Abstract
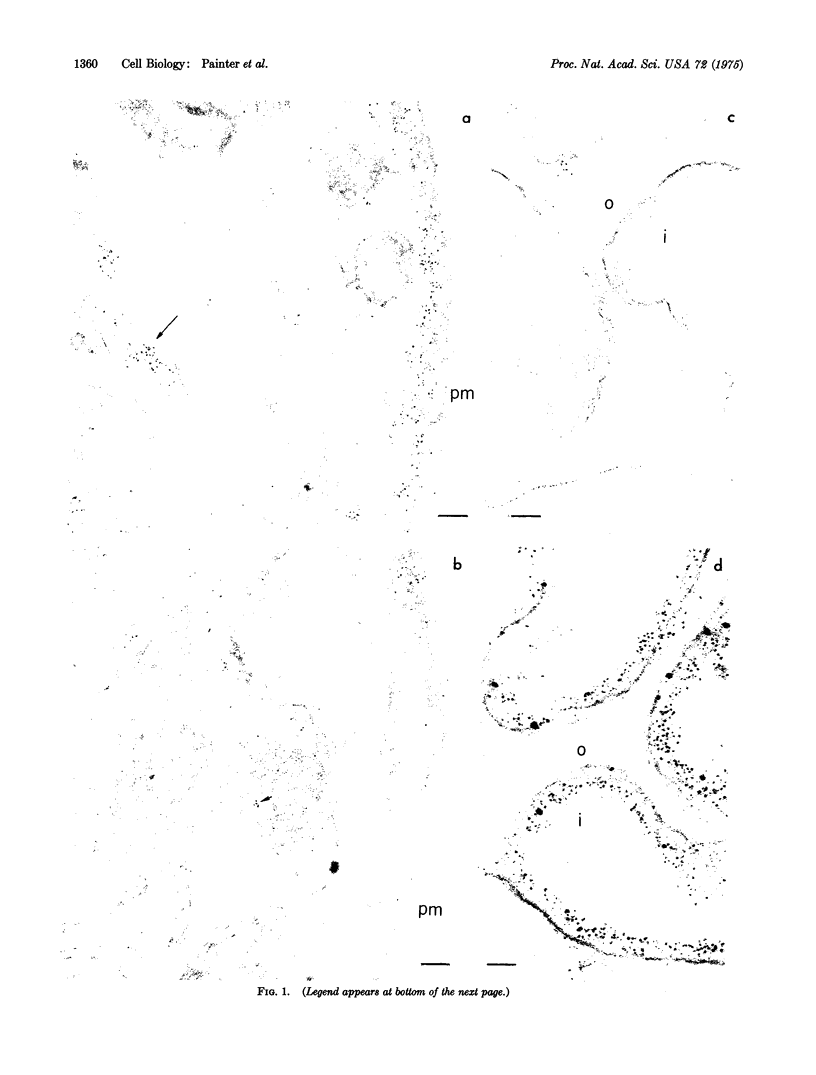
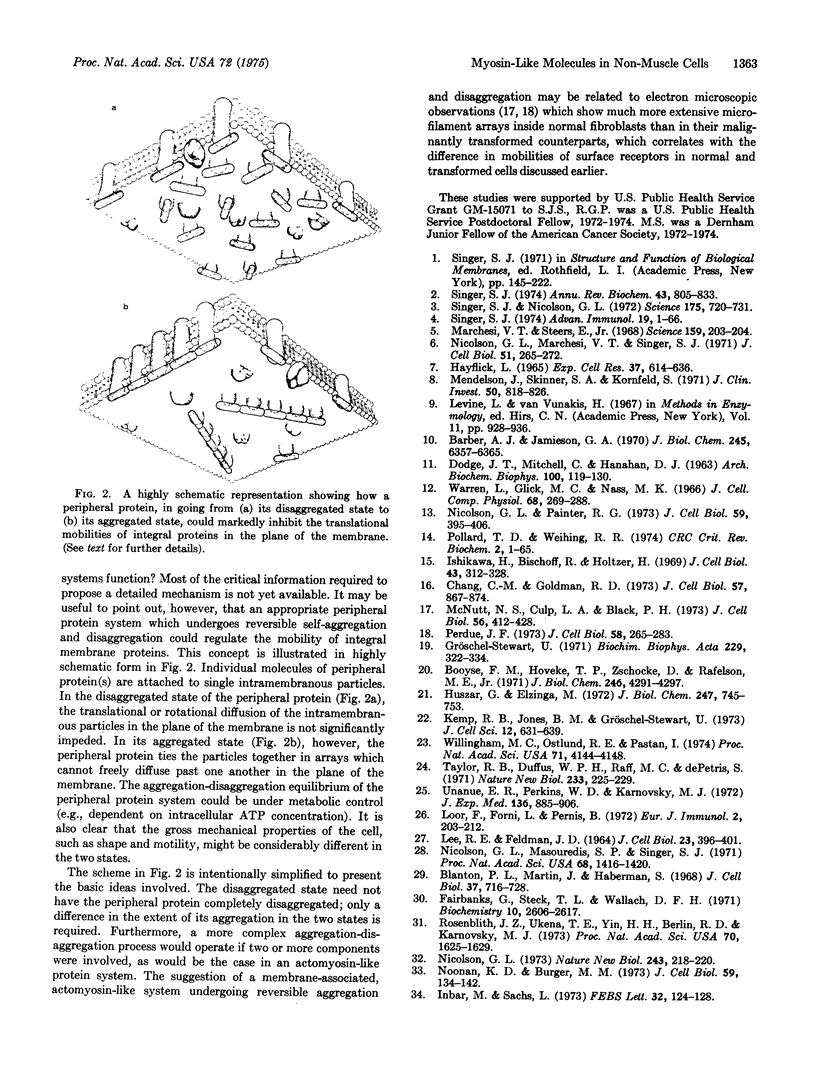
Spectrin, a protein complex which is peripherally attached to the cytoplasmic surface of the human erythrocyte membrane, cannot be detected (by complement fixation with anti-spectrin antibodies) in homogenates of several different human non-muscle cells studied. On the other hand, a protein antigenically identical or similar to human smooth muscle myosin was detected (by complement fixation with antibodies to uterine smooth muscle myosin) in these cells. In the case of human fibroblast line WI38, this smooth muscle myosin like component was shown (by ferritin-antibody experiments in electron microscopy) to be at least partly associated with cytoplasmic surface of the plasma membrane of the cell. It is proposed that the spectrin complex of the erythrocyte membrane and the smooth muscle myosin-like component of the fibroblast membrane play similar roles in regulating the translational mobilities of integral proteins in their respective membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. J., Jamieson G. A. Isolation and characterization of plasma membranes from human blood platelets. J Biol Chem. 1970 Dec 10;245(23):6357–6365. [PubMed] [Google Scholar]
- Blanton P. L., Martin J., Haberman S. Pinocytotic response of circulating erythrocytes to specific blood grouping antibodies. J Cell Biol. 1968 Jun;37(3):716–728. doi: 10.1083/jcb.37.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booyse F. M., Hoveke T. P., Zschocke D., Rafelson M. E., Jr Human platelet myosin. Isolation and properties. J Biol Chem. 1971 Jul 10;246(13):4291–4297. [PubMed] [Google Scholar]
- Chang C. M., Goldman R. D. The localization of actin-like fibers in cultured neuroblastoma cells as revealed by heavy meromyosin binding. J Cell Biol. 1973 Jun;57(3):867–874. doi: 10.1083/jcb.57.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U. Comparative studies of human smooth and striated muscle myosins. Biochim Biophys Acta. 1971 Feb 16;229(2):322–334. doi: 10.1016/0005-2795(71)90191-7. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Huszar G., Elzinga M. Homologous methylated and nonmethylated histidine peptides in skeletal and cardiac myosins. J Biol Chem. 1972 Feb 10;247(3):745–753. [PubMed] [Google Scholar]
- Inbar M., Sachs L. Mobility of carbohydrate containing sites on the surface membrane in relation to the control of cell growth. FEBS Lett. 1973 May 15;32(1):124–128. doi: 10.1016/0014-5793(73)80753-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Formation of arrowhead complexes with heavy meromyosin in a variety of cell types. J Cell Biol. 1969 Nov;43(2):312–328. [PMC free article] [PubMed] [Google Scholar]
- Kemp R. B., Jones B. M., Gröschel-Stewart U. Abolition by myosin and heavy meromyosin of the inhibitory effect of smooth-muscle actomyosin antibodies on cell aggregation in vitro. J Cell Sci. 1973 Mar;12(2):631–639. doi: 10.1242/jcs.12.2.631. [DOI] [PubMed] [Google Scholar]
- LEE R. E., FELDMAN J. D. VISUALIZATION OF ANTIGENIC SITES OF HUMAN ERYTHROCYTES WITH FERRITIN-ANTIBODY CONJUGATES. J Cell Biol. 1964 Nov;23:396–401. doi: 10.1083/jcb.23.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Masouredis S. P., Singer S. J. Quantitative two-dimensional ultrastructural distribution of Rh o (D) antigenic sites on human erythrocyte membranes. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1416–1420. doi: 10.1073/pnas.68.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. The relationship of concanavalin A binding to lectin-initiated cell agglutination. J Cell Biol. 1973 Oct;59(1):134–142. doi: 10.1083/jcb.59.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue J. F. The distribution, ultrastructure, and chemistry of microfilaments in cultured chick embryo fibroblasts. J Cell Biol. 1973 Aug;58(2):265–283. doi: 10.1083/jcb.58.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. Molecular biology of cellular membranes with applications to immunology. Adv Immunol. 1974;19(0):1–66. doi: 10.1016/s0065-2776(08)60251-5. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Ostlund R. E., Pastan I. Myosin is a component of the cell surface of cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4144–4148. doi: 10.1073/pnas.71.10.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]