Abstract
A mutant of E. coli K-12 has been isolated which has only 1-3% of the wild-type lysyl-tRNA synthetase activity [L-lysine:tRNA ligase (AMP forming), EC 6.1.1.6]. Additions of 20 mM L-alanine or 6 mM leucine dipeptides to the culture medium can restore the activity of lysyl-tRNA synthetase in the mutant strain to the wild-type level. Experiments on the in vivo charging of lysine tRNA in the mutant show that in the absence of the metabolites lysine tRNA is charged 15-23%. Upon the addition of 3 mM L-leucyl-L-alanine to the medium the lysyl tRNA synthetase activity increases 25-fold and the in vivo charging of lysine tRNA returns to the wild-type level. Experiments with antibody against lysyl-tRNA synthetase show that the stimulation of lysyl-tRNA synthetase activity by the metabolites is the result of new protein synthesis.
Full text
PDF
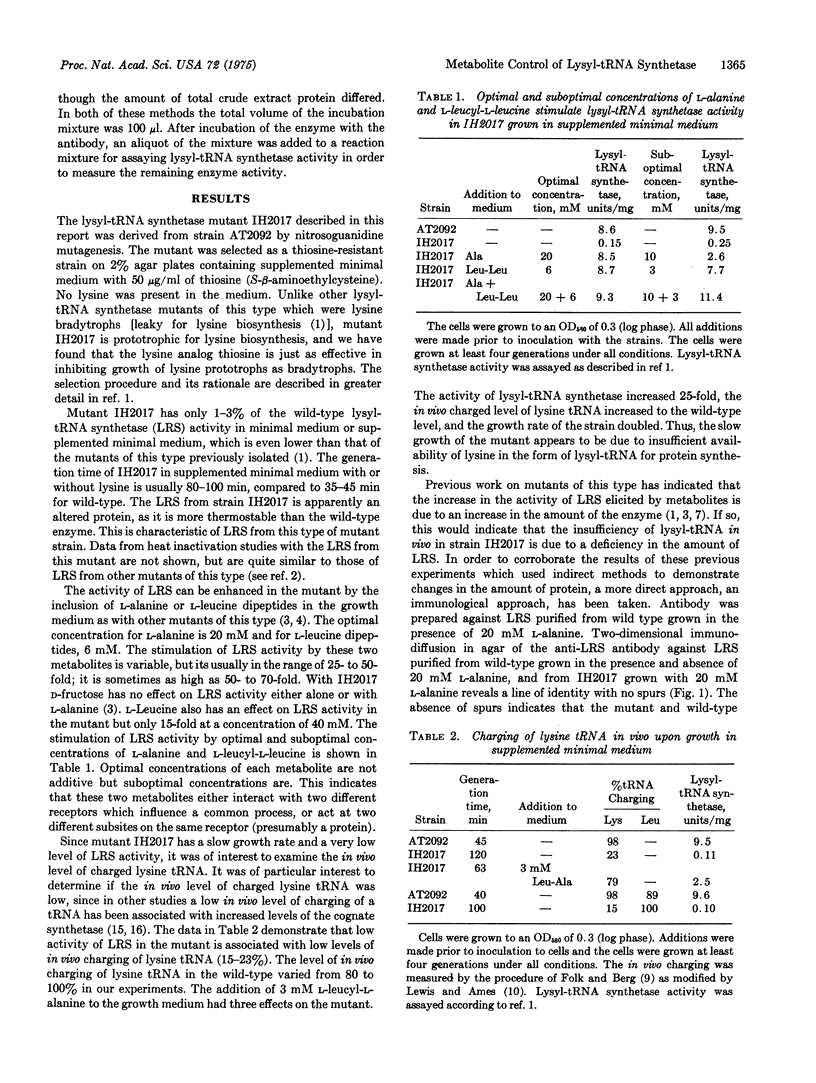
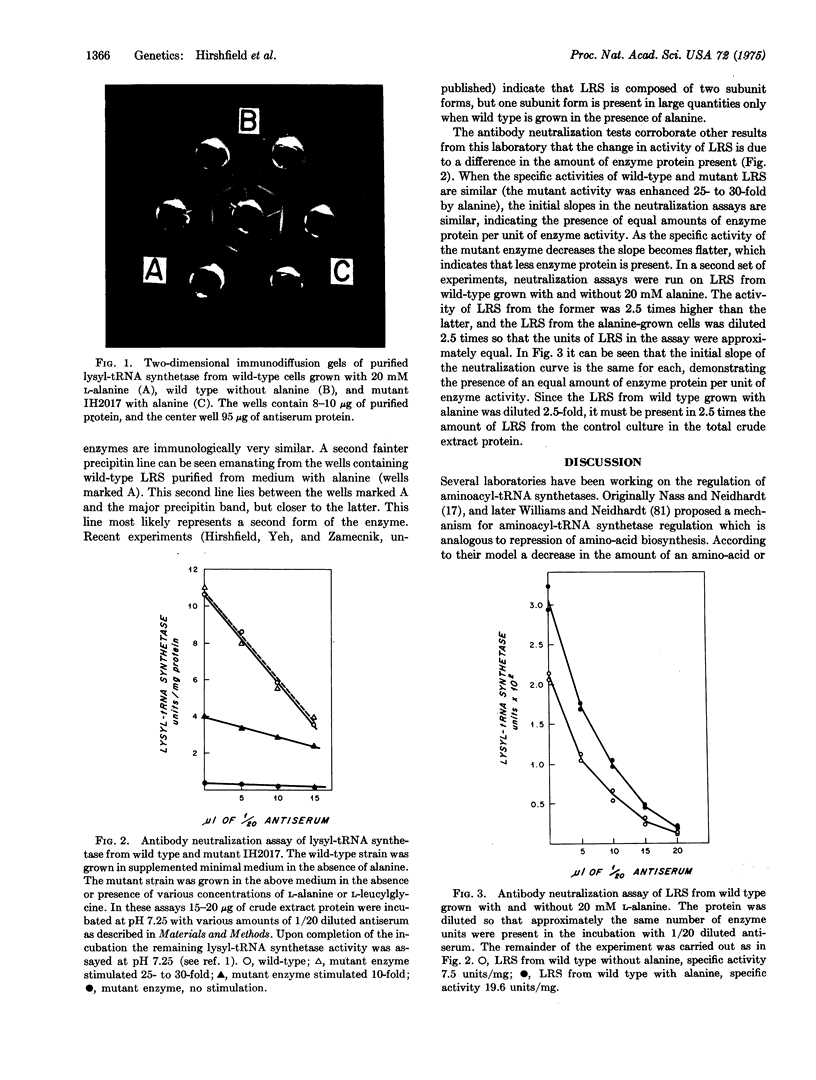

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibold E. R., Williams L. S. Regulation of synthesis of methionyl-, prolyl-, and threonyl-transfer ribonucleic acid synthetases of Escherichia coli. J Bacteriol. 1972 Mar;109(3):1020–1026. doi: 10.1128/jb.109.3.1020-1026.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buklad N. E., Sanborn D., Hirshfield I. N. Particular influence of leucine peptides on lysyl-transfer ribonucleic acid ligase formation in a mutant of Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1477–1478. doi: 10.1128/jb.116.3.1477-1478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C., Guerola N. Mutagenesis of the replication point by nitrosoguanidine: map and pattern of replication of the Escherichia coli chromosome. J Mol Biol. 1968 May 14;33(3):705–719. doi: 10.1016/0022-2836(68)90315-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Bukald N. E. Effect of alanine, leucine and fructose on lysyl-transfer ribonucleic acid ligase activity in a mutant of Escherichia coli K-12. J Bacteriol. 1973 Jan;113(1):167–177. doi: 10.1128/jb.113.1.167-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Tomford J. W., Zamecnik P. C. Thiosine-resistant mutants of Escherichia coli K-12 with growth-medium-dependent lysyl-tRNA synthetase activity.II. Evidence for an altered lysyl-tRNA synthetase. Biochim Biophys Acta. 1972 Feb 15;259(3):344–356. doi: 10.1016/0005-2787(72)90309-7. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Zamecnik P. C. Thiosine-resistant mutants of Escherichia coli K-12 with growth-medium-dependent lysl-tRNA synthetase activity. I. Isolation and physiological characterization. Biochim Biophys Acta. 1972 Feb 15;259(3):330–343. [PubMed] [Google Scholar]
- Kendall F. E. STUDIES ON SERUM PROTEINS. I. IDENTIFICATION OF A SINGLE SERUM GLOBULIN BY IMMUNOLOGICAL MEANS. ITS DISTRIBUTION IN THE SERA OF NORMAL INDIVIDUALS AND OF PATIENTS WITH CIRRHOSIS OF THE LIVER AND WITH CHRONIC GLOMERULONEPHRITIS. J Clin Invest. 1937 Nov;16(6):921–931. doi: 10.1172/JCI100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- McGinnis E., Williams A. C., Williams L. S. Derepression of synthesis of the aminoacyl-transfer ribonucleic acid synthetases for the branched-chain amino acids of Escherichia coli. J Bacteriol. 1974 Aug;119(2):554–559. doi: 10.1128/jb.119.2.554-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis E., Williams L. S. Regulation of histidyl-transfer ribonucleic acid synthetase formation in a histidyl-transfer ribonucleic acid synthetase mutant of Salmonella typhimurium. J Bacteriol. 1972 Sep;111(3):739–744. doi: 10.1128/jb.111.3.739-744.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis E., Williams L. S. Role of histidine transfer ribonucleic acid in regulation of synthesis of histidyl-transfer ribonucleic acid synthetase of Salmonella typhimurium. J Bacteriol. 1972 Feb;109(2):505–511. doi: 10.1128/jb.109.2.505-511.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. An immunological study of the constitutive and the penicillin-induced penicillinases of Bacillus cereus, based on specific enzyme neutralization by antibody. J Gen Microbiol. 1956 Feb;14(1):90–108. doi: 10.1099/00221287-14-1-90. [DOI] [PubMed] [Google Scholar]
- Parker J., Flashner M., Mckeever W. G., Neidhardt F. C. Metabolic regulation of the arginyl and valyl transfer ribonucleic acid synthetases in bacteria. J Biol Chem. 1974 Feb 25;249(4):1044–1053. [PubMed] [Google Scholar]
- Parker J., Neidhardt F. C. Metabolic regulation of aminoacyl-tRNA synthetase formation in bacteria. Biochem Biophys Res Commun. 1972 Oct 17;49(2):495–501. doi: 10.1016/0006-291x(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Waldenström J. Purification and some properties of lysyl ribonucleic acid synthetase from Escherichia coli. Eur J Biochem. 1968 Feb;3(4):483–487. doi: 10.1111/j.1432-1033.1967.tb19556.x. [DOI] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]



