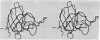Abstract
An electron density map at 3 angstrom resolution has been calculated for Cu2+, Zn2+ superoxide dismutase from bovine erythrocytes, and the course of the main chain has been traced. The dominant structural feature is an 8-stranded barrel of antiparallel beta-pleated sheet. There is one very short helical section and two long loops of non-repetitive structure. The Cu and Zn are bound between the loops and one side of the beta barrel and are about 6 Angstrom apart, with a common histidine ligand. The Cu has four histidine ligands in a somewhat distorted square plane, and the Zn has three histidines and an aspartate in approximately tetrahedral arrangement. The two coppers of a dimer are about 34 Angstrom apart. The two subunits have essentially the same conformation and have an extensive contact area that mainly involves hydrophobic side chain interactions. The overall folding pattern of the polypeptide chain is very similar to that of an immunoglobulin domain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy J. L., Steinman H. M., Hill R. L. Bovine erythrocyte superoxide dismutase. Subunit structure and sequence location of the intrasubunit disulfide bond. J Biol Chem. 1974 Nov 25;249(22):7339–7347. [PubMed] [Google Scholar]
- Beem K. M., Rich W. E., Rajagopalan K. V. Total reconstitution of copper-zinc superoxide dismutase. J Biol Chem. 1974 Nov 25;249(22):7298–7305. [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Geisow M. J., Swan I. D., Rerat C., Rerat B. Strjcture of human plasma prealbumin at 2-5 A resolution. A preliminary report on the polypeptide chain conformation, quaternary structure and thyroxine binding. J Mol Biol. 1974 Sep 5;88(1):1–12. doi: 10.1016/0022-2836(74)90291-5. [DOI] [PubMed] [Google Scholar]
- Fee J. A. Studies on the reconstitution of bovine erythrocyte superoxide dismutase. IV. Preparation and some properties of the enzyme in which Co(II) is substituted for Zn(II). J Biol Chem. 1973 Jun 25;248(12):4229–4234. [PubMed] [Google Scholar]
- Fee J. A., Teitelbaum H. D. Evidence that superoxide dismutase plays a role in protecting red blood cells against peroxidative hemolysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):150–158. doi: 10.1016/0006-291x(72)90022-8. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Evans H. J., Hill R. L., Fridovich I. Histidine at the active site of superoxide dismutase. Biochemistry. 1973 Feb 27;12(5):823–827. doi: 10.1021/bi00729a006. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J Biol Chem. 1973 Apr 25;248(8):2645–2649. [PubMed] [Google Scholar]
- Freeman H. C. Crystal structures of metal-peptide complexes. Adv Protein Chem. 1967;22:257–424. doi: 10.1016/s0065-3233(08)60043-1. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. NADPH-dependen lipid peroxidation catalyzed by purified NADPH-cytochrome C reductase from rat liver microsomes. Biochem Biophys Res Commun. 1972 Aug 21;48(4):789–795. doi: 10.1016/0006-291x(72)90676-6. [DOI] [PubMed] [Google Scholar]
- Richardson D. C., Bier C. J., Richardson J. S. Two crystal forms of bovine superoxide dismutase. J Biol Chem. 1972 Oct 10;247(19):6368–6369. [PubMed] [Google Scholar]
- Rotilio G., Agrò A. F., Calabrese L., Bossa F., Guerrieri P., Mondovì B. Studies of the metal sites of copper proteins. Ligands of copper in hemocuprein. Biochemistry. 1971 Feb 16;10(4):616–621. doi: 10.1021/bi00780a011. [DOI] [PubMed] [Google Scholar]
- Rubin B., Richardson J. S. The simple construction of protein alpha-carbon models. Biopolymers. 1972;11(11):2381–2385. doi: 10.1002/bip.1972.360111116. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M., Hill R. L. Sequence homologies among bacterial and mitochondrial superoxide dismutases. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3725–3729. doi: 10.1073/pnas.70.12.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M., Naik V. R., Abernethy J. L., Hill R. L. Bovine erythrocyte superoxide dismutase. Complete amino acid sequence. J Biol Chem. 1974 Nov 25;249(22):7326–7338. [PubMed] [Google Scholar]
- Thomas K. A., Rubin B. H., Bier C. J., Richardson J. S., Richardson D. C. The crystal structure of bovine Cu2+,Zn2+ superoxide dismutase at 5.5-A resolution. J Biol Chem. 1974 Sep 10;249(17):5677–5683. [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- White H. L., White J. R. Interaction of streptonigrin with DNA in vitro. Biochim Biophys Acta. 1966 Sep;123(3):648–651. doi: 10.1016/0005-2787(66)90241-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Flohé L., Weser U., Hartmann H. J. Inhibition of lipid peroxidation in isolated inner membrane of rat liver mitochondria by superoxide dismutase. FEBS Lett. 1973 Jan 15;29(2):117–120. doi: 10.1016/0014-5793(73)80539-3. [DOI] [PubMed] [Google Scholar]