Abstract
AIM: To analyze the outcomes of self-expandable stent placement for benign esophageal strictures and benign esophageal leaks in the literature.
METHODS: The PubMed, Embase and Cochrane databases were searched for relevant articles published between January 2000 and July 2014. Eight prospective studies were identified that analyzed the outcomes of stent placement for refractory benign esophageal strictures. The outcomes of stent placement for benign esophageal leaks, perforations and fistulae were extracted from 20 retrospective studies that were published after the inclusion period of a recent systematic review. Data were pooled and analyzed using descriptive statistics.
RESULTS: Fully covered self-expandable metal stents (FC SEMS) (n = 85), biodegradable (BD) stents (n = 77) and self-expandable plastic stents (SEPS) (n = 70) were inserted in 232 patients with refractory benign esophageal strictures. The overall clinical success rate was 24.2% and according to stent type 14.1% for FC SEMS, 32.9% for BD stents and 27.1% for SEPS. Stent migration occurred in 24.6% of cases. The overall complication rate was 31.0%, including major (17.7%) and minor (13.4%) complications. A total of 643 patients were treated with self-expandable stents mainly for postsurgical leaks (64.5%), iatrogenic perforations (19.6%), Boerhaave’s syndrome (7.8%) and fistulae (3.7%). FC SEMS and partially covered SEMS were used in the majority of patients. Successful closure of the defect was achieved in 76.8% of patients and according to etiology in 81.4% for postsurgical leaks, 86.0% for perforations and 64.7% for fistulae. The pooled stent migration rate was 16.5%. Stent-related complications occurred in 13.4% of patients, including major (7.8%) and minor (5.5%) complications.
CONCLUSION: The outcomes of stent placement for refractory benign esophageal strictures were poor. However, randomized trials are needed to put this into perspective. The evidence on successful stent placement for benign esophageal leaks, perforations and fistulae is promising.
Keywords: Self-expandable stents, Benign esophageal strictures, Esophageal perforation, Esophageal fistula, Anastomotic leak, Systematic review
Core tip: After a systematic search of the literature, we performed a pooled analysis on the clinical outcomes of self-expandable stent placement for benign esophageal diseases. We analyzed the clinical success, adverse events and removal outcome of stent placement in 232 patients with refractory benign esophageal strictures and 643 patients with benign esophageal leaks, perforations and fistulae. Additional analyses were performed for clinical outcomes according to stent type and etiology.
INTRODUCTION
Esophageal self-expandable stent placement is a well-established, evidence-based treatment for the palliation of malignant dysphagia. By the end of the 90’s self-expandable metal stents have replaced the traditional rigid plastic tubes, because of their superiority in safety and cost-effectiveness[1-6]. Ever since the stent designs have evolved in order to improve their efficacy, durability and safety, and to expand their use for different clinical indications.
Besides malignant indications, esophageal self-expandable stents are nowadays used for refractory benign strictures, benign perforations, postoperative anastomotic leaks and benign fistulae[1,7]. To define the heterogeneous group of patients with refractory benign esophageal strictures Kochman et al[8] have proposed a uniform definition that has been widely accepted. According to Kochman’s criteria an esophageal stricture is refractory or recurrent when it cannot be remediated to a diameter of 14 mm over 5 dilatation sessions at 2-wk intervals, or when a satisfactory luminal diameter cannot be maintained for 4 wk once the target diameter of 14 mm has been achieved[8]. The definition only applies in the absence of active inflammation and neuromuscular dysfunction. In this subgroup of patients with refractory strictures self-expandable stent placement is performed to extend the dysphagia-free period and to reduce the number of dilatations (Figure 1A and B).
Figure 1.
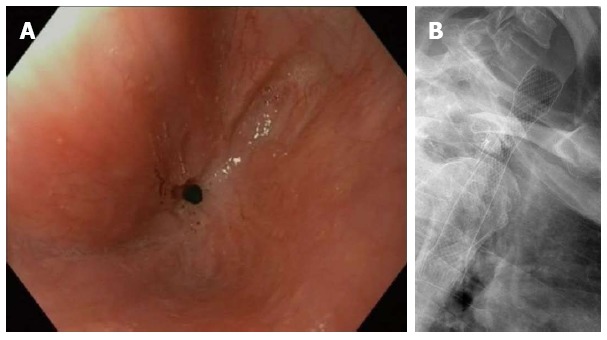
Refractory benign anastomotic stricture after esophagectomy (A) and fully covered self-expandable metal stent placement for a refractory benign esophageal anastomotic stricture (B).
There is a varied offer of esophageal self-expandable stents, that can be divided into four main groups: (1) removable fully covered metal stents (FC SEMS); (2) removable partially covered metal stents (PC SEMS); (3) removable covered plastic stents (SEPS); and (4) biodegradable stents (BD stents). In this literature review we aim to provide an overview of the clinical outcomes of self-expandable stent placement for benign esophageal diseases including a by clinical indication and by stent design breakdown.
MATERIALS AND METHODS
The PubMed, Embase and Cochrane databases were searched for publications from January 2000 to July 2014. Key words that were used included esophagus, stent and benign. Articles were screened by title and abstract for their relevance. Studies were considered for inclusion when they reported on the clinical outcomes of esophageal self-expandable stent placement for benign strictures, benign perforations, anastomotic leaks and/or benign fistulae. The exclusion criteria and search results are shown in Figure 2. The primary endpoint was clinical success, which was defined as the absence of dysphagia at end of follow-up after single stent placement in case of esophageal strictures and successful closure of the defect after single or multiple stent placements in case of an esophageal leak, perforation or fistula. Clinical failures were defined as recurrent dysphagia in case of esophageal strictures and persistent leak or death during stent therapy in case of esophageal leaks, perforations and fistulae. Secondary endpoints were the technical success rates of esophageal stent placement, morbidity rates, mortality rates and stent removal outcome. Technical success was defined as stent placement across the lesion at the end of the procedure, including successful stent repositioning after immediate migration. Successful stent removal was defined as uneventful endoscopic stent extraction without the need for additional interventions or procedures. So stent removal by the stent-in-stent procedure, which is used to induce pressure-necrosis of granulation tissue to facilitate the removal of an embedded stent, was considered an adverse event.
Figure 2.
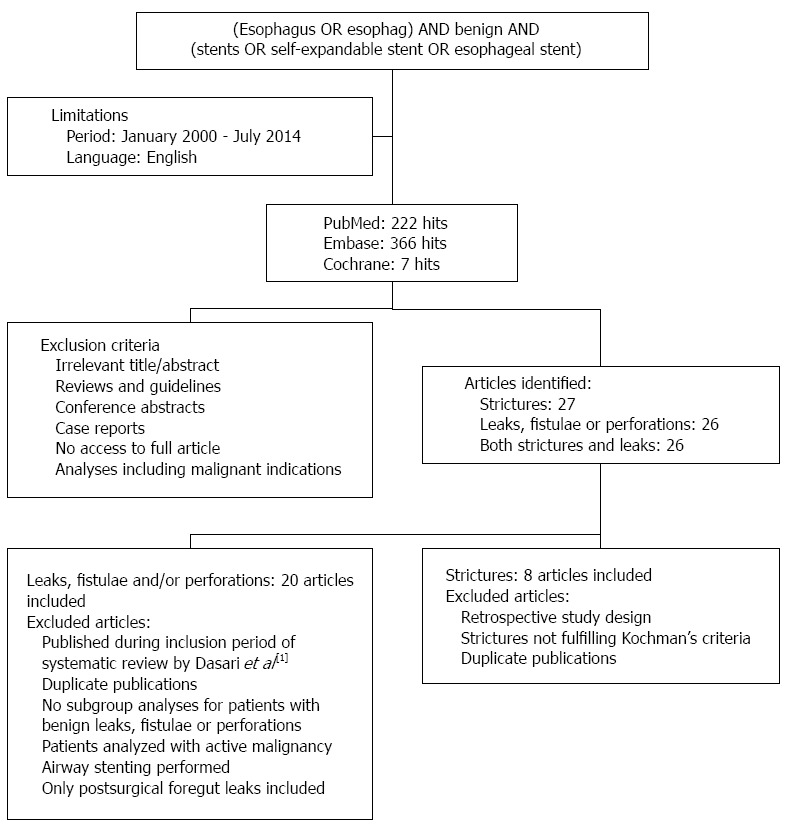
Literature search.
Statistical analysis
This manuscript contains descriptive statistics. Data were pooled and presented as frequency and percentage, so no biostatistical tests were used.
RESULTS
Refractory benign esophageal strictures
After searching the literature no randomized controlled trials (RCTs) were found that studied the outcomes of stent placement for refractory benign esophageal strictures. Twelve prospective, nonrandomized studies were identified that reported on the outcomes of esophageal stent placement for benign strictures (Table 1)[9-20]. One was excluded because of a duplicate publication[20]. To create a homogeneous population only the studies were analyzed that included patients with refractory benign esophageal strictures according to Kochman’s criteria[8]. A total of eight prospective cohort series were included that reported on 232 patients with refractory benign esophageal strictures[9-16]. In 85 patients a FC SEMS was placed, 77 patients received a BD stent and a SEPS was inserted in 70 patients. No PC SEMS were used in any of the included articles. The overall pooled technical success rate of esophageal stent placement was 98.7%. Details on stricture etiology, stent type and clinical outcomes are summarized in Table 2. Analyses by stricture etiology were not possible due to lacking data.
Table 1.
Literature on self-expandable stent placement for refractory benign esophageal strictures
| Ref. | Study design | Patients, indications | Stent type, technical success rate, scheduled removal | Follow-up median (range) | Complications | Successful stent removal | Clinical success (dysphagia-free) |
| Prospective cohort studies including patients with RBES according to Kochman’s criteria | |||||||
| Chaput et al[9] 2013 | Prospect | Patients with recurrent benign strictures after more than 3 dilatations to more than 15 mm during the previous 12 mo: n = 41 1 Anastomotic stricture: 29% (12/41) 2 Peptic stricture: 39% (16/41) 3 Caustic stricture: 7% (3/41) 4 Radiation stricture: 20% (8/41) 5 Others: 5% (2/41) | Standard FC SEMS: 100% (24/24) - 4 wk Multilayer silicone FC SEMS: 100% (17/17) - 3 mo | 24 mo | Overall complications: Stent migration: 29.3% (12/41) Chest pain requiring stent removal or repositioning: 9.8% (4/41) Chest pain resolved with conservative management: 2.4% (1/41) Vomiting: 2.4% (1/41) Pneumonia: 2.4% (1/41) | FC SEMS: 100% (41/41) | Overall clinical success: 9.8% (4/41) |
| Canena et al[10] 2012 | Prospect | Patients with RBES according to Kochman criteria: n = 30 1 Anastomotic stricture: 43% (13/30) 2 Peptic stricture: 23% (7/30) 3 Caustic stricture: 10% (3/10) 4 Radiation stricture: 7% (2/30) 5 Idiopathic stricture: 17% (5/30) | BD stent: 100% (10/10) SEPS: 100% (10/10) - 12 wk FC SEMS: 100% (10/10) - 12 wk | 23.4 (8-66) mo | Patients with complications (P = 0.38): BD stent 50%, SEPS 70%, FC SEMS 60% Stent migration (P = 0.16): BD stent 20%, SEPS 60%, FC SEMS 30% Tissue hyperplasia (P = 0.09): BD stent 30%, SEPS 0%, FC SEMS 0% Associated with one major bleeding and recurrent dysphagia in two patients Minor complications in 17% (5/30) of patients: 1 Globus sensation: BD stent 0%, SEPS 0%, FC SEMS 10% 2 Moderate chest pain: BD stent 0%, SEPS 20%, FC SEMS 10% 3 Reflux: BD stent 0%, SEPS 10%, FC SEMS 10% Major complications in 7% (2/30) of patients: 1 Major bleeding: BD stent 10%, SEPS 0%, FC SEMS 0% 2 Severe chest pain: BD stent 10%, SEPS 0%, FC SEMS 0% | SEPS: 100% (10/10) FC SEMS: 100% (10/10) | Overall: 27% (8/30) Stent type (P = 0.27): 1 BD stent: 30% (3/10) 2 SEPS: 10% (1/10) 3 FC SEMS: 40% (4/10) |
| Hirdes et al[11] 2012 | Prospect | Patients with RBES according to Kochman criteria: n = 28 1 Peptic stricture: 32% (9/28) 2 Anastomotic stricture: 25% (7/28) 3 Radiation stricture: 11% (3/28) 4 Caustic stricture: 7% (2/28) 5 Others: 11% (3/28) 6 Unknown origin: 14% (4/28) | Single BD stent: n = 15 Sequential BD stent: n = 13 Technical success: 100% (28/28) In total 59 BD stent placed | 630 (21-1121) d | Stent migration: 10.7% (3/28) Food impaction: 10.7% (3/28) Major complications of 59 stent placements in 28 patients: 29% (8/28) of patients 1 Retrosternal pain and vomiting: 7.1% (2/28) 2 Retrosternal pain: 7.1% (2/28) 3 Bleeding: 7.1% (2/28) 4 Fever and vomiting: 3.6% (1/28) 5 Aspiration pneumonia: 3.6% (1/28) Minor complications of 59 stent placements in 28 patients: 14% (4/28) of patients 1 Retrosternal pain: 7.1% (2/28) 2 Reflux: 3.6% (1/28) 3 Vomiting: 3.6% (1/28) One patient (3.6%) died of aspiration pneumonia, relation to stent unclear | Not applicable | At 6 mo after: First stent: 25% (7/28) Second stent: 15% (2/13) Third stent: 0% (0/7) |
| Hirdes et al[12] 2012 | Prospect | Patients with RBES according to Kochman criteria: n = 15 1 Peptic stricture: 40% (6/15) 2 Caustic stricture: 20% (3/15) 3 Radiation stricture: 13% (2/15) 4 Other: 7% (1/15) 5 Unknown cause: 20% (3/15) | FC SEMS: 100% (15/15) 109 d (87-222) | After stent removal: 86 (14-330) d | Stent migration: 33% (5/15) Tissue overgrowth: 20% (3/15) Major complications in 20% (3/15) of patients: 1 Severe pain requiring stent removal: 7% (1/15) 2 Severe persistent odynophagia: 7% (1/15) 3 Nausea/vomiting: 13% (2/15) 4 Aspiration pneumonia: 7% (1/15) Minor complications: 1 Pain: 20% (3/15) | 93% (14/15) Stent-in-stent: 7% | 0% (0/15) |
| Eloubeidi et al[13] 2011 | Pro- and retrospect | Patients with benign esophageal lesions treated with Alimaxx-E stent: n = 35 Leaks/fistulae: n = 12 Perforations: n = 4 RBES: n = 19 1 Anastomotic stricture: 37% (7/19) 2 Peptic stricture: 21% (4/19) 3 Caustic stricture: 11% (2/19) 4 Radiation stricture: 11% (2/19) 5 Others: 21% (4/19) | FC SEMS: 100% (19/19) In situ for: 64 ± 74 d (range 6-300) | 161 ± 111 (range 24-360) d | Stent migration: 36.8% (7/19) Minor complications in patients with RBES: 1 Stent infolding/invagination: 16% (3/19) 2 Chest pain: 5% (1/19) 3 Abdominal pain: 11% (2/19) 4 Globus sensation: 5% (1/19) 5 Fever: 5% (1/19) Major complications in patients with RBES: 1 Arrhythmia: 5% (1/19) | 97% (34/35) Stent fracture: 3% | 21% (4/19) |
| Van Boeckel et al[14] 2011 | Prospect | Patients with RBES according to Kochman criteria: n = 38 1 Anastomotic stricture: 34% (13/38) 2 Peptic stricture: 18% (7/38) 3 Radiation stricture: 18% (7/38) 4 Caustic stricture: 16% (6/38) 5 Others: 11% (4/38) 6 Unknown etiology: 3% (1/38) | BD stent: 100% (18/18) SEPS: 95% (19/20) - 6 wk | BD stent: 166 (21-559) d SEPS: 385 (77-924) d | Major complications: 15.8% (6/38) 1 Hemorrhage: SEPS 5%, BD stent 11% 2 Perforation: SEPS 5%, BD stent 0% 3 Severe pain requiring opiates: SEPS 0%, BD stent 11% Minor complications: 10.5% (4/38) 1 Reflux: SEPS 0%, BD stent 6% 2 Nausea/vomiting: SEPS 5%, BD stent 11% Stent migration: SEPS 25% (5/20), BD stent 22% (4/18) Food impaction: SEPS 0%, BD stent 11% (2/18) Tissue in-/overgrowth: SEPS 0%, BD stent 11% (2/18) A FC SEMS was placed in both patients | SEPS: 100% (16/16) | Stent type (P = 0.83): 1 SEPS: 30% (6/20) 2 BD stent: 33% (6/18) |
| Repici et al[15] 2010 | Prospect | Patients with RBES according to Kochman criteria: n = 21 1 Peptic stricture: 33% (7/21) 2 Anastomotic stricture: 24% (5/21) 3 Radiation stricture: 24% (5/21) 4 Caustic stricture: 10% (2/21) 5 Other: 5% (1/21) 6 Idiopathic stricture: 5% (1/21) | BD stent: 100% (21/21) | 53 (25-88) wk | Stent migration: 9.5% (2/21) Severe thoracic pain requiring analgesics: 14.3% (3/21) Minor bleeding: 4.8% (1/21) Dysphagia caused by hyperplastic tissue: 4.8% (1/21) | Not applicable | 45% (9/20) |
| Dua et al[16] 2008 | Prospect | Patients with RBES according to Kochman criteria: n = 40 1 Anastomotic stricture: 30% (12/40) 2 Caustic stricture: 20% (8/40) 3 Radiation stricture: 18% (7/40) 4 Peptic stricture: 5% (2/40) 5 Others: 28% (11/40) | SEPS: 95% (38/40) 4 wk | 53 (11-156) wk | Stent migration: 22.2% (8/36) Severe chest pain requiring medication: 11.1% (4/36) Fistula: 2.8% (1/36) Perforation: 5.6% (2/36) Gastroesophageal reflux: 5.6% (2/36) Bleeding: 8.3% (3/36) Stent-related mortality: 2.8% (1/36) Massive bleeding probably due to stent eroding into major vessel | 94% (31/33) Inability to remove stent: 6% (2/33) | 30% (12/40) |
| Remaining prospective cohort studies | |||||||
| Van Hooft et al[17] 2011 | Prospect | Patients with an esophagogastric anastomotic stricture who did not have had any endoscopic treatment: n = 10 | BD stent: 100% (10/10) | 6 mo | Food impaction: 10% (1/10) Hyperplasia-induced obstruction: 20% (2/10) | Not applicable | 60% (6/10) |
| Evrard et al[18] 2004 | Prospect | SEMS-induced stricture: n = 5 Esophagocolonic anastomotic stricture: n = 4 Refractory benign strictures after a median of 6 (range 1-12) dilation sessions per year: n = 8 Anastomotic leak: n = 4 | SEPS: 100% (21/21) Range 2 d–18 mo | After stent removal: 21 (8-39) mo | Stent migration: 57.1% (12/21) Stridor due to tracheal compression: 4.8% (1/21) Inflammatory epiglottic stenosis: 4.8% (1/21) | 100% (21/21) | 76% (13/17) |
| Repici et al[19] 2004 | Prospect | Patients with persisting benign esophageal strictures after at least 6 dilation sessions: n = 15 1 Caustic stricture: 33% 2 Anastomotic stricture: 27% 3 Radiation stricture: 27% | SEPS: 100% (15/15) 6 wk | Mean: 22.7 (19-27) mo | Severe chest pain requiring analgesics: 33% (5/15) Mild/moderate mucosal hyperproliferation: 27% (4/15) Stent migration: 7% (1/15) | 100% (15/15) | 80% (12/15) |
RBES: Refractory benign esophageal strictures; SEPS: Self-expandable plastic stent; BD stent: Biodegradable stent; FC SEMS: Fully covered self-expandable metal stent.
Table 2.
Pooled analysis of 232 patients with refractory benign esophageal strictures according to Kochman’s criteria treated with self-expandable stent placement n (%)
| Stricture etiology | |
| Anastomotic strictures | 69 (29.7) |
| Peptic strictures | 58 (25.0) |
| Radiation strictures | 36 (15.5) |
| Caustic strictures | 29 (12.5) |
| Others | 26 (11.2) |
| Unknown | 14 (6.0) |
| Stent type | |
| FC SEMS | 85 (36.6) |
| BD stent | 77 (33.2) |
| SEPS | 70 (30.2) |
| PC SEMS | 0 (0) |
| Technical success | |
| Overall | 229 (98.7) |
| FC SEMS | 85 (100) |
| BD stent | 77 (100) |
| SEPS | 67 (95.7) |
| Clinical success | |
| Overall (n = 231) | 56 (24.2) |
| FC SEMS (n = 85) | 12 (14.1) |
| BD stent (n = 76) | 25 (32.9) |
| SEPS (n = 70) | 19 (27.1) |
FC SEMS: Fully covered self-expandable metal stent; BD stent: Biodegradable stent; SEPS: Self-expandable plastic stent; PC SEMS: Partially covered self-expandable metal stent.
Clinical success: The overall clinical success rate after single stent placement was 24.2%. The clinical success rates per type of stent are presented in Table 2. The time to recurrence of dysphagia after failed stent therapy varied widely. Stricture recurrence after FC SEMS removal was reported by three studies after median periods ranging from 15 d to 1.7 mo[9,10,12]. Recurrence of dysphagia after SEPS removal was reported by one study after a mean of 4 (range 2-9) wk[10]. After BD stent placement dysphagia recurred after mean periods ranging from 4 wk to 19 wk[10,15].
Stent migration, reactive tissue formation and food impaction: The overall pooled stent migration rate was 24.6% (57/232). By stent type migration rates were 31.8% (27/85) for FC SEMS, 14.3% (11/77) for BD stents and 27.1% (19/70) for SEPS.
Tissue hyperplasia was reported in 4.3% (10/232) of patients, causing recurrent dysphagia in 5 patients (2.2%) who all had received a BD stent. Hyperplastic tissue growth according to stent type was 3.5% (3/85) for FC SEMS, 7.8% (6/77) for BD stents and 1.4% (1/70) for SEPS. Food impaction was reported in 2.2% (5/232) of patients and occurred only in patients with a BD stent (6.5%, 5/77).
Adverse events: Excluding stent migration, reactive tissue formation and food impaction which were analyzed separately, 72 (31.0%) patients suffered a total of 77 complications due to stent placement. Major complications were reported in 17.7% of patients and minor complications in 13.4%. Major and minor complication rates per stent design are presented in Table 3. There was one (0.4%) stent-related death from a massive bleeding probably due to SEPS erosion into a major vessel[16]. Another patient with a BD stent died of aspiration pneumonia, which may have been caused by stricture recurrence[11].
Table 3.
Pooled analysis of adverse events in patients with refractory benign esophageal strictures n (%)
| Overall complications | 72 (31.0) |
| Overall major complications | 41 (17.7) |
| FC SEMS (n = 85) | 9 (10.6)1 |
| Severe retrosternal pain | 5 (5.9) |
| Severe nausea and vomiting | 2 (2.4) |
| Aspiration pneumonia | 2 (2.4) |
| Arrhythmia | 1 (1.2) |
| Percutaneous endoscopic gastrostomy because of impaired intake caused by severe, persistent odynophagia | 1 (1.2) |
| BD stents (n = 77) | 22 (28.6)1 |
| Severe retrosternal pain | 10 (13.0) |
| Hyperplasia-induced stenosis | 5 (6.5) |
| Bleeding, hematemesis | 5 (6.5) |
| Severe nausea and vomiting | 3 (3.9) |
| Aspiration pneumonia | 1 (1.3) |
| SEPS (n = 70) | 10 (14.3) |
| Severe retrosternal pain | 4 (5.7) |
| Perforation | 3 (4.3) |
| Bleeding, hematemesis2 | 2 (2.9) |
| Stent-induced fistula | 1 (1.4) |
| Overall minor complications | 31 (13.4) |
| FC SEMS (n = 85) | 15 (17.6)1 |
| Retrosternal pain | 6 (7.1) |
| Stent infolding/invagination | 3 (3.5) |
| Abdominal pain | 2 (2.4) |
| Globus sensation | 2 (2.4) |
| Reflux symptoms | 1 (1.2) |
| Vomiting | 1 (1.2) |
| Fever | 1 (1.2) |
| BD stents (n = 77) | 8 (10.4) |
| Nausea and vomiting | 3 (3.9) |
| Retrosternal pain | 2 (2.6) |
| Reflux symptoms | 2 (2.6) |
| Minor bleeding | 1 (1.3) |
| SEPS (n = 70) | 8 (11.4) |
| Reflux symptoms | 3 (4.3) |
| Retrosternal pain | 2 (2.9) |
| Minor bleeding | 2 (2.9) |
| Nausea and vomiting | 1 (1.4) |
Patients can have more than one complication;
Including one stent-related death from massive bleeding. FC SEMS: Fully covered self-expandable metal stent; BD stent: Biodegradable stent; SEPS: Self-expandable plastic stent; PC SEMS: Partially covered self-expandable metal stent.
Stent removal: Removal of SEPS or FC SEMS was scheduled after 4 to 12 wk and BD stents were left in place to dissolve. Stent removal was attempted in 92.9% (144/155) of patients with a SEPS or FC SEMS. Successful stent removal was achieved in 97.2% (140/144) of patients; FC SEMS (97.6%, 83/85) and SEPS (96.6%, 57/59). Two SEPS were removed during surgery, because one migrated SEPS could not be pulled through the ileocecal valve in a patient with a colon – ileocecal valve – terminal ileum interposition, and one was partially embedded by granulation tissue above the stent[16]. One FC SEMS had to be removed by a stent-in-stent procedure due to severe reactive tissue growth through the disrupted cover of the stent[12]. Another FC SEMS fractured during removal and was retrieved in two pieces[13].
Benign esophageal leaks, perforations and fistulae
The literature search revealed a recently published systematic review that analyzed the clinical outcomes of self-expandable stent placement for benign esophageal leaks and perforations in the literature published from 1990 to 2012[1]. Therefore, the studies published after the systematic review of Dasari et al[1] were considered for analysis. No RCTs that focused on the outcomes of stent placement for benign esophageal leaks, perforations or fistulae were identified. A total of 28 studies were selected from the literature, but after more careful reading of the articles eight more studies were excluded because they analyzed patients with active malignancy[21], performed a double stent strategy including airway stenting[22-24], included only postsurgical foregut leaks[25], did not perform subgroup analyses for patients with benign esophageal leaks, perforations or fistulae[26,27], or because of duplicate publication[28]. Ultimately, 20 studies were included for analysis, all with a retrospective study design (Table 4)[13,29-47]. A total of 643 patients with benign esophageal leaks, perforations and fistulae were considered for analysis. A total of 852 stents were inserted in 573 patients. In the remaining 70 patients the number of stents used was not reported. The main indications for self-expandable stent placement were postsurgical leaks (64.5%), iatrogenic perforations (19.6%), Boerhaave’s syndrome (7.8%) and fistulae (3.7%). The majority of inserted stents were FC SEMS (41.0%) and PC SEMS (37.7%). Stent placement was technically successful in 99.9% of cases. Further details are summarized in Table 5. Data on concurrent drainage of fluid collections were available for 425 patients, of whom 57.4% (244/425) underwent drainage procedures.
Table 4.
Literature on self-expandable stent placement for benign esophageal leaks, perforations and fistulae
| Ref. | Study design | Patients | Stent type, technical success (%) and time to removal | Additional sepsis-related procedures, follow-up | Complications and mortality | Successful stent removal | Clinical success |
| Dua et al[29] 2014 | Pro- and retrospect | Patients treated with a non-foreshortening FC SEMS for benign esophageal leaks: n = 6 1 Postsurgical leaks: n = 5 2 Esophagopericardial fistula: n = 1 Single stent: 83% (5/6) Multiple stents: 17% (1/6) | FC SEMS: 100% (7/7) Median time to removal: 50 (49-56) d | Unknown FU: unknown | Minor complications: Pneumoperitoneum during endoscopy secondary to air insufflation: 17% (1/6) Stent migration: 17% (1/7) Mortality rate: 33.3% (2/6) - cerebral embolism: 16.7% (1/6) - sepsis-related: 16.7% (1/6) | FC SEMS: 100% (4/4) | Overall: 67% (4/6) -Postsurgical leaks: 80% (4/5) -Fistula: 0% (0/1) |
| El Hajj et al[30] 2014 | Retrospect | Patients with attempted stent placement for esophageal leaks, fistulae and perforations: 1 Postsurgical leaks: n = 29 Single stent: 72% (21/29) Multiple stents: 28% (8/29) Excluded from analysis because patients were included with active malignancy: 1 Perforations: n = 10 2 Fistulae: n = 15 | PC SEMS: 100% (19/19) - 4 to 6 wk FC SEMS: 100% (30/30) - 6 to 8 wk SEPS: 100% (15/15) - 6 to 8 wk | Not analyzed for subgroup of patients with anastomotic leaks ≥ 3 mo FU: 100% | No subgroup analysis for patients with esophageal leaks 1 Chest pain 2 GI Bleed 3 Pneumothorax 4 Increase size of leak during deployment 5 Breakage of stent 6 Dysphagia 7 Aspiration pneumonia Stent migration not analyzed for subgroup of patients with esophageal leaks Mortality rate: 0% (0/29) | No subgroup analysis for patients with postsurgical leaks -Stent-in-stent procedure: 2 -Breakage of stent: 1 | Overall: 82.8% (24/29) -Primary closure rate: 72% (21/29) -Secondary closure rate: 38% (3/8) |
| Freeman et al[31] 2014 | Retrospect | Patients with intrathoracic leak after surgical repair of an acute iatrogenic esophageal perforation: n = 29 Single stent: 100% (29/29) | SEPS: 100% (24/24) FC SEMS: 100% (5/5) Mean time to removal: 22 (13-41) d | PEG: 10.3% (3/29) Thoracoscopic decortication: 10.3% (3/29) Jejunostomy: 3.4% (1/29) Median FU: 6 wk | No stent-related complications. Stent migration: 17.2% (5/29) - not analyzed according to stent type Mortality rate: 0% (0/29) | 100% (25/25) Not analyzed according to stent type | 86.2% (25/29) |
| Gubler et al[32] 2014 | Retrospect | Patients with benign (gastro)esophageal leaks, fistulae or perforations: n = 85 1 Iatrogenic perforations: n = 32 2 Anastomotic leaks: n = 31 3 Fistulae: n = 7 4 Boerhaave: n = 7 5 Other perforations: n = 8 Single stent: 78% (66/85) Multiple stents: 22% (19/85) | Total SEMS: n = 113 PC SEMS: n = 72 FC SEMS: n = 28 Unknown: n = 13 Technical success: 100% Average time to removal: 15 (1-111) d | Percutaneous/thoracoscopic drainage: 55.3% (47/85) OTSC: 2.4% (2/85) Vacuum-therapy: 2.4% (2/85) FU: at least 4 wk after stent removal | Stent migration: 8.8% (10/113) - not analyzed according to stent type Food bolus obstruction: 0.9% (1/113) - not analyzed according to stent type Mortality rate: 9.4% (8/85) 1 Unrelated to in situ stent: 3.5% (3/85) 2 Multi-organ failure: 2.4% (2/85) 3 Acute respiratory distress syndrome: 1.2% (1/85) 4 Heart insufficiency: 1.2% (1/85) 5 Aortic dissection: 1.2% (1/85) | 98.2% (107/109) - Irremovable: 2 - Spontaneous passage after migration: 1 Not analyzed according to stent type | Overall: 79% (67/85) -Postsurgical leaks: 74% (23/31) -Fistulae: 43% (3/7) -Iatrogenic: 94% (30/32) -Boerhaave: 71% (5/7) -Others: 75% (6/8) PC SEMS: 68% (49/72) FC SEMS: 54% (15/28) |
| Orive-Calzada et al[33] 2014 | Pro- and retrospect | Patients treated with FC SEMS for benign upper gastrointestinal fistulae and perforations: n = 56 1 Postsurgical leaks: n = 44 2 Iatrogenic perforations: n = 6 3 Boerhaave syndrome: n = 4 4 Other perforations: n = 2 Single stent: 59% (33/56) Multiple stents: 41% (23/56) | FC SEMS: 100% (87/87) PC SEMS: 100% (1/1) Median time to removal: 42 (9-1460) d | Surgical drainage: 30% (17/56) Percutaneous drainage: 41% (23/56) FU: unknown | Minor complications: Atrial fibrillation: 1.8% (1/56) Major complications: Stent-related perforation: 5.4% (3/56) Stent migration: 20.5% (18/88) - FC SEMS: 20.7 (18/87) - PC SEMS: 0% (0/1) Mortality rate: 16% (9/56) - cerebrovascular accident: 1.8% (1/56) - nosocomial pneumonia: 1.8% (1/56) - neoplasia: 1.8% (1/56) - secondary to sepsis: 10.7% (6/56) | FC SEMS: 100% (87/87) PC SEMS: 0/1 -Stent-in-stent procedure: 1 | Overall: 79% (44/56) -Postsurgical leaks: 78% (36/46) -Perforations: 80% (8/10) |
| Persson et al[34] 2014 | Retrospect | Patients with benign spontaneous, iatrogenic or traumatic esophageal perforations: n = 40 1 Iatrogenic perforation: n = 16 2 Boerhaave syndrome: n = 23 3 Other perforations: n = 1 Single stent: missing Multiple stents: missing | Total No. of stents missing Stent type missing Time to removal: 4-6 wk | Unknown FU: unknown | No stent-related complications Stent migration not analyzed according to stent type Mortality rate: 7.5% (3/40) 1 Multi-organ failure: 5% (2/40) 2 Respiratory insufficiency without sepsis: 2.5% (1/40) | Stent type and no. of stents removed missing -Removal during second procedure: 1 | 82.5% (33/40) No subgroup analysis according to etiology |
| Sharaiha et al[35] 2014 | Retrospect | Patients treated with stent placement for benign upper GI leaks: n = 18 1 Postsurgical leaks: n = 12 2 Iatrogenic perforation: n = 1 3Other fistulae: n = 5 Single stent: 28% (5/18) Multiple stents: 72% (13/18) | Total stents: n = 47 1 FC SEMS 2 PC SEMS 3 SEPS 4 Uncovered Technical success: 100% Mean time to removal: 54 (18-118) d | Clip/endoloop: 27.8% (5/18) Dilation: 33.3% (6/18) Surgery: 16.7% (3/18) FU: median 283 d (IQR 38-762) | Overall 9 complications in 5 patients 5 minor complications in 4 patients: - reflux/esophagitis: 16.7% (3/18) - abdominal pain: 5.6% (1/18) - collapsed stent: 5.6% (1/18) 4 Major complications in 3 patients: - aspiration pneumonia: 11.1% (2/18) - perforation: 5.6% (1/18) - stricture: 5.6% (1/18) Tissue hyperplasia: 5.6% (1/47) - stent type unknown Food impaction/bezoar: 11.1% (2/47) - stent type unknown Stent migration not analyzed for subgroup of patients with esophageal leaks Overall mortality rate: 5.6% (1/18) Not specified | No subgroup analysis for patients with benign leaks -Stent-in-stent procedure: 7 -Irremovable uncovered stent: 1 | Overall: 47% (7/15) -Postsurgical leaks: (5/11) -Fistula: 67% (2/3) -Iatrogenic: 0% (0/1) |
| Shim et al[36] 2014 | Retrospect | Patients who underwent endoscopic treatment for anastomotic leakage after total gastrectomy: n = 27 1 FC SEMS: n = 13 2 Non stent therapy: n = 14 Single stent: 85% (11/13) Multiple stents: 15% (2/13) | FC SEMS: 100% (15/15) PC SEMS: 100% (1/1) Median time to removal: 38 (0-69) d | Concurrent fluid drainage: 61.5% (8/13) FU: unknown | Minor complication: Stent malposition: 6.3% (1/13) Stent migration: 25% (4/16) - FC SEMS: 26.7% (4/15) - PC SEMS: 0% (0/1) Tissue in- or overgrowth: 6.3% (1/16) - FC SEMS: 6.7% (1/15) Mortality rate: 15.4% (2/13) - sepsis related: 7.7% (1/13) - non-stent related bleeding: 7.7% (1/13) | FC SEMS: 100% (11/11) PC SEMS: 100% (1/1) | Overall: 67% (8/12) -Primary closure rate: 67% (8/12) -Secondary closure rate: 0% (0/4) |
| Brangewitz et al[37] 2013 | Retrospect | Patients with esophageal defects treated with stent placement: n = 39 1 Postsurgical leaks: n = 31 2 Iatrogenic perforations: n = 6 3 Boerhaave syndrome: n = 2 Single stent: 100% (39/39) | FC SEMS: 100% (39/39) Median time to removal: 33 (9-132) d | Unknown FU: unknown | Minor complications: - stent-related ulcers: 12.8% (5/39) Major complications: - severe bleed at upper end of stent: 2.6% (1/39) - death due to esophageal necrosis at proximal end of stent: 2.6% (1/39) Stent migration: 15.4% (6/39) Mortality rate: 25.6% (10/39) - esophageal necrosis at proximal stent end: 2.6% (1/39) - not specified: 23.1% (9/39) | FC SEMS: 90.3% (28/31) -Self-limiting bleed: 2 -Migrated stent requiring surgical removal: 1 | 53.8% (21/39) No subgroup analysis according to etiology |
| Leenders et al[38] 2013 | Retrospect | Patients with anastomotic leakage after esophageal resection or bariatric surgery: n = 26 Single stent: 81% (21/26) Multiple stents: 19% (5/26) | FC SEMS: 100% (31/31) PC SEMS: 100% (2/2) Mean time to removal: 11 (1-63) wk | Unknown FU: range 2-144 wk | Minor complications: - stent disintegration all with FC SEMS: 11.5% (3/26) Major complications: - stent-related perforation with FC SEMS: 3.8% (1/26) Stent migration: 24.2% (8/33) - FC SEMS: 25.8% (8/31) Tissue ingrowth: 6.1% (2/33) - PC SEMS: 100% (2/2) Mortality rate: 19.2% (5/26) - sepsis-related: 19.2% (5/26) | FC SEMS: 100% (26/26) PC SEMS: 0% (0/2) -Traumatic removal due to tissue ingrowth: 2 | 80.8% (21/26) |
| Wilson et al[39] 2013 | Retrospect | Patients treated with FC SEMS placement for benign esophagogastric diseases: n = 33 1 Perforation: n = 7 2 Anastomotic leak: n = 14 3 Sleeve gastrectomy leak: n = 6 4 Fistula: n = 6 Single stent: missing Multiple stents: missing | FC SEMS: 100% (40/40) Average time to removal: 47 d | Drainage procedure: 66.7% (22/33) VATS/open: 36.4% (12/33) Tube thoracostomy: 21.2% (7/33) Percutaneous: 9.1% (3/33) FU: unknown | Major complications: - severe hemorrhage from aorta-esophageal fistula: 3.0% (1/33) No subgroup analysis for patients with esophageal leaks, fistulae and perforations: - stent migration - food impaction Mortality rate: 0% (0/33) | No subgroup analysis for patients with esophageal leaks, fistulae and perforations -Stent fracture: 2 | 94% (31/33) avoided open repair -Postsurgical leaks: 95% (19/20) -Perforations: 86% (6/7) -Fistulae: 100% (6/6) |
| Van Boeckel et al[40] 2012 | Retrospect | Patients treated with a SEMS or SEPS for sealing a benign esophageal rupture or anastomotic leak: n = 52 1 Anastomotic leak: n = 32 2 Iatrogenic perforation: n = 13 3 Boerhaave syndrome: n = 4 4 Others: n = 3 Single stent: missing Multiple stents: missing | PC SEMS: 98% (60/61) FC SEMS: 100% (15/15) SEPS: 100% (7/7) Median time to removal: 25 (1-197) d | Concurrent fluid drainage: 46.2% (24/52) Median FU: 470 (25-1200) d | Major complications: Severe retrosternal pain: 3.8% (2/52) - all PC SEMS Hemorrhage: 3.8% (2/52) - FC SEMS: stent-related death 1.9% (1/52) - PC SEMS: required adrenaline injections: 1.9% (1/52) Ruptured stent cover: 7.2% (6/83) - PC SEMS: 9.8% (6/61) Tissue in-/overgrowth: 9.6% (8/83) - PC SEMS: 13.1% (8/61) Stent migration: 12.0% (10/83) - FC SEMS: 20% (3/15) - PC SEMS: 10% (6/61) | 88.7% (63/71) Tissue in- and/or overgrowth at removal of 8 PC SEMS -Stent-in-stent procedure: 4 -Esophageal rupture: 2 -Second endoscopic procedure: 1 -Esophagectomy: 1 Not analyzed according to stent type | 65.4% (34/52) No subgroup analysis according to etiology PC SEMS: 69% FC SEMS: 56% SEPS: 71% |
| - SEPS: 14% (1/7) Food obstruction: 3.6% (3/83) - PC SEMS: 4.9% (3/61) Mortality rate: 13.5% (7/52) - severe stent-related hemorrhage: 1.9% (1/52) - sepsis related: 7.7% (4/52) - malignancy: 1.9% (1/52) - active euthanasia: 1.9% (1/52) | |||||||
| Buscaglia et al[41] 2011 | Retrospect | Patients treated for benign esophageal conditions by FC SEMS placement: - fistula or leak: n = 15 Single stent: 67% (10/15) Multiple stents: 33% (5/15) | FC SEMS: 100% (24/24) Median time to removal: 42.5 (3-122) d | Unknown FU: unknown | Stent migration: 33.3% (8/24) Further complications not analyzed for subgroup of patients with fistulae and leaks - chest pain - globus sensation Mortality rate: 6.7% (1/15) - paraspinal abscess related to persistent fistula | No subgroup analysis for patients with esophageal leaks and fistulae -Removal during surgery: 1 -Stent-in-stent procedure: 1 | 79% (11/14) No subgroup analysis according to etiology |
| Dai et al[42] 2011 | Retrospect | Patients treated with SEPS for: - postoperative esophageal anastomotic leaks: n = 30 Single stent: missing Multiple stents: missing Excluded from analysis because patients were included with active malignancy: - esophageal perforations: n = 6 - fistulae: n = 5 | Total no. of SEPS missing Technical success: 100% Mean time to stent removal: 30 (7-62) d | Interventional drainage: 40% (12/30) Tracheotomy: 43% (13/30) Mean FU: 12.8 (1-61) mo | Major complications: - stent dislocation and inability to place new stent requiring rethoracotomy: 3.3% (1/30) Stent migration not analyzed for subgroup of patients with esophageal leaks Mortality rate: (2/30) - persistent sepsis and multi-organ failure: 6.7% (2/30) | No. of removed stents missing One migrated stent in a patient with an esophago-colonic anastomotic leak could not be removed | 90% (27/30) |
| David et al[43] 2011 | Pro- and retrospect | Patients treated with SEMS for esophageal or gastric perforation and intrathoracic contamination: n = 30 - postsurgical leak: n = 13 - boerhaave syndrome: n = 6 - iatrogenic perforation: n = 6 - fistulae: n = 4 - other perforation: n = 1 Single stent: 50% (15/30) Multiple stents: 50% (15/30) | At least 62 stents - FC SEMS - PC SEMS Technical success: 100% Average duration of stenting: 29 d | Chest tube thoracostomy: - Alone: 23.3% (7/30) - Additional intervention: 76.7% (23/30) Pleural decortication: 56.7% (17/30) Muscle-flap reinforcement: 36.7% (11/30) Average FU: 8.1 mo | Minor complications: - pain: 6.7% (2/30) - hiccups: 3.3% (1/30) - nausea: 3.3% (1/30) Major complications: - bowel obstruction: 6.7% (2/30) - erosion: 3.3% (1/30) - left atrial compression: 3.3% (1/30) Stent migration: 6.7% (2/30) - not analyzed according to stent type Mortality rate: 10% (3/30) - multi-organ failure: 3.3% (1/30) - multiple emboli caused by esophago-atrial fistula: 3.3% (1/30) - aspiration during contrast study: 3.3% (1/30) | No. of removed stents missing Not analyzed according to stent type | 76.7% (23/30) No subgroup analysis according to etiology |
| Eloubeidi et al[13] 2011 | Pro- and retrospect | Patients with benign esophageal lesions treated with Alimaxx-E stent: n = 16 - postsurgical leaks: n = 11 - fistula: n = 1 - iatrogenic perforations: n = 3 - other: n = 1 Single stent: 81% (13/16) Multiple stents: 19% (3/16) | FC SEMS: 100% (16/16) In situ for: 51 ± 45 d (range 9-163) | Dilation: 6.3% (1/16) PEG placement: 6.3% (1/16) FU: unknown | Minor complications: 1 Stent infolding/invagination: 6.3% (1/16) 2 Chest pain: 6.3% (1/16) 3 Dysphagia: 6.3% (1/16) 4 Globus sensation: 6.3% (1/16) Major complications: 1 Respiratory compromise: 6.3% (1/16) 2 Aspiration pneumonia: 12.5% (2/16) Stent migration: 31.3% (5/16) Mortality rate: 0% (0/16) | FC SEMS: 100% (16/16) One stent was retrieved in two pieces | 43.8% (7/16) No subgroup analysis according to etiology |
| Freeman et al[44] 2011 | Unknown | Hospitalized patients with an anastomotic leak after esophagectomy: n = 17 Single stent: 100% (17/17) | SEPS: 100% (14/14) FC SEMS: 100% (3/3) Mean time to removal: 17 (12-27) d | VATS pleural drainage: 29.4% (5/17) Pharyngostomy: 5.9% (1/17) Tube jenunostomy: 5.9% (1/17) FU: at least 3 mo after stent removal | No complications associated with stent placement or removal Stent migration: 17.6% (3/17) - not analyzed according to stent type Mortality rate: 0% (0/17) | SEPS: 100% (14/14) FC SEMS: 100% (3/3) | 94% (16/17) |
| Nguyen et al[45] 2011 | Retrospect | Patients who developed postoperative leaks after minimally invasive esophagectomy: n = 18 - conventional treatment: n = 9 - FC SEMS placement: n = 9 Single stent: 100% (9/9) | FC SEMS: 100% (9/9) Removal after 6 wk | Percutaneous drainage: 22% (2/9) Tracheostomy: 11% (1/9) FU: unknown | No stent-related complications Mortality rate: 0% (0/9) | FC SEMS: 100% (9/9) | 100% (9/9) |
| Schweigert et al[46] 2011 | Retrospect | Patients treated with stent placement for intrathoracic leak after esophagectomy: n = 12 Single stent: 100% (12/12) | PC SEMS: 100% (12/12) Median time to removal: 48 (16-99) d | Tube thoracostomy: 100% (12/12) FU: unknown | Major complications: 1 Death by hemorrhage from stent-related erosion into the aorta: 8.3% (1/12) 2 Stent-related fistula after removal: 8.3% (1/12) Stent migration: n = missing Mucosal hyperproliferation: n = missing Mortality rate: 16.7% (2/12) 1 Stent-related death by hemorrhage: 8.3% (1/12) 2 Pulmonary aspiration after stent removal and successful healing of the leak: 8.3% (1/12) | PC SEMS: 100% (10/10) | 81.8% (9/11) |
| Swinnen et al[47] 2011 | Retrospect | Patient treated with PC SEMS placement for benign upper GI leaks or perforations: n = 88 - postsurgical leaks: n = 65 - boerhaave syndrome: n = 4 - iatrogenic perforation: n = 14 - other perforations: n = 5 | PC SEMS: 100% (153/153) Median time to removal for 33 PC SEMS: 23 d Median time to removal for 99 PC SEMS: 69 d | Drainage of collections: 47.7% (42/88) - Surgical: 26.1% (23/88) - Percutaneous: 15.9% (14/88) - Endoscopic: 5.7% (5/88) | Minor complications: - transient stent-related dysphagia: 11.4% (10/88) Major complications: - bleeding requiring intervention: 5.7% (5/88) - stent-related perforation: 1.1% (1/88) | PC SEMS: 24.4% (33/135) Stent-in-stent procedure: 73.3% (99/135) Removal during surgery: 2.2% (3/135) | 77.6% (59/76) No subgroup analysis according to etiology |
| Single stent: 58% (51/88) Multiple stents: 42% (37/88) | Follow-up after removal: 3 mo: 83% 7 mo: 81% 1 yr: 72% | - tracheal compression: 1.1% (1/88) - dysphagia due to tissue hyperplasia: 18.2% (16/88); PC SEMS: 10.5% (16/153) Stent migration: 11.1% (17/153) of PC SEMS mortality rate: 10.2% (9/88) 1 Sepsis related: 3.4% (3/88) 2 Pulmonary embolism: 1.1% (1/88) 3 Full-blown AIDS: 1.1% (1/88) 4 Cardiac disease: 1.1% (1/88) Three additional deaths during first 3 mo after treatment: 1 Sepsis after surgery: 1.1% (1/88) 2 Tension pneumothorax: 1.1% (1/88) 3 Pneumonia: 1.1% (1/88) |
FC SEMS: Fully covered self-expandable metal stent; FU: Follow-up; PC SEMS: Partially covered self-expandable metal stent; SEPS: Self-expandable plastic stent; VATS: Video-assisted thoracic surgery; PEG: Percutaneous endoscopic gastrostomy; OTSC: Over-the-scope-clips; IQR: Interquartile range.
Table 5.
Pooled analysis of 643 patients with benign esophageal leaks, perforations and fistulae treated with self-expandable stent placement
| Etiology | |
| Postsurgical leaks | 415 (64.5) |
| Iatrogenic perforations | 126 (19.6) |
| Boerhaave’s syndrome | 50 (7.8) |
| Fistulae | 24 (3.7) |
| Others/not specified | 28 (4.4) |
| Stent type of 852 stents used in 573 patients1 | |
| FC SEMS | 349 (41.0) |
| PC SEMS | 321 (37.7) |
| SEPS | 60 (7.0) |
| Stent type unknown | 122 (14.3) |
| Technical success | |
| Overall | 851 (99.9) |
| FC SEMS | 349 (100) |
| PC SEMS | 320 (99.7) |
| SEPS | 60 (100) |
| Stent type unknown | 122 (100) |
| No. of stents per patient | |
| Single stent placement | 357 (55.5) |
| Multiple stents inserted | 131 (20.4) |
| Unknown | 155 (24.1) |
| Clinical success | |
| Overall (n = 625) | 480 (76.8) |
| According to etiology (n = 358) | |
| Postsurgical leaks (n = 247) | 201 (81.4) |
| Perforations2 (n = 86) | 74 (86.0) |
| Fistulae (n = 17) | 11 (64.7) |
| Others/not specified (n = 8) | 6 (75.0) |
In two studies including 70 patients the total number of stents used was not reported;
Including iatrogenic and spontaneous perforations. FC SEMS: Fully covered self-expandable metal stent; PC SEMS: Partially covered self-expandable metal stent; SEPS: Self-expandable plastic stent.
Clinical success: The overall clinical success rate of esophageal stent placement for benign leaks, perforations and fistulae was 76.8% (480/625). Subgroup analysis according to etiology was possible for 358 patients. The highest clinical success rate was achieved in patients with perforations (86.0%), followed by postsurgical leaks (81.4%) and fistulae (64.7%) (Table 5). When solely FC SEMS were used, clinical success was achieved in 73.0% (135/185) of patients. Solely PC SEMS were used in two studies with a pooled clinical success rate of 78.2% (68/87). Only one study focused on the outcomes of SEPS placement and reported clinical success in 90% (27/30) of patients with anastomotic leaks.
Stent migration, reactive tissue formation and food impaction: Stent migration could be analyzed in 320 patients who received a total of 468 self-expandable stents. The overall pooled stent migration rate was 16.5% (77/468). By stent type migration rates were 21.8% (53/243) for FC SEMS and 10.6% (23/218) for PC SEMS. Data were insufficient to analyze the stent migration rate of SEPS.
Pooled analysis of tissue hyperplasia was possible for 384 patients in whom 530 stents were inserted. Tissue hyperplasia was directly reported or deduced out of context (e.g., stent-in-stent procedure for removal) in 111 cases (28.9% of patients and 20.9% of stents). The rate of reactive tissue formation according to stent type was 0.4% (1/267) for FC SEMS, 50.5% (110/218) for PC SEMS and 0% (0/45) for SEPS.
Of the 812 stents that were inserted in 540 patients, food impaction was reported in 6 cases (1.1% of patients and 0.7% of stents). Data were insufficient to analyze food impaction according to type of stent.
Adverse events and mortality: Two studies including 44 patients and 88 stent placements could not be analyzed because of missing subgroup analyses for patients with benign esophageal leaks[30,41]. So adverse events were evaluated for 599 patients. Excluding stent migration, reactive tissue formation and food impaction, which were analyzed separately, 80 (13.4%) patients suffered a total of 82 complications due to stent placement. The overall pooled major and minor complication rate were 7.8% and 5.5%, respectively. Complication rates according to stent type are presented in Table 6.
Table 6.
Pooled analysis of adverse events in patients with benign esophageal leaks, perforations and fistulae
| Total number of patients analyzed: n = 599 | No. of patients (n = 599) | No. of FC SEMS (n = 295) | No. of PC SEMS (n = 302) | No. of SEPS (n = 75)1 | Stent type unknown (n = 162)2 |
| Overall complications | 803 (13.4) | 26 (8.8) | 38 (12.6) | 1 (1.3) | 17 (10.5) |
| Overall major complications | 473 (7.8) | 11 (3.7) | 28 (9.3) | 1 (1.3) | 8 (4.9) |
| Hyperplasia-induced stenosis | 16 (2.7) | 0 | 16 | 0 | 0 |
| Hemorrhage4 | 8 (1.3) | 24 | 6 | 0 | 0 |
| Stent-related perforation | 6 (1.0) | 4 | 1 | 0 | 1 |
| Aspiration pneumonia | 4 (0.7) | 2 | 0 | 0 | 2 |
| Respiratory compromise/ tracheal compression | 2 (0.3) | 1 | 1 | 0 | 0 |
| Severe retrosternal pain | 2 (0.3) | 0 | 2 | 0 | 0 |
| Bowel obstruction | 2 (0.3) | 0 | 0 | 0 | 2 |
| Erosion4 | 2 (0.3) | 0 | 14 | 0 | 1 |
| Hemorrhage from aorta-esophageal fistula | 1 (0.2) | 1 | 0 | 0 | 0 |
| Stricture formation | 1 (0.2) | 0 | 0 | 0 | 1 |
| Stent-related fistula | 1 (0.2) | 0 | 1 | 0 | 0 |
| Stent dislocation and inability to place new stent requiring rethoracotomy | 1 (0.2) | 0 | 0 | 1 | 0 |
| Left atrial compression | 1 (0.2) | 0 | 0 | 0 | 1 |
| Death due to esophageal necrosis at proximal stent end | 1 (0.2) | 1 | 0 | 0 | 0 |
| Overall minor complications | 333 (5.5) | 15 (5.1) | 10 (3.3) | 0 (0) | 9 (5.6) |
| Transient stent-related dysphagia | 11 (1.8) | 1 | 10 | 0 | 0 |
| Stent-related ulcers | 5 (0.8) | 5 | 0 | 0 | 0 |
| Reflux/esophagitis | 3 (0.5) | 0 | 0 | 0 | 3 |
| Chest pain | 3 (0.5) | 1 | 0 | 0 | 2 |
| Stent disintegration | 3 (0.5) | 3 | 0 | 0 | 0 |
| Stent collapse/invagination | 2 (0.3) | 1 | 0 | 0 | 1 |
| Pneumoperitoneum during endoscopy secondary to air insufflation | 1 (0.2) | 1 | 0 | 0 | 0 |
| Atrial fibrillation related to sedation | 1 (0.2) | 1 | 0 | 0 | 0 |
| Stent malposition | 1 (0.2) | 1 | 0 | 0 | 0 |
| Abdominal pain | 1 (0.2) | 0 | 0 | 0 | 1 |
| Nausea | 1 (0.2) | 0 | 0 | 0 | 1 |
| Globus sensation | 1 (0.2) | 1 | 0 | 0 | 0 |
| Hiccups | 1 (0.2) | 0 | 0 | 0 | 1 |
Including 30 patients in whom the number of SEPS used was not reported;
Including 40 patients in whom the number of stents used was not reported;
Patients can have more than one complication;
Including one stent-related death. FC SEMS: Fully covered self-expandable metal stent; PC SEMS: Partially covered self-expandable metal stent; SEPS: Self-expandable plastic stent.
Mortality during the course of stent therapy was reported in 10.0% of all patients with benign leaks, perforations and fistulae. Stent-related mortality was reported in three cases (0.5%). One was caused by severe bleeding in a patient treated with a FC SEMS who refused further interventions[40]. In another case esophageal necrosis at the proximal end of a FC SEMS resulted in a fatal outcome[37]. The third stent-related death was due to massive hemorrhage caused by erosion of a PC SEMS into the aorta[46]. Other non-stent-related causes for mortality are summarized in Table 7.
Table 7.
Overall mortality in 643 patients treated with self-expandable stents for benign esophageal leaks, perforations and fistulae n (%)
| Overall mortality | 64 (10.0) |
| Stent-related | 3 (0.5) |
| Sepsis-related | 23 (3.6) |
| Multi-organ failure | 5 (0.8) |
| Cerebral embolism/cerebrovascular accident | 2 (0.3) |
| Heart insufficiency/cardiac disease | 2 (0.3) |
| Pneumonia | 2 (0.3) |
| Malignancy | 2 (0.3) |
| Non stent-related bleeding | 1 (0.2) |
| Respiratory insufficiency without sepsis | 1 (0.2) |
| Pulmonary embolism | 1 (0.2) |
| Acute respiratory distress syndrome | 1 (0.2) |
| Pulmonary aspiration after healing of leak | 1 (0.2) |
| Aortic dissection | 1 (0.2) |
| Tension pneumothorax | 1 (0.2) |
| Paraspinal abscess related to persistent fistula | 1 (0.2) |
| Full-blown AIDS | 1 (0.2) |
| Aspiration during contrast study | 1 (0.2) |
| Multiple emboli caused by esophago-atrial fistula | 1 (0.2) |
| Active euthanasia | 1 (0.2) |
| Not specified | 13 (2.0) |
Stent removal: The outcome of stent removal could be analyzed in 13 studies in which 555 of the 615 inserted stents were subsequently removed. The overall pooled successful removal rate was 78.7% (437/555). Causes of failure were stent embedding by granulation tissue requiring stent-in-stent procedures for removal (n = 104, 18.7%), surgical removal (n = 5, 0.9%), esophageal ruptures (n = 2, 0.4%), irremovable stent (n = 2, 0.4%), self-limiting bleedings (n = 2, 0.4%), traumatic removal due to tissue ingrowth (n = 2, 0.4%) and additional endoscopic procedures (n = 1, 0.2%). When uneventful stent-in-stent procedures were considered as successful removals, the overall successful stent removal rate increased up to 97.5% (541/555). Stent removal outcome could be analyzed for 187 FC SEMS of which 84% (158/187) were removed after a median period of 5 to 7 wk. FC SEMS removal was successful in 98.4% (184/187) of procedures, including one fractured stent that was retrieved in two pieces. There were two cases of a self-limiting bleeding and one migrated FC SEMS required surgical removal. The majority of PC SEMS (73%, 109/149) were removed after a median period of 7 to 10 wk. One study accounted for 91% (135/149) of the PC SEMS removals[47]. Successful endoscopic removal of PC SEMS was achieved in 29.5% (44/149) of cases and in 96.6% (144/149) after stent-in-stent procedures. Three PC SEMS were removed during surgery and two removals were traumatic due to tissue ingrowth. The two cases of esophageal rupture also occurred during the removal of PC SEMS, but could not be included in the pooled analysis because the overall number of removed PC SEMS was not reported[40]. The ruptures were successfully treated with another stent. The outcome of SEPS removal could be extracted from one study and was successful in 100% (14/14) of cases after a mean stent time of 17 d[44].
DISCUSSION
This pooled analysis of the literature showed that the overall clinical success rate of self-expandable stent placement was 24.2% for refractory benign esophageal strictures and 76.8% for benign esophageal leaks, perforations and fistulae. With regard to refractory benign strictures, the meta-analysis by Thomas et al[7] found sustained improvement of dysphagia in 46.2% of patients treated with self-expandable stents, which is almost twice as high as the clinical success rate (24.2%) in this pooled analysis. Also the systematic review by Repici et al[48] reported a much higher clinical success rate of 52% after SEPS placement for benign esophageal strictures. The difference in clinical success between our pooled analysis and the aforementioned systematic reviews may be explained by the etiology and the severity of the strictures. The study population of Thomas et al[7] mainly included corrosive (43%), postsurgical (25%) and radiation (11%) strictures. The etiologies of the patients in the systematic review on SEPS treatment mainly included postsurgical (38%), corrosive (25%) and radiation (15%) strictures[48]. In our analysis, peptic strictures accounted for 25.0% of patients, while corrosive strictures represented only 12.5%. However, the literature data were insufficient to analyze the clinical outcomes of stent placement according to stricture etiology. With regard to the severity of the strictures, Thomas et al[7] included three studies, that accounted for 50% of weight in the meta-analysis, in which patients had a history of two or less dilatations before stent placement. The review by Repici et al[48] did not provide details on the number of previous dilatations, but included mainly retrospective studies with heterogeneous definitions of refractory or recurrent strictures. We think that the more homogeneous population included in this analysis of prospective studies, that fulfilled Kochman’s criteria[8], had more severe strictures and therefore a poorer outcome of stent therapy. Thomas et al[7] reported a significantly higher clinical success rate for Polyflex stents (55.3%) compared with nitinol stents (36.7%). Our results also showed a lower clinical success rate with the use of FC SEMS (14.1%) compared with SEPS (27.1%) and BD stents (32.9%). We do not have a good explanation for this finding. Complication, stent migration and tissue response rates were not significantly higher with the use of FC SEMS.
Safety analyses in patients treated with self-expandable stents for refractory benign strictures showed an overall complication rate of 31.0%, including a major complication rate of 17.7%. That complications are frequent during the course of stent therapy has also been demonstrated by several retrospective studies that were not included in our analyses[49-53]. The major complication rate of BD stents (28.6%) was twice as high as those of SEPS (14.3%) and FC SEMS (10.6%), because they caused more retrosternal pain, hyperplasia-induced stenoses and bleedings. Severe retrosternal pain occurred in 13.0% of patients who received a BD stent. Pain after stent placement has been postulated to be caused by the radial force of the stent against the tight stricture and is mainly reported within the first week after stent placement[9,11,15,49,53]. However, in vitro analysis of the radial and axial forces of 23 esophageal stent models showed that BD stents had a relatively low radial force and high axial force[54]. Therefore, it is more likely that because of the rigid stent design BD stents interact less well with the peristalsis of the esophagus causing more spasm and pain. In this analysis clinically relevant hyperplastic tissue growth was reported in 7.8% of BD stents. Two case series not included in this review also showed that reactive tissue formation is common after BD stent placement (Figure 3)[17,55]. The occurrence of tissue growth may be explained as a reaction to the chemical processes of degradation, which may also trigger bleedings from the affected esophageal mucosa. So one should be aware that the higher efficacy of BD stent placement is attended by an increased risk of complications.
Figure 3.
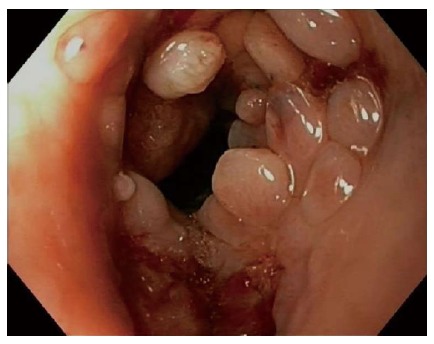
Endoscopic image of granulation tissue growth 4 mo after biodegradable stent placement for a refractory benign esophageal anastomotic stricture.
The clinical success rate (76.8%) of self-expandable stent placement for benign esophageal leaks, perforations and fistulae found in this pooled analysis is comparable with the 81% of the systematic review by Dasari et al[1] (Figure 4A and B). In contrast to our analysis, the latter review excluded patients with leaks from the gastric staple line after sleeve gastrectomy and did not analyze patients with fistulae. In our study clinical success according to etiology was 81.4% for postsurgical leaks, 86.0% for perforations and 64.7% for fistulae. Though derived from retrospective series, these results seem promising. Patients with esophageal leaks or ruptures are usually in poor condition with elevated septic parameters and require invasive management, like drainage procedures, surgery and ICU care. This is reflected by the increased mortality rate of 7.2%-25.8% after postsurgical esophageal leakage[56-58]. Several treatment strategies have been described for the management of esophageal leaks, such as endoscopic vacuum therapy, nose fistula tube drainage, surgical repair and conservative management[59-61]. Retrospective comparison of 41 patients with an anastomotic leak after esophagectomy who were matched by clinical status, showed that endoscopic vacuum therapy resulted in a lower mortality rate (12%, 2/17) compared with surgical treatment (50%, 9/18) and stent placement (83%, 5/6) in systemically ill patients[58]. Another retrospective study reported a significantly higher closure rate after endoscopic vacuum therapy (84%) compared with stent therapy (53.8%) in 71 patients with esophageal defects[37]. However, one should keep in mind that success of stent placement depends on the size of the esophageal lesion, the delay between diagnosis and stent placement and if the patient has elevated septic parameters[30,33,34,47]. Stent placement is most likely to fail in a large lesion (> 15 mm), that exists for several weeks in a septic patient. Therefore, patients with an esophageal leak should receive a multidisciplinary patient-tailored approach.
Figure 4.
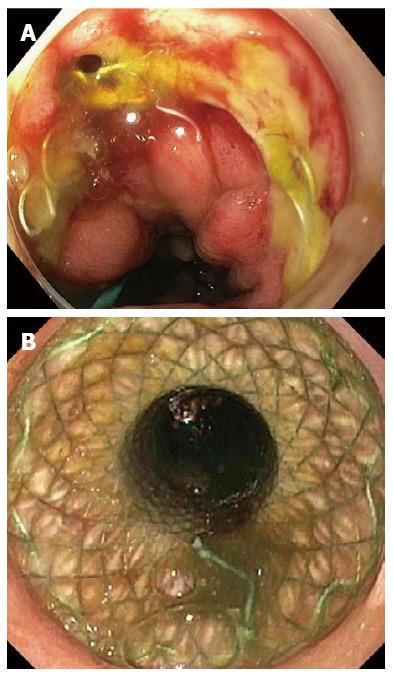
A small leak at the anastomosis of the esophagus and the gastric tube 5 d after esophagectomy (A) and esophageal fully covered self-expandable metal stent placement for a small anastomotic leak (B).
The removability of self-expandable stents was safe and feasible with an overall successful removal rate of 97.2% in patients with refractory strictures and 78.7% in patients with esophageal leaks. The fact that PC SEMS were used in 38% of patients with esophageal leaks resulted in a lower overall successful removal rate. PC SEMS removal was often complicated by stent embedding requiring stent-in-stent procedures to induce pressure-necrosis of the granulation tissue to facilitate the removal procedure. The vast majority of stent-in-stent procedures were reported in the study by Swinnen et al[47]. The removal of FC SEMS and SEPS removal was much safer with successful removal rates of 96.6% up to 98.4%. The relation between the use of PC SEMS and complicated stent removal has also been demonstrated by several large retrospective series[62,63].
This pooled analysis of the literature has several limitations. The prospective data on the outcomes of stent placement for refractory benign esophageal strictures reflect a patient population with various causes for stricture formation. Data were insufficient to provide analyses according to stricture etiology. The studies that were analyzed on the outcomes of esophageal stent placement for benign leaks, perforations and fistulae were all retrospective, causing heterogeneity and underreporting of adverse outcomes.
In conclusion, the outcomes of self-expandable stent placement for refractory benign esophageal strictures were poor with a clinical success rate of 24.4% and a major complication rate of 17.7%. However, randomized trials are needed to put these outcomes into perspective. Although derived from retrospective series, the evidence on stent placement for benign esophageal leaks, perforations and fistulae is promising with an overall clinical success rate of 76.8%.
COMMENTS
Background
Self-expandable stents in various types are increasingly being used for the treatment of refractory benign esophageal strictures and benign esophageal leaks, perforations and fistulae.
Research frontiers
It is hypothesized that esophageal stent placement for benign refractory strictures prolongs the dysphagia-free period compared with conventional dilatation therapy. Besides application for the treatment of strictures, esophageal stents are also used to seal leaks, perforations and fistulae.
Innovations and breakthroughs
The literature on esophageal stent placement for benign indications is heterogeneous and usually includes small samples. In this systematic review we performed a pooled analysis on the treatment outcomes of 232 patients with refractory strictures and 643 patients with leaks, perforations and fistulae.
Applications
This pooled analysis may be helpful for the endoscopist in the decision-making on the indication for esophageal stent placement and also to inform the patient on the risks and benefits of stent therapy.
Terminology
Biodegradable stents are only used for strictures, because they have the property to dissolve. Nitinol metal stents (SEMS) have an outer membrane of silicone or polytetrafluoroethylene to prevent hyperplastic tissue ingrowth. Because they are covered, they are removable and can also be used to seal leaks. Partially covered SEMS usually become partially embedded by granulation tissue, which prevents stent migration, but makes them harder to remove. Self-expandable plastic stents consist of plastic instead of metal and are fully covered.
Peer-review
This review is well-written and comprehensive review about this subject.
Footnotes
Conflict-of-interest: Emo van Halsema had no conflict of interest. Jeanin van Hooft: consultancy work for Cook Medical, Boston Scientific, Abbott and Covidien.
Data sharing: All data in this systematic review were extracted from the original articles and are presented in the literature Tables 1 and 4. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 3, 2014
First decision: November 19, 2014
Article in press: December 17, 2014
P- Reviewer: Bustamante-Balen M, Chen MJ, Nishida T S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
References
- 1.Dasari BV, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg. 2014;259:852–860. doi: 10.1097/SLA.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 2.Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329:1302–1307. doi: 10.1056/NEJM199310283291803. [DOI] [PubMed] [Google Scholar]
- 3.De Palma GD, di Matteo E, Romano G, Fimmano A, Rondinone G, Catanzano C. Plastic prosthesis versus expandable metal stents for palliation of inoperable esophageal thoracic carcinoma: a controlled prospective study. Gastrointest Endosc. 1996;43:478–482. doi: 10.1016/s0016-5107(96)70290-0. [DOI] [PubMed] [Google Scholar]
- 4.Siersema PD, Hop WC, Dees J, Tilanus HW, van Blankenstein M. Coated self-expanding metal stents versus latex prostheses for esophagogastric cancer with special reference to prior radiation and chemotherapy: a controlled, prospective study. Gastrointest Endosc. 1998;47:113–120. doi: 10.1016/s0016-5107(98)70342-6. [DOI] [PubMed] [Google Scholar]
- 5.Davies N, Thomas HG, Eyre-Brook IA. Palliation of dysphagia from inoperable oesophageal carcinoma using Atkinson tubes or self-expanding metal stents. Ann R Coll Surg Engl. 1998;80:394–397. [PMC free article] [PubMed] [Google Scholar]
- 6.Birch JF, White SA, Berry DP, Veitch PS. A cost-benefit comparison of self-expanding metal stents and Atkinson tubes for the palliation of obstructing esophageal tumors. Dis Esophagus. 1998;11:172–176. doi: 10.1093/dote/11.3.172. [DOI] [PubMed] [Google Scholar]
- 7.Thomas T, Abrams KR, Subramanian V, Mannath J, Ragunath K. Esophageal stents for benign refractory strictures: a meta-analysis. Endoscopy. 2011;43:386–393. doi: 10.1055/s-0030-1256331. [DOI] [PubMed] [Google Scholar]
- 8.Kochman ML, McClave SA, Boyce HW. The refractory and the recurrent esophageal stricture: a definition. Gastrointest Endosc. 2005;62:474–475. doi: 10.1016/j.gie.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Chaput U, Heresbach D, Audureau E, Vanbiervliet G, Gaudric M, Bichard P, Bauret P, Coumaros D, Ponchon T, Fumex F, et al. Comparison of a standard fully covered stent with a super-thick silicone-covered stent for the treatment of refractory esophageal benign strictures: A prospective multicenter study. United European Gastroenterol J. 2013;1:93–102. doi: 10.1177/2050640613476501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canena JM, Liberato MJ, Rio-Tinto RA, Pinto-Marques PM, Romão CM, Coutinho AV, Neves BA, Santos-Silva MF. A comparison of the temporary placement of 3 different self-expanding stents for the treatment of refractory benign esophageal strictures: a prospective multicentre study. BMC Gastroenterol. 2012;12:70. doi: 10.1186/1471-230X-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirdes MM, Siersema PD, van Boeckel PG, Vleggaar FP. Single and sequential biodegradable stent placement for refractory benign esophageal strictures: a prospective follow-up study. Endoscopy. 2012;44:649–654. doi: 10.1055/s-0032-1309818. [DOI] [PubMed] [Google Scholar]
- 12.Hirdes MM, Siersema PD, Vleggaar FP. A new fully covered metal stent for the treatment of benign and malignant dysphagia: a prospective follow-up study. Gastrointest Endosc. 2012;75:712–718. doi: 10.1016/j.gie.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Eloubeidi MA, Talreja JP, Lopes TL, Al-Awabdy BS, Shami VM, Kahaleh M. Success and complications associated with placement of fully covered removable self-expandable metal stents for benign esophageal diseases (with videos) Gastrointest Endosc. 2011;73:673–681. doi: 10.1016/j.gie.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 14.van Boeckel PG, Vleggaar FP, Siersema PD. A comparison of temporary self-expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clin Gastroenterol Hepatol. 2011;9:653–659. doi: 10.1016/j.cgh.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Repici A, Vleggaar FP, Hassan C, van Boeckel PG, Romeo F, Pagano N, Malesci A, Siersema PD. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72:927–934. doi: 10.1016/j.gie.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Dua KS, Vleggaar FP, Santharam R, Siersema PD. Removable self-expanding plastic esophageal stent as a continuous, non-permanent dilator in treating refractory benign esophageal strictures: a prospective two-center study. Am J Gastroenterol. 2008;103:2988–2994. doi: 10.1111/j.1572-0241.2008.02177.x. [DOI] [PubMed] [Google Scholar]
- 17.van Hooft JE, van Berge Henegouwen MI, Rauws EA, Bergman JJ, Busch OR, Fockens P. Endoscopic treatment of benign anastomotic esophagogastric strictures with a biodegradable stent. Gastrointest Endosc. 2011;73:1043–1047. doi: 10.1016/j.gie.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Evrard S, Le Moine O, Lazaraki G, Dormann A, El Nakadi I, Devière J. Self-expanding plastic stents for benign esophageal lesions. Gastrointest Endosc. 2004;60:894–900. doi: 10.1016/s0016-5107(04)02278-3. [DOI] [PubMed] [Google Scholar]
- 19.Repici A, Conio M, De Angelis C, Battaglia E, Musso A, Pellicano R, Goss M, Venezia G, Rizzetto M, Saracco G. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc. 2004;60:513–519. doi: 10.1016/s0016-5107(04)01882-6. [DOI] [PubMed] [Google Scholar]
- 20.Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol. 2009;104:1374–1381. doi: 10.1038/ajg.2009.133. [DOI] [PubMed] [Google Scholar]
- 21.Biancari F, Saarnio J, Mennander A, Hypén L, Salminen P, Kuttila K, Victorzon M, Böckelman C, Tarantino E, Tiffet O, et al. Outcome of patients with esophageal perforations: a multicenter study. World J Surg. 2014;38:902–909. doi: 10.1007/s00268-013-2312-2. [DOI] [PubMed] [Google Scholar]
- 22.Elbe P, Lindblad M, Tsai J, Juto JE, Henriksson G, Agustsson T, Lundell L, Nilsson M. Non-malignant respiratory tract fistula from the oesophagus. A lethal condition for which novel therapeutic options are emerging. Interact Cardiovasc Thorac Surg. 2013;16:257–262. doi: 10.1093/icvts/ivs478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweigert M, Solymosi N, Dubecz A, Stadlhuber RJ, Muschweck H, Ofner D, Stein HJ. Endoscopic stent insertion for anastomotic leakage following oesophagectomy. Ann R Coll Surg Engl. 2013;95:43–47. doi: 10.1308/003588413X13511609956255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweigert M, Dubecz A, Beron M, Muschweck H, Stein HJ. Management of anastomotic leakage-induced tracheobronchial fistula following oesophagectomy: the role of endoscopic stent insertion. Eur J Cardiothorac Surg. 2012;41:e74–e80. doi: 10.1093/ejcts/ezr328. [DOI] [PubMed] [Google Scholar]
- 25.Donatelli G, Dhumane P, Perretta S, Dallemagne B, Vix M, Mutter D, Dritsas S, Doffoel M, Marescaux J. Endoscopic placement of fully covered self expanding metal stents for management of post-operative foregut leaks. J Minim Access Surg. 2012;8:118–124. doi: 10.4103/0972-9941.103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seven G, Irani S, Ross AS, Gan SI, Gluck M, Low D, Kozarek RA. Partially versus fully covered self-expanding metal stents for benign and malignant esophageal conditions: a single center experience. Surg Endosc. 2013;27:2185–2192. doi: 10.1007/s00464-012-2738-x. [DOI] [PubMed] [Google Scholar]
- 27.Dubecz A, Watson TJ, Raymond DP, Jones CE, Matousek A, Allen J, Salvador R, Polomsky M, Peters JH. Esophageal stenting for malignant and benign disease: 133 cases on a thoracic surgical service. Ann Thorac Surg. 2011;92:2028–232; discussion 2028-232;. doi: 10.1016/j.athoracsur.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Schweigert M, Dubecz A, Stadlhuber RJ, Muschweck H, Stein HJ. Risk of stent-related aortic erosion after endoscopic stent insertion for intrathoracic anastomotic leaks after esophagectomy. Ann Thorac Surg. 2011;92:513–518. doi: 10.1016/j.athoracsur.2011.02.083. [DOI] [PubMed] [Google Scholar]
- 29.Dua KS, Latif SU, Yang JF, Fang TC, Khan A, Oh Y. Efficacy and safety of a new fully covered self-expandable non-foreshortening metal esophageal stent. Gastrointest Endosc. 2014;80:577–585. doi: 10.1016/j.gie.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 30.El Hajj II, Imperiale TF, Rex DK, Ballard D, Kesler KA, Birdas TJ, Fatima H, Kessler WR, DeWitt JM. Treatment of esophageal leaks, fistulae, and perforations with temporary stents: evaluation of efficacy, adverse events, and factors associated with successful outcomes. Gastrointest Endosc. 2014;79:589–598. doi: 10.1016/j.gie.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Freeman RK, Ascioti AJ, Dake M, Mahidhara RS. An analysis of esophageal stent placement for persistent leak after the operative repair of intrathoracic esophageal perforations. Ann Thorac Surg. 2014;97:1715–1719; discussion 1715-1719. doi: 10.1016/j.athoracsur.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Gubler C, Bauerfeind P. Self-expandable stents for benign esophageal leakages and perforations: long-term single-center experience. Scand J Gastroenterol. 2014;49:23–29. doi: 10.3109/00365521.2013.850735. [DOI] [PubMed] [Google Scholar]
- 33.Orive-Calzada A, Calderón-García Á, Bernal-Martínez A, Díaz-Roca AB, Barrio-Beraza I, Cabriada-Nuño JL, Orive-Cura VM. Closure of benign leaks, perforations, and fistulas with temporary placement of fully covered metal stents: a retrospective analysis. Surg Laparosc Endosc Percutan Tech. 2014;24:528–536. doi: 10.1097/SLE.0b013e318293c4d8. [DOI] [PubMed] [Google Scholar]
- 34.Persson S, Elbe P, Rouvelas I, Lindblad M, Kumagai K, Lundell L, Nilsson M, Tsai JA. Predictors for failure of stent treatment for benign esophageal perforations - a single center 10-year experience. World J Gastroenterol. 2014;20:10613–10619. doi: 10.3748/wjg.v20.i30.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharaiha RZ, Kim KJ, Singh VK, Lennon AM, Amateau SK, Shin EJ, Canto MI, Kalloo AN, Khashab MA. Endoscopic stenting for benign upper gastrointestinal strictures and leaks. Surg Endosc. 2014;28:178–184. doi: 10.1007/s00464-013-3150-x. [DOI] [PubMed] [Google Scholar]
- 36.Shim CN, Kim HI, Hyung WJ, Noh SH, Song MK, Kang DR, Park JC, Lee H, Shin SK, Lee YC, et al. Self-expanding metal stents or nonstent endoscopic therapy: which is better for anastomotic leaks after total gastrectomy? Surg Endosc. 2014;28:833–840. doi: 10.1007/s00464-013-3228-5. [DOI] [PubMed] [Google Scholar]
- 37.Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer J, Manns MP, Schneider AS, Wedemeyer J. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy. 2013;45:433–438. doi: 10.1055/s-0032-1326435. [DOI] [PubMed] [Google Scholar]
- 38.Leenders BJ, Stronkhorst A, Smulders FJ, Nieuwenhuijzen GA, Gilissen LP. Removable and repositionable covered metal self-expandable stents for leaks after upper gastrointestinal surgery: experiences in a tertiary referral hospital. Surg Endosc. 2013;27:2751–2759. doi: 10.1007/s00464-013-2802-1. [DOI] [PubMed] [Google Scholar]
- 39.Wilson JL, Louie BE, Farivar AS, Vallières E, Aye RW. Fully covered self-expanding metal stents are effective for benign esophagogastric disruptions and strictures. J Gastrointest Surg. 2013;17:2045–2050. doi: 10.1007/s11605-013-2357-4. [DOI] [PubMed] [Google Scholar]
- 40.van Boeckel PG, Dua KS, Weusten BL, Schmits RJ, Surapaneni N, Timmer R, Vleggaar FP, Siersema PD. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol. 2012;12:19. doi: 10.1186/1471-230X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buscaglia JM, Ho S, Sethi A, Dimaio CJ, Nagula S, Stavropoulos SN, Gonda TA, Poneros JM, Stevens PD. Fully covered self-expandable metal stents for benign esophageal disease: a multicenter retrospective case series of 31 patients. Gastrointest Endosc. 2011;74:207–211. doi: 10.1016/j.gie.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Dai Y, Chopra SS, Kneif S, Hünerbein M. Management of esophageal anastomotic leaks, perforations, and fistulae with self-expanding plastic stents. J Thorac Cardiovasc Surg. 2011;141:1213–1217. doi: 10.1016/j.jtcvs.2010.07.096. [DOI] [PubMed] [Google Scholar]
- 43.David EA, Kim MP, Blackmon SH. Esophageal salvage with removable covered self-expanding metal stents in the setting of intrathoracic esophageal leakage. Am J Surg. 2011;202:796–801; discussion 801. doi: 10.1016/j.amjsurg.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 44.Freeman RK, Vyverberg A, Ascioti AJ. Esophageal stent placement for the treatment of acute intrathoracic anastomotic leak after esophagectomy. Ann Thorac Surg. 2011;92:204–208; discussion 208. doi: 10.1016/j.athoracsur.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen NT, Rudersdorf PD, Smith BR, Reavis K, Nguyen XM, Stamos MJ. Management of gastrointestinal leaks after minimally invasive esophagectomy: conventional treatments vs. endoscopic stenting. J Gastrointest Surg. 2011;15:1952–1960. doi: 10.1007/s11605-011-1658-8. [DOI] [PubMed] [Google Scholar]
- 46.Schweigert M, Dubecz A, Stadlhuber RJ, Muschweck H, Stein HJ. Treatment of intrathoracic esophageal anastomotic leaks by means of endoscopic stent implantation. Interact Cardiovasc Thorac Surg. 2011;12:147–151. doi: 10.1510/icvts.2010.247866. [DOI] [PubMed] [Google Scholar]
- 47.Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, Devière J. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc. 2011;73:890–899. doi: 10.1016/j.gie.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Repici A, Hassan C, Sharma P, Conio M, Siersema P. Systematic review: the role of self-expanding plastic stents for benign oesophageal strictures. Aliment Pharmacol Ther. 2010;31:1268–1275. doi: 10.1111/j.1365-2036.2010.04301.x. [DOI] [PubMed] [Google Scholar]
- 49.Dan DT, Gannavarapu B, Lee JG, Chang K, Muthusamy VR. Removable esophageal stents have poor efficacy for the treatment of refractory benign esophageal strictures (RBES) Dis Esophagus. 2014;27:511–517. doi: 10.1111/j.1442-2050.2012.01432.x. [DOI] [PubMed] [Google Scholar]
- 50.Bakken JC, Wong Kee Song LM, de Groen PC, Baron TH. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest Endosc. 2010;72:712–720. doi: 10.1016/j.gie.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 51.Senousy BE, Gupte AR, Draganov PV, Forsmark CE, Wagh MS. Fully covered Alimaxx esophageal metal stents in the endoscopic treatment of benign esophageal diseases. Dig Dis Sci. 2010;55:3399–3403. doi: 10.1007/s10620-010-1415-y. [DOI] [PubMed] [Google Scholar]
- 52.Kim JH, Song HY, Choi EK, Kim KR, Shin JH, Lim JO. Temporary metallic stent placement in the treatment of refractory benign esophageal strictures: results and factors associated with outcome in 55 patients. Eur Radiol. 2009;19:384–390. doi: 10.1007/s00330-008-1151-2. [DOI] [PubMed] [Google Scholar]
- 53.Holm AN, de la Mora Levy JG, Gostout CJ, Topazian MD, Baron TH. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest Endosc. 2008;67:20–25. doi: 10.1016/j.gie.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Hirdes MM, Vleggaar FP, de Beule M, Siersema PD. In vitro evaluation of the radial and axial force of self-expanding esophageal stents. Endoscopy. 2013;45:997–1005. doi: 10.1055/s-0033-1344985. [DOI] [PubMed] [Google Scholar]
- 55.Karakan T, Utku OG, Dorukoz O, Sen I, Colak B, Erdal H, Karatay E, Tahtaci M, Cengiz M. Biodegradable stents for caustic esophageal strictures: a new therapeutic approach. Dis Esophagus. 2013;26:319–322. doi: 10.1111/j.1442-2050.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 56.Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96:1919–1926. doi: 10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 57.Rutegård M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol. 2012;19:99–103. doi: 10.1245/s10434-011-1926-6. [DOI] [PubMed] [Google Scholar]
- 58.Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C, et al. Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc. 2013;27:3883–3890. doi: 10.1007/s00464-013-2998-0. [DOI] [PubMed] [Google Scholar]
- 59.Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, Broering DC, Schniewind B. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42:693–698. doi: 10.1055/s-0030-1255688. [DOI] [PubMed] [Google Scholar]
- 60.Hu Z, Yin R, Fan X, Zhang Q, Feng C, Yuan F, Chen J, Jiang F, Li N, Xu L. Treatment of intrathoracic anastomotic leak by nose fistula tube drainage after esophagectomy for cancer. Dis Esophagus. 2011;24:100–107. doi: 10.1111/j.1442-2050.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 61.Kotzampassakis N, Christodoulou M, Krueger T, Demartines N, Vuillemier H, Cheng C, Dorta G, Ris HB. Esophageal leaks repaired by a muscle onlay approach in the presence of mediastinal sepsis. Ann Thorac Surg. 2009;88:966–972. doi: 10.1016/j.athoracsur.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 62.van Halsema EE, Wong Kee Song LM, Baron TH, Siersema PD, Vleggaar FP, Ginsberg GG, Shah PM, Fleischer DE, Ratuapli SK, Fockens P, et al. Safety of endoscopic removal of self-expandable stents after treatment of benign esophageal diseases. Gastrointest Endosc. 2013;77:18–28. doi: 10.1016/j.gie.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 63.van Heel NC, Haringsma J, Wijnhoven BP, Kuipers EJ. Endoscopic removal of self-expandable metal stents from the esophagus (with video) Gastrointest Endosc. 2011;74:44–50. doi: 10.1016/j.gie.2011.02.020. [DOI] [PubMed] [Google Scholar]


