;80:1557–1570. doi: 10.1161/01.cir.80.6.1557. [DOI] [PubMed] [Google Scholar]
- 46.Van Gelder IC, Crijns HJ, Van Gilst WH, Van Wijk LM, Hamer HP, Lie KI. Efficacy and safety of flecainide acetate in the maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation or atrial flutter. Am J Cardiol. 1989;64:1317–1321. doi: 10.1016/0002-9149(89)90574-2. [DOI] [PubMed] [Google Scholar]
- 47.Pietersen AH, Hellemann H. Usefulness of flecainide for prevention of paroxysmal atrial fibrillation and flutter. Danish-Norwegian Flecainide Multicenter Study Group. Am J Cardiol. 1991;67:713–717. doi: 10.1016/0002-9149(91)90527-r. [DOI] [PubMed] [Google Scholar]
- 48.Carunchio A, Fera MS, Mazza A, Burattini M, Greco G, Galati A, Ceci V. A comparison between flecainide and sotalol in the prevention of recurrences of paroxysmal atrial fibrillation. G Ital Cardiol. 1995;25:51–68. [PubMed] [Google Scholar]
- 49.van Wijk LM, den Heijer P, Crijns HJ, van Gilst WH, Lie KI. Flecainide versus quinidine in the prevention of paroxysms of atrial fibrillation. J Cardiovasc Pharmacol. 1989;13:32–36. doi: 10.1097/00005344-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Naccarelli GV, Dorian P, Hohnloser SH, Coumel P. Prospective comparison of flecainide versus quinidine for the treatment of paroxysmal atrial fibrillation/flutter. The Flecainide Multicenter Atrial Fibrillation Study Group. Am J Cardiol. 1996;77:53A–59A. doi: 10.1016/s0002-9149(97)89118-7. [DOI] [PubMed] [Google Scholar]
- 51.Aliot E, Denjoy I. Comparison of the safety and efficacy of flecainide versus propafenone in hospital out-patients with symptomatic paroxysmal atrial fibrillation/flutter. The Flecainide AF French Study Group. Am J Cardiol. 1996;77:66A–71A. doi: 10.1016/s0002-9149(97)89120-5. [DOI] [PubMed] [Google Scholar]
- 52.Chimienti M, Cullen MT, Casadei G. Safety of flecainide versus propafenone for the long-term management of symptomatic paroxysmal supraventricular tachyarrhythmias. Report from the Flecainide and Propafenone Italian Study (FAPIS) Group. Eur Heart J. 1995;16:1943–1951. doi: 10.1093/oxfordjournals.eurheartj.a060852. [DOI] [PubMed] [Google Scholar]
- 53.Hohnloser SH, Zabel M. Short- and long-term efficacy and safety of flecainide acetate for supraventricular arrhythmias. Am J Cardiol. 1992;70:3A–9A; discussion 9A-10A. doi: 10.1016/0002-9149(92)91071-b. [DOI] [PubMed] [Google Scholar]
- 54.Gulizia M, Mangiameli S, Chiarandà G, Spadola V, Di Giovanni N, Colletti A, Bulla V, Circo A, Pensabene O, Vasquez L, et al. Design and rationale of a randomized study to compare amiodarone and Class IC anti-arrhythmic drugs in terms of atrial fibrillation treatment efficacy in patients paced for sinus node disease: the PITAGORA trial. Europace. 2006;8:302–305. doi: 10.1093/europace/eul003. [DOI] [PubMed] [Google Scholar]
- 55.Gulizia M, Mangiameli S, Orazi S, Chiarandà G, Piccione G, Di Giovanni N, Colletti A, Pensabene O, Lisi F, Vasquez L, et al. A randomized comparison of amiodarone and class IC antiarrhythmic drugs to treat atrial fibrillation in patients paced for sinus node disease: the Prevention Investigation and Treatment: A Group for Observation and Research on Atrial arrhythmias (PITAGORA) trial. Am Heart J. 2008;155:100–107, 107.e1. doi: 10.1016/j.ahj.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 56.Kirchhof P, Andresen D, Bosch R, Borggrefe M, Meinertz T, Parade U, Ravens U, Samol A, Steinbeck G, Treszl A, et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet. 2012;380:238–246. doi: 10.1016/S0140-6736(12)60570-4. [DOI] [PubMed] [Google Scholar]
- 57.Alboni P, Botto GL, Boriani G, Russo G, Pacchioni F, Iori M, Pasanisi G, Mancini M, Mariconti B, Capucci A. Intravenous administration of flecainide or propafenone in patients with recent-onset atrial fibrillation does not predict adverse effects during ‘pill-in-the-pocket’ treatment. Heart. 2010;96:546–549. doi: 10.1136/hrt.2009.187963. [DOI] [PubMed] [Google Scholar]
- 58.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:746–837. doi: 10.1093/europace/eul108. [DOI] [PubMed] [Google Scholar]
- 59.Galimberti ES, Knollmann BC. Efficacy and potency of class I antiarrhythmic drugs for suppression of Ca2+ waves in permeabilized myocytes lacking calsequestrin. J Mol Cell Cardiol. 2011;51:760–768. doi: 10.1016/j.yjmcc.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, Yin H, Knollmann BC. Inhibition of cardiac Ca2+ release channels (RyR2) determines efficacy of class I antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–135. doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, Shimizu W, Sumitomo N, Fish FA, Bhuiyan ZA, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe H, van der Werf C, Roses-Noguer F, Adler A, Sumitomo N, Veltmann C, Rosso R, Bhuiyan ZA, Bikker H, Kannankeril PJ, et al. Effects of flecainide on exercise-induced ventricular arrhythmias and recurrences in genotype-negative patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2013;10:542–547. doi: 10.1016/j.hrthm.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marai I, Khoury A, Suleiman M, Gepstein L, Blich M, Lorber A, Boulos M. Importance of ventricular tachycardia storms not terminated by implantable cardioverter defibrillators shocks in patients with CASQ2 associated catecholaminergic polymorphic ventricular tachycardia. Am J Cardiol. 2012;110:72–76. doi: 10.1016/j.amjcard.2012.02.049. [DOI] [PubMed] [Google Scholar]


