Abstract
A familiar challenge for neuroradiologists and neuro-oncologists is differentiating between radiation treatment effect and disease progression in the CNS. Both entities are characterized by an increase in contrast enhancement on MRI and present with similar clinical signs and symptoms that may occur either in close temporal proximity to the treatment or later in the disease course. When radiation-related imaging changes or clinical deterioration are mistaken for disease progression, patients may be subject to unnecessary surgery and/or a change from otherwise effective therapy. Similarly, when disease progression is mistaken for treatment effect, a potentially ineffective therapy may be continued in the face of progressive disease. Here we describe the three types of radiation injury to the brain based on the time to development of signs and symptoms – acute, subacute and late – and then review specific imaging changes after intensity-modulated radiation therapy, stereotactic radiosurgery and brachytherapy. We provide an overview of these phenomena in the treatment of a wide range of malignant and benign CNS illnesses. Finally, we review the published data regarding imaging techniques under investigation to address this well-known problem.
Keywords: pseudoprogression, radiation treatment effect, radionecrosis, radiosurgery
Radiation therapy (RT) is an effective treatment for various intracranial pathologies, including primary CNS malignancies, brain metastases, meningiomas and vestibular schwannomas. Serial contrast-enhanced MRI represents the current mainstay for monitoring treatment response following therapy based on the assumption that enlarging lesions reflect increasing disease burden. However, radiation neurotoxicity may closely resemble recurrent or progressive disease. There are many shared characteristics between the two entities including: presence of contrast enhancement; location near site of original disease and, therefore, highest radiation dose; growth over time; mass effect; vasogenic edema; and clinical symptoms. When radiation-related imaging changes are mistaken for disease progression, patients may be subject to unneeded surgery and discontinuation of otherwise effective systemic therapy. This may also pose a dilemma in clinical trials for new systemic therapies, where such imaging changes may cause the patient to be falsely labeled as having progressive disease. This review summarizes the types of radiation injury (acute, subacute and late), and then discusses common clinical scenarios and relevant literature associated with three modalities of RT commonly used to treat CNS pathology – intensity-modulated RT (IMRT), stereotactic radiosurgery (SRS) and brachytherapy. Finally, we discuss recent advances in imaging techniques that show promise in the noninvasive differentiation between radiation treatment effect and disease progression.
Radiation injury
Three distinct types of radiation injury are recognized and can be identified on the basis of the time of presentation: acute (during or shortly after radiation), subacute or early-delayed (typically up to 12 weeks after radiation), and late (months to years after completion of radiation) [1]. A complete understanding of the pathophysiology of CNS injury after RT is lacking, however, it is clear that multiple variables are relevant to its occurrence, including total dose, fraction size, time between fractions, treatment volume and concurrent chemotherapy [2–8].
Acute effects
Acute radiation effects occur during or immediately after the course of radiation. Clinically, acute encephalopathy often manifests as signs of increased intracranial pressure with headache, nausea/vomiting and/or mental status changes. It is not uncommon for symptoms or signs caused by the lesion to progressively worsen in the setting of acute edema secondary to RT. Symptoms often improve with dexamethasone, however, it is unclear whether such therapy actually affects long-term outcome or if there is clear benefit to treating acute radiation-related edema or other imaging changes in the absence of symptoms. Acute toxicity is thought to be secondary to radiation-induced cytokine release and vasodilation resulting in increased edema and disruption of the blood–brain barrier. The MRI in acute radiation injury is usually unchanged but occasionally can demonstrate diffuse brain swelling [9]. With conventional fractionation (1.8–2 Gy per fraction) to doses up to approximately 60 Gy, symptoms of acute radiation toxicity are typically mild and self-limiting [8] and will completely resolve without therapeutic intervention. Occasionally patients with acute radiation injury may develop progressive symptoms similar to late radiation injury; in this case, there is clinical benefit to the use of dexamethasone and surgery may be life saving. Although no longer part of current treatment regimens, high doses of radiation (e.g., >6 Gy fractions or twice-daily treatment), particularly when delivered to the whole brain, may result in death secondary to acute neurotoxicity [10–12]. For these reasons, it is possible that imaging progression as well as clinical deterioration during brain radiotherapy may represent treatment effects rather than actual tumor progression.
Subacute (early-delayed) effects
Subacute reactions appear from a few weeks to a few months after radiation and can be operationally defined as treatment-related changes that often stabilize or diminish over time, with or without clinical symptoms. A specific entity, known as somnolence syndrome may also occur in the subacute period and is characterized by fatigue and lethargy, and may or may not be associated with imaging changes. Somnolence syndrome is thought to be secondary to demyelination and is more frequent in children, particularly when concurrent methotrexate is administered with whole brain radiation [13].
When the subacute or early-delayed type of injury occurs, MRI findings can vary from edema to an increase in size of the contrast enhancing lesion(s) within the area of prior irradiation. Effects of radiation on the robustness and function of the blood–brain barrier also contributes to the observed imaging changes. As with most treatment-related effects, the occurrence of these effects depends on total dose and fraction size. Although these symptoms are mostly reversible, the imaging changes do not always demonstrate complete resolution, particularly if they progress to late effects, such as radionecrosis [14,15]. It may be appropriate to warn patients that development or recurrence of symptoms in the months after radiation treatment may very well be related to the treatment rather than progression of disease, especially for conditions where radiotherapy is highly successful.
Late effects
Radiation late effects include white matter changes, radionecrosis and other vascular lesions, such as lacunar infarcts and parenchymal calcifications [16]. The interval between radiation and time to occurrence varies from a few months to several years. CNS toxicity, as demonstrated by subclinical white matter changes, is relatively common and can even manifest after chemotherapy alone [15,17–18]. After RT for gliomas, white matter changes can occur in up to 50% of patients. This mechanism is felt to be secondary to cerebral edema and these changes may be confused with tumor progression, given that a high-grade glioma itself may appear similar to radiation white matter changes on MRI. The clinical situation may even be more difficult in the follow-up for patients with infiltrating low-grade glioma. In this case the tumor itself may be present on pretreatment imaging as an ill-defined white matter infiltrating process that is difficult to differentiate from later radiation-induced white matter changes that may develop over years of follow-up. Such slowly progressive changes in the radiation field may be considered appropriate for conservative follow-up, especially without progressive symptoms or evidence of mass effect. These changes are more often associated with cognitive decline, but can also be asymptomatic.
Radionecrosis, on the other hand, is a potentially devastating and irreversible late complication that will be an additional important focus of this review as it may also closely mimic tumor progression and thereby create a clinical management dilemma. Radionecrosis is felt to be a more severe form of the white matter changes associated with leukoencephalopathy. There is likely a physiologic and temporal relationship between white matter changes and frank radionecrosis, and white matter changes and radionecrosis are not mutually exclusive [19]. One must also keep in mind that as a general pathologic term, necrosis is also identified in the subacute or early-delayed period as well as a component of the primary tumor in high-grade gliomas. Patients with subacute radiation effect (up to 12 weeks after radiation), despite showing evidence of necrosis if taken to surgery, will usually have improvement in imaging findings over time without surgical intervention. This is in contrast to late necrosis, which often requires surgical intervention.
Radionecrosis
Radionecrosis typically occurs months to years after radiation, but may be seen as early as a few weeks after and has been reported as late as 19 years after the completion of RT. Approximately 80% of cases occur within 3 years from the completion of RT. In 1972, Glass et al. at the MD Anderson Cancer Center (TX, USA) reported their experience of cerebral radionecrosis following RT for extracranial neoplasms. They reported that the risk increases significantly with increasing radiation dose, fraction size and administration of chemotherapy (either concurrent or subsequent) [5]. It is accepted that a dose of 60 Gy administered in 200 cGy fractions over a period of 6 weeks is considered safe. However, even with this schedule and dose, cases of cerebral radiation necrosis will inevitably occur. A 5% risk of radionecrosis within 5 years after radiotherapy has been estimated to occur after a total dose of 50 Gy to two thirds of the total brain volume and after 60 Gy to a third of the total brain volume using standard fractionation [4]. It is unlikely to occur at doses below 50 Gy in 25 fractions [6].
Incidence
It is difficult to estimate the true incidence of radionecrosis. Early studies, including dose escalation and hyperfractionation trials, report a 3–9% incidence of radionecrosis. Most neuro-oncologists believe that these studies underestimate the true incidence of radiation damage and necrosis because they were conducted before the MRI era [2–3,14,20–21]. Furthermore, most investigators calculate the incidence of radionecrosis according to the number of patients treated, rather than the number of patients at risk (i.e., patients alive). This method also underestimates the true incidence, as some patients will have died from their disease prior to the development of necrosis. In the modern era, the incidence of true radionecrosis is approximately <5% when 60 Gy of radiation is delivered to the brain in daily 2 Gy fractions [8], and it is rarely seen in doses less than 50 Gy when delivered via standard fractionation. A sharp increase in this rate is seen with twice-daily fractionation, and the incidence and severity of radiotoxicity is unpredictable for fraction sizes larger than 2.5 Gy [8,22].
Pathophysiology
The precise mechanism of radionecrosis of the brain remains to be elucidated, but two popular theories exist – one based on radiation damage to blood vessels and endothelial cells and the other based on radiation damage to glial cells. The vascular hypothesis posits that RT damages endothelial cells and causes local cytokine release, leading to an increase in capillary permeability and extracellular edema. Demyelination and other injury to the brain is due to small -and medium-sized blood vessel damage, which ultimately leads to tissue necrosis as a result of ischemia [23,24]. This is thought to be similar to occlusive vascular diseases after the blood vessel walls become thickened and occluded secondary to hyalinization [25]. Corroborating evidence to support this hypothesis is the histopathologic features of radionecrosis, which include perivascular parenchymal coagulative necrosis and fibrinoid necrosis of blood vessel walls [19,24]. Animal studies have indeed demonstrated that vascular abnormalities occur before the development of parenchymal changes in the brain [26]. In addition to thickened vascular walls with necrosis, there may also be clusters of telangiectasias within the regions of smaller blood vessels similar to the late effects of radiation observed in other parts of the body, such as the skin. The glial hypothesis, on the other hand, suggests that radionecrosis results from direct damage to glial cells, in particular the oligodendroglial cells. There is preclinical evidence to suggest that oligodendrocytes are very sensitive to radiation and demyelination ensues after their destruction [27,28]. Neurons are thought to be insensitive to RT, but white matter changes and a reduction in the volume of parenchyma that is often seen with radiation effect, can be attributed to damage to the oligodendrocytes [29]. It is likely that both theories are correct to some degree. An additional promising theory that has gained acceptance in recent years is the role of the host’s immune response and inflammatory cytokines [19]. Other downstream effects of radiation on the brain are well known, such as derangements in the fibrinolytic system, and are likely contributors to the development of radionecrosis [25,29–30].
Diagnosis
Distinguishing radionecrosis and other forms of treatment effect from recurrent tumor poses a significant challenge as both entities present with similar imaging findings and clinical features. The gold standard diagnostic test for radiation effect is surgical resection followed by extensive pathological evaluation. Any other investigation lacks sensitivity and specificity, including tissue biopsy, which may lead to sampling error. In radionecrosis there is often an enhancing lesion with central area of necrosis on T1-weighted images, and on T2-weighted images, the solid portion will display a low signal intensity while the necrotic central portion will reveal increased signal intensity [26,31]. Kumar et al. published a thorough characterization of imaging findings in radionecrosis after examining MRIs from approximately 150 patients with malignant gliomas who received an aggressive treatment regimen with accelerated partial brain irradiation and concurrent carboplatin followed by procarbazine, lomustine and vincristine. In total, 52 patients had radiation-induced enhancement of white matter, 20 patients had pure radionecrosis, 16 patients had a mixture of predominantly radionecrosis with limited residual tumor, and two patients had radionecrosis of the cranial nerves. Kumar et al. coined two terms to describe the imaging features commonly seen in cases of radionecrosis – the ‘soap bubble’ and ‘Swiss cheese’ signs. The soap bubble sign refers to an area of contrast enhancement that contains a heterogeneous non-enhancing necrotic center, which resembles soap bubbles. The Swiss cheese pattern refers to scattered areas of necrosis of various sizes. Cerebral radionecrosis usually occurs within the radiation field, as did all cases of pathologically confirmed radionecrosis in this cohort, but other less common patterns do exist, such as multiple lesions and lesions in the contralateral hemisphere or sites distant from the radiation field [5,25,32].
Radiation-induced white matter changes are felt to represent a milder form of brain injury and affects white matter at variable distances from the radiation field. The pattern of white matter changes on MRI may be nodular, curvilinear or linear enhancement [3]. Typically these lesions are not biopsied unless they progress to overt necrosis.
Management
Radionecrosis is the end point of late radiation injury and is frequently irreversible and often progressive [27,33]. If not treated successfully, it can cause serious neurologic injury secondary to cerebral edema and mass effect. Patients often develop progressive lesions that may require surgical intervention to remove the cause of an advancing front of destructive inflammation. Initial management includes corticosteroids with dexamethasone, which will often lead to prompt improvement in symptoms. Although some patients will have resolution of their symptoms with conservative management and eventually taper off steroids, the majority of patients will require long-term steroid use and will be at risk of complications such as osteoporosis, infection and proximal muscle weakness. When the process is not arrested by dexamethasone, steroids can also be used in certain cases as a bridge to surgery. Surgery may be the only therapy that halts the process or leads to improvement of symptoms. We recommend consideration of surgery with a progressive symptomatic process not halted by steroids as the procedure may be beneficial for either progressive tumor or necrosis.
In addition to corticosteroids, many nonoperative treatment approaches have been used in an attempt to treat radionecrosis, including anticoagulants [34], pentoxyfyline, desferioxamine, pentobarbital, hyperbaric oxygen and, more recently, bevacizumab, a monoclonal antibody targeted against VEGF. Bevacizumab has demonstrated promising results in a number of case reports and a small prospective randomized trial [35–38], but its efficacy beyond short-term relief of symptoms is unknown and caution is required, as the drug is associated with significant risks that should be considered in each patients individual situation. In a randomized trial, there was a reduction in both fluid attenuated inversion recovery (FLAIR) and T1-weighted postcontrast abnormalities and improvement in neurologic signs and symptoms in all 14 patients that received bevacizumab [35]. Hyperbaric oxygen therapy has also demonstrated benefit in radionecrosis of bone and soft tissue, although no prospective randomized study has demonstrated benefit in CNS radionecrosis [39,40].
A recent case report suggests that laser interstitial thermal therapy (LITT), as a form of minimally invasive heat therapy that has been established for the treatment of deep-seated intracranial tumors, may hold promise for cases of refractory unresectable radionecrosis [41]. The mechanism of action in the treatment of radionecrosis is thought to involve the replacement of proliferating endothelium and surrounding inflammatory tissue with thrombosed vessels. The case report describes an elderly gentleman with diabetes mellitus who developed progressive radionecrosis after SRS for a deep-seated, unresectable brain metastasis. Prolonged treatment with corticosteroids and/or bevacizumab was not possible given the patient’s comorbidities. LITT was performed after progressively worsening symptoms warranted urgent nonsurgical intervention. After treatment the patient experienced early symptomatic improvement and corticosteroids were successfully discontinued. Postprocedure imaging 7 weeks after treatment demonstrated an initial increase in the size of the lesion, but subsequent imaging 3 months after treatment showed a reduction in the size of the lesion consistent with appropriate response to treatment. This treatment modality warrants further prospective evaluation as a means of nonoperative management in cerebral radionecrosis.
Radiation treatment effect in various radiation modalities
In the following section, we discuss imaging findings associated with radiotoxicity after three different radiation modalities: IMRT, SRS and brachytherapy. We highlight clinical scenarios in which there is often difficulty distinguishing between treatment effect and disease progression, including treatment for glioblastoma multiforme (GBM), brain metastases, vestibular schwannomas and meningiomas.
IMRT
The treatment of primary CNS malignancies often incorporates partial brain irradiation delivered via IMRT, an advanced form of 3D conformal RT that is delivered with varied intensity to different parts of the treatment volume in order to spare dose to normal tissue. As described previously, there is a relationship between the incidence of radionecrosis and total radiation dose. The total dose that leads to a 5% risk of significant toxicity at 5 years (TD 5/5; defined as radionecrosis or cognitive decline at 5 years) for fractionated RT is 72 Gy according to the QUANTEC report published by Lawrence et al. in 2010 [8]. For most cancers in the CNS, there is little reason to give doses higher than 60 Gy, and an incidence of 5% radiation necrosis at 5 years would be unacceptably high in most situations, therefore, the conventional upper limit of RT delivered to the brain with standard fractionation (i.e., 1.8–2 Gy per day) is 60 Gy. At this dose, the risk of frank radionecrosis is felt to be much less than 5%. One must keep in mind, however, that the brain is particularly sensitive to fraction sizes >2.5 Gy and to fractions delivered more frequently than once daily. Other factors that increase the risk of radiation toxicity, specifically radionecrosis, include concurrent chemotherapy, interstitial brachytherapy and re-irradiation [8,14,31].
GBM & pseudoprogression
GBM is the most common primary CNS malignancy in adults in the USA and is nearly universally fatal [42]. In the 1970s a randomized controlled trial showed an overall survival benefit for postoperative whole-brain RT to 60 Gy [43]. Better imaging and improvement in radiation delivery techniques allowed the use smaller fields (i.e., focal or involved field radiation), which translated into less neurotoxicity. The current standard of care for newly diagnosed GBM is maximum safe resection followed by focal RT with IMRT to 60 Gy in 200 cGy daily fractions with concurrent and adjuvant temozolomide (TMZ). This treatment regimen was found to significantly improve overall survival compared with radiation alone in a landmark trial published by Stupp et al. [44]. A specific treatment-related phenomenon that mimics tumor progression is seen after partial brain irradiation and chemotherapy for GBM and is termed pseudoprogression. As opposed to actual tumor progression, these imaging changes, which may or may not be associated with clinical deterioration, resolve spontaneously. The typical time to onset is within the first 3 months after completion of radiation, and although most patients will have improvement over time without treatment, some may progress to frank radionecrosis. Pseudoprogression is thought to represent a form of toxicity on the continuum between subacute and late toxicity.
Even though early trials with patients with malignant gliomas described findings consistent with pseudoprogression, such as temporary contrast enhancement and edema on imaging that occur in the first 6 months after chemoradiation [45–47], the incidence of pseudoprogression has increased over time. Some argue this is mainly attributed to higher quality imaging with MRI compared to CT and more frequent imaging studies to monitor response over the past 20 years. This is likely contributing to the heightened awareness of patients who have what appear to be progressive lesions shortly after chemoradiation but who do not ultimately suffer from tumor progression in that area. On the other hand, the addition of TMZ to RT may have led to a true increase in this exaggerated early necrosis secondary to increased cell kill and is a manifestation of the positive effects of combined modality therapy with TMZ. It has been reported that there is a higher incidence of radionecrosis in patients who undergo surgical resection in the first 6 months after chemoradiation with TMZ than was previously reported. De Wit et al. report a 9% rate of pseudoprogression in a cohort of patients treated in two prospective Phase III trials with radiation alone prior to the incorporation of concurrent TMZ [48]. In a more recent study with concurrent TMZ and radiation, Taal et al. report a 21% rate of pseudoprogression [49]. In another prospective study, 26 patients (51%) who underwent RT with concurrent TMZ for GBM demonstrated imaging changes suggestive of disease progression within 6 months of completion of radiation. In total, 15 patients underwent re-operation, and seven (47%) had pathologic evidence of necrosis without evidence of tumor [50]. It is currently estimated that pseudoprogression explains approximately half of all cases of increasing contrast enhancement after treatment and a total of 20–30% of patients undergoing their first postradiation MRI will have evidence of pseudoprogression [9].
There are provocative data to suggest that pseudoprogression is more frequent in patients who harbor a methylated MGMT promoter [51]. Brandes et al. report the results of 103 patients with GBM treated with TMZ and RT after resection. MGMT promoter methylation was present in 35% of patients, and of those, pseudoprogression was recorded in 91% (21 out of 23). This is in stark contrast to 41% of patients with unmethylated MGMT promoter [51]. It remains to be established whether these findings are a result of higher sensitivity of these tumors to treatment or whether this was simply due to more frequent progression of tumor in patients with unmethylated promoters [52].
Pseudoprogression may lead to discontinuation of effective therapy, complicate study end points and pose challenges in selecting appropriate patients in studies for recurrent GBM. Because it is a retrospective diagnosis that can only be made after disease progression has been ruled out, there is a critical need to improve our diagnostic techniques in order to optimize the management of these patients. Historically, tumor response had been measured using the MacDonald criteria, originally published in 1990 [53]. These criteria provided an objective assessment of tumor response based primarily on the size of the enhancing tumor area (i.e., the product of maximal cross-sectional enhancing diameters), and also took into account clinical status and use of corticosteroids. The assumption required to apply this criteria was that the areas of tumor are depicted as the contrast-enhancing component of the MRI due to having abnormal vascular permeability and architecture. For the most part, this assumption is correct. The 6-month progression-free survival was demonstrated to predict for overall survival in a series of Phase II studies in the North American Brain Tumor Consortium (NABTC) [54]. However, contrast enhancement and edema are nonspecific and primarily reflect the passage of material across the disrupted blood–brain barrier, and do not necessarily accurately define the extent of a tumor mass. The Response Assessment in Neuro-oncology (RANO) working group recently outlined a proposal for updating the Response Assessment Criteria for High Grade Gliomas [55]. One significant change to the response criteria was that patients who demonstrated an enlargement in the area of contrast enhancement in the irradiated brain within the first 12 weeks after therapy are not considered to have recurrent or progressive disease without histopathologic confirmation or further follow-up to determine the behavior of the new abnormality. In general, we recommend caution in diagnosing recurrence in the first 3 months after radiation for high-grade glioma. In our experience, pseudoprogression may present after 3 months following completion of radiation and should be considered in the differential diagnosis. If the presumed area of growth is within the radiation field, a period of continued follow-up may be warranted to determine the clinical course. Where uncertainty exists, biopsy confirmation of disease state may be considered, and it may be useful to consider MGMT promoter methylation status. Although pseudoprogression has not been well described for low-grade gliomas, it may be more significant as the probability of early true recurrence is lower in this indolent disease.
Stereotactic radiosurgery
SRS has emerged as a safe and efficacious treatment for many intracranial pathologies, including primary and metastatic tumors, and vestibular schwannomas. The goal of stereotactic RT (or stereotactic ablative radiation [SABR]) is to induce tumor-specific, double-stranded DNA damage irrespective of cell cycle phase. By delivering radiation from multiple directions that converge at the site of intracranial pathology, irreversible damage can be inflicted on tumor cells while sparing normal brain parenchyma. Ablation of tumor microvasculature by the spatially well-localized high-dose radiation may contribute substantially to both tumor killing and the imaging findings after treatment.
While protocols and radiation dose differ among various intracranial pathologies, it is clear that adequate interpretation of imaging after stereotactic radiation is required to assess response to therapy and prevent unnecessary re-treatment. As with GBMs, serial MRI scans are the standard method of assessing response following radiosurgery. While many patients show regression of their lesion following treatment, others appear to show a transient growth, and thus radiographic failure, despite a later decrease in size. This temporary phenomenon, similar to pseudoprogression in GBMs, is not infrequent in early post-treatment imaging, presents as an early-delayed or subacute reaction, and is usually asymptomatic. For common indications, control rates after radiosurgery are quite high and the imaging effects of this intensive treatment may be more frequent, such that abnormalities may indeed represent the consequences of treatment without any tumor likely to grow in the future.
The most common imaging change following radiosurgery is peritumoral edema with or without contrast enhancement as illustrated in Figure 1. The time course of edema and increased contrast enhancement following radiosurgery typically peaks in 6–8 months, although it has been reported as far out as 23 months [56,57]. The presence of post-treatment edema has been correlated with pre-treatment peritumoral edema, larger tumor volume, and doses greater than 16 Gy delivered in a single fraction [58].
Figure 1. Treatment effect after stereotactic radiosurgery.
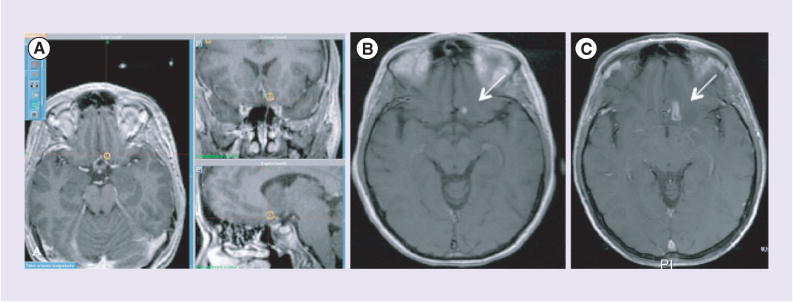
Four brain metastases were treated with stereotactic radiosurgery in a single fraction, 18 Gy × 1. (A) Radiation therapy treatment plan. (B) Pretreatment T1-weighted postcontrast MRI. (C) 6 months post-treatment, with increased T1 enhancement.
The mechanism of treatment effect after radiosurgery is not well understood, although general hypotheses have been proposed that include a pronounced tissue reaction with inflammation, edema and abnormal vessel permeability leading to new or enhanced contrast uptake. The release of toxins from damaged cells in conjunction with disruption of the leptomeninges is thought to lead to the spread of vasogenic fluid into the brain parenchyma. Furthermore, because the brain is devoid of lymphatic drainage, it may take longer to clear cellular debris, leading to an increased inflammatory reaction as tumor cells die. The literature reporting the observed effects for several commonly treated entities are described below.
Brain metastases
In brain metastases treated with radiosurgery, approximately a third to one half of patients will experience transient growth of the imaging abnormality up to 2 years following treatment (Figure 1) [56,59]. Furthermore, homogenous ring enhancement may also occur during this time period. The largest series of quantitative analysis of the radiographic response is reported by Patel et al. who followed 500 brain metastases that were treated with SRS [56]. A third of the lesions had a transient increase in size of the lesion after radiosurgery. A total of 23 patients underwent surgery for pathologic confirmation when there was growth on MRI and suspicious findings on PET CT or MRI spectroscopy. Of those, 96% of patients (22 out of 23) demonstrated treatment effect without confirmation of active tumor on pathology. Stockham et al. evaluated 67 patients who underwent radiosurgery for brain metastases followed by histopathologic analysis via biopsy and/or surgery in the setting of imaging findings concerning for disease progression versus radionecrosis. Pathology revealed that 60% of these patients demonstrated evidence of tumor progression, 28% had necrosis only, 11% had mixed histology (necrosis + viable tumor), and 2% were indeterminate. No MRI criteria to distinguish these entities could be developed from this cohort [57]. Another series included patients with glioma or brain metastases treated with radiosurgery [59]. Of 30 gliomas, 73% were larger at a mean of 13 weeks after treatment, and of 35 metastatic tumors, 22% were larger at a mean of 22 weeks after treatment.
The question of whether imaging changes after radiosurgery represents treatment effect or tumor growth is of particular importance for brain metastasis. The control of individual metastatic lesions by radiosurgery, in contrast to GBM, is quite high, such that there is a higher probability that post-treatment imaging changes represent radiation effect rather than true recurrence. Similar to GBM, this presumed progression might cause discontinuation of efficacious systemic therapies and influence outcomes of studies examining new systemic treatments. We recommend that imaging changes be interpreted with reference to radiosurgery treatment dosimetry, and that asymptomatic patients be followed at short intervals prior to intervention. Patients with symptoms resolving on steroids may be managed conservatively with repeat MRI. If the patient’s systemic disease is well controlled elsewhere in the body, we recommend continuation of systemic therapy until clarity about the clinical course of the imaging findings over time or pathological confirmation of tumor progression is obtained.
Meningiomas
Meningiomas are benign neoplasms arising from the meninges. They are often asymptomatic, but when symptomatic are usually treated with surgical resection or SRS depending on the location of the tumor, resectability and patient preference. SRS is an effective and widely utilized treatment modality for intracranial meningiomas, and similar treatment-related imaging abnormalities have been reported for this benign tumor as we have described for brain metastases. Tumor control has been reported in 84–100% of patients, and a transient increase in the size of the lesion after RT is a well-known phenomenon that is found in 40–60% of cases [60]. Novotny et al. retrospectively reviewed 368 patients with 381 meningiomas that were treated with radiosurgery. The actuarial tumor control rate was 97.9% at 5 years, but peritumoral edema after SRS occurred in 51 patients (15.4%). In total, 32 patients (9.7%) were symptomatic and 2.7% had permanent symptoms associated with the edema. Multiple factors were identified as predictors for occurrence of intracranial edema after SRS: previous surgery, presence of edema before treatment, tumor volume greater than 10 cm3, tumor location in the anterior fossa, and higher doses delivered to the tumor margin [61]. Cai et al. published similar results. In their series of patients with meningioma treated with SRS, the amount of tumor–brain contact interface area and the presence of pre-existing edema were the most significant risk factors for post-SRS peritumoral edema [58]. For this reason special care should be utilized in presuming recurrence in this clinical circumstance, especially given the excellent long-term control rates with radiation treatment.
Vestibular schwannomas
A vestibular schwannoma, also known as an acoustic neuroma, is a benign neoplasm arising from the 8th cranial nerve as a result of overproliferation of Schwann cells. Long-term control rates after radiosurgery for vestibular schwannomas are also quite high and, therefore, treatment effects are a meaningful cause of observed imaging changes after SRS. Nakamura et al. report the results of 78 patients with vestibular schwannoma treated with SRS. They report a control rate of 91%. However, 41% of patients had temporary tumor enlargement, which may be quite substantial. These changes were generally within the first 2 years after therapy [62]. Flickinger et al. treated 134 patients with 12–20 Gy to the tumor periphery and found that tumors regressed in 42% and enlarged in only 3% at a mean of 24 months [63]. As the tumor is rarely life threatening and surgical salvage for vestibular schwannoma after RT is fraught with high risks of complications, including facial nerve damage, we also recommend great caution and conservative follow-up for an enlarging lesion, especially within the first 2 years of treatment [62].
Brachytherapy
Glioblastoma
Intensified local therapy for the treatment of GBMs has been studied in an attempt to improve long-term control. Methods have included standard approaches, such as implantation of radioactive material (brachytherapy) and slow-release chemotherapy wafers, as well as investigational approaches, such as infusion or direct injection of novel drugs, biologic therapies or gene therapies [64]. These approaches may enhance the imaging consequences of radiotherapy, impacting not only the management of individual patients but also the early assessment of potential efficacy of novel approaches.
The GliaSite brachytherapy system utilizes a balloon inserted into the resection cavity at the time of surgery, which may be later accessed through a subcutaneous port. RT is delivered locally by temporarily inflating the balloon with an aqueous solution of organically bound iodine-125 (I-125). Typically a dose of 45–60 Gy is prescribed to a distance of 0.5–1.0 cm from the balloon edge and is usually administered in addition to the 60 Gy of standard radiotherapy. Even higher doses may be utilized for recurrent disease [65].
The tumor control results from clinical trials using GliaSite were difficult to interpret because in the months after treatment it was common for T1-weighted images to demonstrate symmetric enhancement, which met criteria for imaging diagnosis of recurrence. In one study, a total of 25 patients with recurrent GBM underwent repeat resection and subsequent GliaSite brachytherapy [66]. After brachytherapy, all patients developed some degree of enhancement around the resection cavity on T1 and T2/FLAIR imaging. At clinical progression the common findings on MRI included increased T2 signal hyperintensity, vasogenic edema and mass effect. Patients with T1 enhancement over 1 cm during the period before clinical progression had a median survival of 19.3 months. In contrast, those with T1 enhancement under 1 cm before clinical progression had a median survival of 8.4 months (p = 0.004). The subset of patients with T1 enhancement over 1 cm with a concomitant increase in T2 hyperintensity had a median survival of only 10.1 months, suggesting that the development of T1-postcontrast enhancement alone (without T2 hyperintensity) did not necessarily indicate disease progression and may have been an indication of a positive effect of treatment [66]. Clinical trials to optimize the dosing with this device were discontinued as it was felt there was a high rate of early progression. However, review of the survival outcomes suggest that treatment-related imaging changes were more likely the cause of the increase in contrast enhancement rather than true tumor progression. Future studies with similar local therapies should consider toxicity or survival as the primary outcome rather than disease progression based on imaging changes.
Other local therapies aimed at treatment intensification include carmustine (BCNU)-impregnated wafers (Gliadel®; Eisai Inc., Tokyo, Japan) that are implanted into the resection cavity at the time of initial resection or later recurrence. They have been demonstrated in randomized trials to improve overall survival in patients with GBM [67,68]. When Gliadel is incorporated into the initial management along with radiotherapy for GBM at our institution, 15 out of 45 patients (33%) had reoperation for progression of a contrast-enhancing lesion at a median of 7.4 months after RT, and five out of 15 cases (33%) revealed necrosis or treatment effect without evidence of active tumor [69]. These findings suggest that a significant portion of locally recurrent contrast-enhancing lesions after the addition of Gliadel to chemoradiation will represent radiation treatment effect or necrosis rather than true disease progression. The precise extent to which the local chemotherapy contributes to imaging changes and treatment effect is unclear because studies with Gliadel wafer without RT do not specifically differentiate between increase in contrast enhancement due to tumor progression versus treatment effect [70]. Even though systemic chemotherapy can result in white matter changes similar to that of radiation [17], the most likely explanation of our findings is that chemotherapy augments the effects of radiation similar to the results reported with concurrent TMZ and carboplatin [9,25,50,71].
Meningioma
In addition to surgery and external-beam RT, another treatment option for meningioma includes brachytherapy with permanently implanted I-125 sources [72,73]. This strategy has been employed for both grade 1 (i.e., the classic benign meningioma), and grade 2 and 3 (i.e., atypical and malignant) tumors. In one series, 13 patients with newly diagnosed or recurrent meningioma received I-125 brachytherapy to a total dose of 70–170 Gy as definitive therapy [74]. Of the three patients who received a total dose greater than 100 Gy, all of them had evidence of vasogenic edema and/or radiation necrosis at follow-up. This was compared with only 25% of the patients who received less than 100 Gy. One patient treated to 100 Gy eventually underwent craniotomy for a progressive lesion and pathology confirmed radiation necrosis. The majority of patients (67%) showed a decrease in the size of their tumors on MRI at first follow-up. At the time of last follow-up (median 25 months), 45% of patients continued to demonstrate a reduction in size of the tumor. The remaining patients were in stable condition without evidence of disease progression or additional late effects. In a similarly designed retrospective cohort of 21 patients with recurrent atypical or malignant meningioma [75], patients were treated with repeat surgical resection followed by implantation of I-125 sources. The dose delivered to these heavily pretreated patients ranged from 7–45 Gy. Four patients (27%) experienced radionecrosis, and half of those (two out of four patients) required surgical resection due to progressive symptoms. The average time to radiation necrosis in this study was 11 months. Most patients that developed an increase in the size of lesion in this cohort did in fact have progressive disease, as opposed to the first cohort of patients with low-grade meningiomas. In summary, radionecrosis is not uncommon after I-125 therapy and the likelihood of an increasing lesion representing true disease progression relies on the aggressiveness of the underlying tumor as well as the dose delivered.
Investigational imaging techniques for distinguishing progression from treatment effects
Because distinguishing radiation treatment effects from true tumor progression is of great clinical importance, there has been interest in developing imaging approaches to noninvasively distinguish these entities. The promising approaches are reviewed below. Unfortunately, no approach has yet been validated to be sufficiently accurate. Limitations of studies include the lack of rigorous sequential imaging prior to and after treatment to allow for the evaluation of changes over time, and the infrequency of pathologic confirmation with biopsy or surgical resection.
T1/T2 matching
One proposed method of differentiating between tumor progression and radiation effect was to examine the ‘T1/T2 match’. Desquada et al. reported that the ratio of the lesion seen on T2 imaging (maximum cross-sectional area) to the total-enhancing area on T1 imaging had high predictive value, sensitivity and specificity for identifying the presence of radionecrosis [76]. Kano et al., repeated this in a retrospective analysis of 71 patients who required delayed surgical resection with serial MRIs from a series of more than 3000 patients who had brain metastases and underwent radiosurgery. They were able to demonstrate that distinct lesion margin on T2- and a contrast-enhanced margin on T1-weighted images (a finding known as the T1/T2 match) was highly correlated to tumor progression rather than treatment effect (p < 0.0001) [77]. When the lesion border on T2-weighted images did not correspond to the contrast-enhanced T1 volume (T1/T2 mismatch), the pathology was more likely to be associated with necrosis (p < 0.0001). The sensitivity of the T1/T2 mismatch in identifying necrosis was 83.3% and the specificity was 91.2%. In terms of identifying tumor recurrence, the sensitivity of T1/T2 match was 93.9%, and the specificity was 76.9%. One major downfall is that the assessment was purely subjective. There is no mathematical formula and prospective validation studies have not been performed.
Alternative MRI sequences
Advanced imaging techniques are currently being studied to differentiate between pseudoprogression/subacute treatment effect, radionecrosis and true progression. The Neuro-Oncology Working Group concluded that these techniques continue to require rigorous validation studies before they are incorporated into widespread use [55]. The most promising methods include dynamic contrast imaging, perfusion imaging and spectroscopy.
Dynamic contrast imaging
The principle of dynamic perfusion imaging is to take advantage of the differences in the degree of vascularity between malignant processes and necrotic or healthy tissue. Tumors are characterized by increased angiogenesis and higher vascularity compared with necrotic or healthy tissue. Measurement of relative cerebral blood volume (CBV), an indirect measure of blood flow within a given region of interest, is achieved by T2* dynamic susceptibility technique following injection of contrast with bolus tracking. The area under the signal curve yields the CBV for the region of interest [78]. Multiple studies have shown that perfusion MRI is capable of distinguishing between tumor recurrence and postradiation necrosis because tumor contains an abundance of vasculature while necrotic tissue does not [79]. In the evaluation of 18 patients, 6 weeks and 3 months following radiosurgery, Essig et al. showed that this method is highly sensitive and specific for outcome prediction. There was 90% sensitivity in differentiating tumor progression from treatment effect when dynamic perfusion imaging was performed 6 weeks following RT; The delayed postcontrast imaging (non-dynamic) sensitivity was only 64% [80]. With improved imaging techniques and higher magnetic fields, recent studies demonstrate even higher diagnostic value for perfusion imaging in the setting of postradiation changes. In a recent study by Mitsuya et al., 27 patients were followed by serial MRI after radiosurgery for metastatic brain tumors in 1–2-month intervals. The relative CBV value of 2.1 (signal intensity with the enhancing region of interest divided by signal intensity of the contralateral normal brain tissue) yields a sensitivity and specificity of 100% and 95.2%, respectively [81]. Nevertheless, this technique requires further prospective evaluation.
An additional way to analyze perfusion-weighted imaging analysis is to develop a parametric response map (PRM), which relies on voxel-by-voxel comparison of perfusion maps through image co-registration of pretreatment images with those obtained at short time intervals after treatment initiation. This method has been shown to be a superior to conventional contrast perfusion analysis (i.e., relative CBV [rCBV]), for prognosis of survival after treatment [82]. The enhancement pattern may also be evaluated with the use of T1-weighted imaging such as fast spin echo (FSE) sequences to plot the enhancement pattern as proposed by Hazle et al. [83]. In this method the signal intensity of the lesion after injection of contrast is plotted, and the maximum and delayed rates of uptake are calculated. Based on these values, the vascular endothelial contrast transfer constant (Ktrans), also known as the transfer constant between intra- and extravascular extracellular space (i.e., the ‘leakiness’ of the blood vessels). Using the same principles, Bisdas et al. demonstrated that using a Ktrans cutoff of 0.19, the sensitivity and specificity of detecting recurrent glioma was 100% and 83%, respectively [84].
Another area of research in the field of perfusion imaging is the development of contrast agents with more blood pool accumulation capacities in order to facilitate more accurate rCBV measurements in the setting of a compromised blood–brain barrier. One of these contrast agents is ferumoxytol, an ultra-small superparamagnetic iron oxide nanoparticle, which acts as a blood pool agent for minutes to hours. An additional benefit is that its vascular localization is not compromised by a violated blood–brain barrier, as is the case in both treatment effect and disease progression [85].
Diffusion imaging
Diffusion weighted imaging (DWI) is based on the ability of the MRI to detect random, thermal energy induced, Brownian motion of water molecules within the microenvironment [86]. The motion of water molecules is affected by several factors, including the structure of intra -and extra-cellular space and degree of cellularity. In patients with true recurrence, there is a high concentration of malignant cells at the site of recurrence with increased intracellular space. Increased intracellular space means less free random motion of water molecules due to closely packed cell walls, hence more restricted diffusion of molecules and a higher (i.e., brighter) signal on images. This restricted diffusion can be quantified with apparent diffusion coefficient (ADC; mm2/s), which is a measure of the motion of water molecules within the medium at the pixel level. This constellation of pixel values is represented as an ADC map. More restricted diffusion translates into a lower ADC value; therefore, areas of recurrence appear dark on an ADC map.
There is a growing body of evidence that DWI can differentiate between recurrence and radiation-induced changes. Using a combination of DWI and contrast-enhanced MRI in 18 patients with high-grade gliomas treated with RT, Hein et al., demonstrated that the mean ADC value of the recurrence group was significantly lower compared with the non-recurrence group [87]. More recently, Cha et al., investigated the role of combined ADC and rCBV in differentiation of radiation-induced changes and tumor progression in 16 patients with enlarging areas of enhancement following radiosurgery. They concluded that if rCBV larger than 2.6 was used to differentiate radiation necrosis and tumor progression, the sensitivity was 100% but specificity was only 56%. If the lesions with moderately increased rCBV (2.6–4.1) were excluded from tumor progression, the sensitivity and specificity increased to 100% [88].
Figure 2 illustrates tumor progression associated with restricted diffusion in a 56-year-old woman who presented with left upper and lower extremity weakness. T1-weighted postcontrast MRI demonstrated a right supratentorial peripherally enhancing mass with associated T2 FLAIR hyperintensity. CT of the chest revealed a left upper lobe lung mass. She underwent right craniotomy with tumor resection and pathology revealed metastatic poorly differentiated adenocarcinoma consistent with a lung primary. She received adjuvant stereotactic radiation to the tumor bed to 18 Gy in three fractions. MRI performed 5 months after surgery showed new contrast enhancement. At this time she was asymptomatic and imaging changes were attributed to treatment effect. The decision was made to continue with surveillance MRIs and 5 months later (10 months postradiation), MRI showed progression of contrast-enhancing lesion. Corresponding DWI showed restricted diffusion (H). A month later, the patient presented with tremulousness, anxiety and a shuffling gate with progressive changes on MRI. She underwent re-do craniotomy and pathology revealed metastatic adenocarcinoma. She received postoperative whole brain radiation and 6 months after whole brain radiation had no evidence of CNS progression.
Figure 2. Restricted diffusion associated with tumor progression after resection of brain metastases followed by radiosurgery.
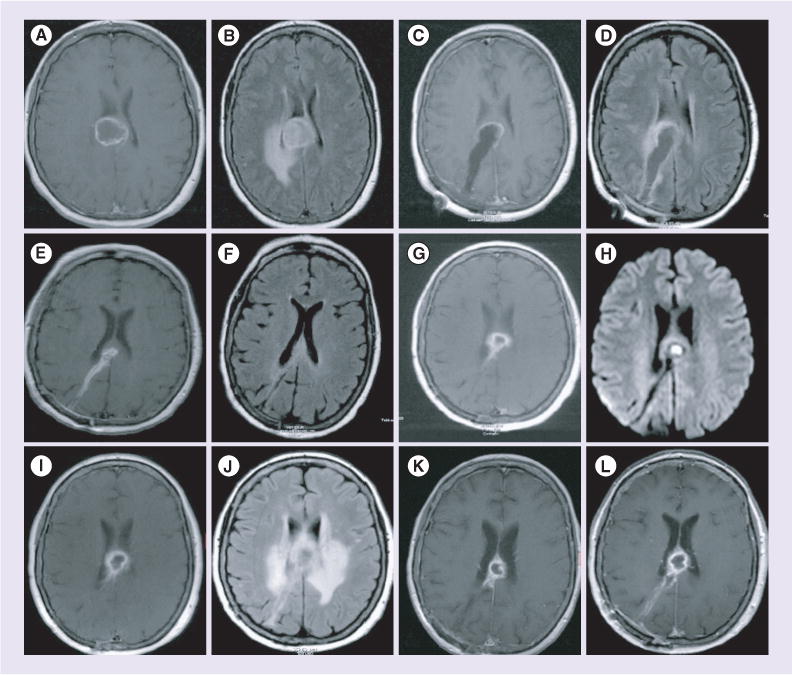
(A) T1-weighted postcontrast MRI demonstrating right supratentorial peripherally enhancing mass with (B) associated T2 fluid attenuated inversion recovery (FLAIR) hyperintensity. Pathology revealed metastatic poorly differentiated adenocarcinoma consistent with a lung primary. (C) Postoperative T1-weighted postcontrast. (D) Postoperative T2 FLAIR. Five months after adjuvant stereotactic radiosurgery, MRI showed (E) new contrast enhancement on T1 weighted postcontrast imaging. (F) T2 FLAIR with associated hyperintensity and 5 months later (10 months postradiation), MRI showed (G) progression of contrast-enhancing lesion. (H) Diffusion weighted imaging showed restricted diffusion. MRI 1 month later showed (I) progression of enhancement and (J) worsening T2 FLAIR hyperintensity. At this point she developed symptoms and underwent re-do craniotomy. Pathology revealed metastatic adenocarcinoma. (K) T1-weighted postcontrast MRI 3 months following whole brain radiation therapy (RT). (L) T1-weighted postcontrast MRI 3 months following whole brain RT 6 months postwhole brain RT demonstrating no evidence of CNS progression.
Figure 3 demonstrates a case of radionecrosis in a man with GBM after adjuvant chemoradiation with lack of restricted diffusion. The patient underwent maximum safe resection followed by RT and concurrent TMZ followed by adjuvant TMZ. First follow-up MRI 2 months after irradiation showed a new area of contrast enhancement just inferior to the tumor bed without evidence of restricted diffusion (G and H). The patient was asymptomatic and underwent surgical resection. Pathology revealed gliosis with focal necrosis, without evidence of tumor.
Figure 3. Lack of restricted diffusion in radionecrosis after surgery and chemoradiation for glioblastoma multiforme.
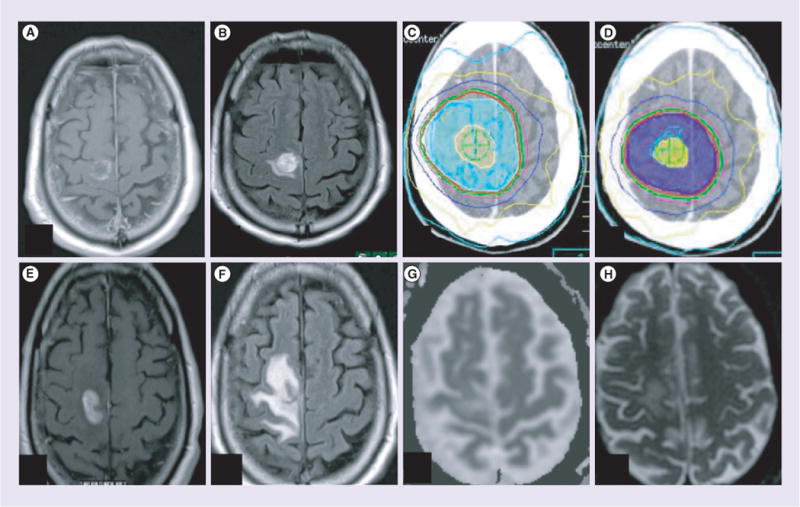
(A) Postoperative T1 postcontrast. (B) Postoperative T2 fluid attenuated inversion recovery (FLAIR). (C) Radiation therapy (RT) plan, initial volume to 46 Gy. (D) RT treatment plan, cone down to 60 Gy. First follow-up MRI 2 months after irradiation showed a new area of contrast enhancement just inferior to the tumor bed without evidence of restricted diffusion. (E) T1 postcontrast; (F) T2 FLAIR; (G) apparent diffusion coefficient map; (H) diffusion weighted imaging: b = 800. The man was asymptomatic and underwent resection. Final path: gliosis with focal necrosis. No evidence of tumor.
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) provides valuable information regarding the chemical composition of tissues. This method can quantify molecules within tissue, including glucose (tumor metabolism), choline (membrane turnover), creatine (energy homeostasis), lactate (necrosis) and N-acetyl-aspartate (NAA; intact glioneural structures) [78]. Data can be obtained with 2D or 3D chemical shift imaging sequences in single or multiple voxels. Measurements of each of these metabolites have been studied for evaluation of tumor response versus disease progression. For example, a decrease in NAA following radiotherapy is a sign of radiation-induced necrosis even before morphologic changes on conventional MRI images can be detected [89,90]. An increase in choline concentration has been shown to be correlated with markers of cell proliferation (progression) and reduced levels of choline can be seen following radiosurgery [91,92]. Another way of approaching the metabolite levels within tissue is to present the data as a ratio of metabolites. Weybright et al. investigated the feasibility of 2D chemical shift imaging spectroscopy for evaluation of recurrence versus radiation-induced brain injury using choline (Cho)/NAA, Cho/creatine (Cr) and NAA/Cr ratios [93]. They demonstrated that Cho/NAA and Cho/Cr ratios were significantly higher and the NAA/Cr ratio was significantly lower in tumor compared with radionecrosis. Using a cutoff of 1.8 for either Cho/Cr or Cho/NAA ratios, they were able to correctly classify 27 out of 28 patients. Elias et al. also evaluated 25 patients with MRS and demonstrated the highest discriminating ability using Cho/NAA and NAA/Cr ratios with sensitivity and specificities of 86%/90% and 93%/70%, respectively [94].
A major drawback with MR spectroscopy is volume averaging within the voxel. This is more troublesome in small volumes typical in early recurrence of tumor following radiation when there may be coexisting inflammatory changes within the adjacent tissue. This problem is more pronounced when the single voxel technique is being utilized as opposed to the 2D chemical shift technique. In the single voxel technique the increased choline and NAA metabolite profiles in a true tumor recurrence might be averaged, thus giving a picture of pure inflammatory changes rather than disease progression. Other limitations of MRS is lengthy acquisitions time due to low signal to noise ratio.
PET & single-photon emission computed tomography
Other imaging modalities, such as PET and single-photon emission computed tomography (SPECT), have been evaluated as tools to differentiate between radiation effect and disease progression [95–97]. Nuclear medicine imaging using radiotracers relies on certain molecular characteristics of normal and cancer cells. For example, in the case of 18-flurodeoxyglucose PET (FDG-PET) imaging, the presence of glucose transporters leads to uptake and entrapment of FDG in the cell. Higher levels of cellular activity, such as in cancer cells, requires more metabolism of glucose, hence more glucose transporters and more uptake of the radiotracer [98]. High-grade gliomas have been shown to have more FDG uptake compared with low-grade or well-differentiated neoplasms, and FDG-PET can be useful in making a distinction between low-grade and high-grade gliomas [99]. Using the same principle, high FDG uptake in a previously low-grade glioma may indicate anaplastic transformation [100]. However, the application of PET imaging in the diagnosis of recurrence versus radionecrosis is more complicated. During the early postradiation period (up to 6 months), high concentration of inflammatory cells at the site of radiation lead to increased uptake of radiotracer similar to recurrence [98,101]. While FDG-PET enjoys high sensitivity of up to 86% for distinguishing recurrence from radiation-induced necrosis, the specificity of this imaging modality can be as low as 40%, making the distinction often difficult [102]. Fusion of MRI images with PET images can improve the ability of PET to distinguish between radionecrosis and recurrence, using the aforementioned techniques [78]. Another technique that has been proposed as a useful tool in this regard is dual-phase or delayed FDG-PET imaging. This method takes advantage of higher phosphorylation levels in certain malignancies, including gliomas, leading to trapping of the radiotracer within the cancer cells when imaging is performed 3–8 h after injection of contrast [103].
The development of new tumor-specific radiotracers is another area of active and promising research. One new radiotracer, 3-deoxy-3-[18F] fluorothymidine (FLT), is a molecule that is processed by thymidine kinase-1 during the S-phase of mitosis. This tracer is unique in the degree to which there is uptake in the setting of a disrupted blood–brain barrier, which makes it very useful in determining the grade of brain tumors since higher-grade cancers are associated with more disruption of the blood–brain barrier [104,105]. Another group of promising tracers includes amino acid radiotracers, such as 11C-methionine, 18F-fluoro-L-phenylalanince (18F-FDOPA) and 18F-fluoroethyl-L-thyrosin (18F-FET). These molecules get actively get transported into the cells regardless of disruption of the blood–brain barrier, therefore, they are able to show uptake within both low- and high-grade gliomas [104]. Amino acid radiotracers generally have a higher specificity compared with other radiotracers for distinguishing radionecrosis from tumor recurrence with reported specificity of 100% for 11C-Met [104], 93.5% for 18F-FET [106], and 86% for 18F-FDOPA [107]. For hexamethylpropylene amine oxime (HMPAO) SPECT imaging, low uptake of both thallium and HMPAO is associated with radiation effects, whereas, increased uptake of both agents are associated with tumor progression. However, due to lack of widespread clinical availability as well as high false-positive rates, it is unlikely that PET or HMPAO will be an efficacious method of determining radiation effect versus treatment effect without substantial future research efforts aimed towards this goal [95–97].
Conclusion
Radiation-induced imaging changes may mimic recurrence and can be difficult to distinguish from true disease progression across the entire spectrum of benign and malignant CNS conditions that are treated with radiotherapy, regardless of the radiation technique utilized. Knowledge of the treatment administered, the clinical course and the probability of actual recurrence are used to guide decisions regarding clinical management in the absence of reliable noninvasive approaches. Table 1 summarizes the clinical and radiographic differences between disease progression and radiation effect. In the event that the radiographic changes are equivocal, it is often most appropriate to continue planned treatment and observe the patient closely. Review of the relationship of the new abnormalities to the actual radiotherapy treatment plan is essential. When the cause of the imaging changes are not clear, we recommend reimaging at approximately 4-week intervals, perhaps with more frequent clinical evaluations. Ideally patients should be scanned with the same contrast protocol and magnet strength to reduce difficulties in comparison and interpretation. The other factors that should be considered (and are included in the updated Revised Assessment in Neuro-Oncology [RANO] criteria) are the following: the nonenhancing component (T2/FLAIR); presence of new lesions; corticosteroid use; and clinical status. Progressive symptoms not resolving with medications, including steroids, is an indication for surgery. Resection would be therapeutic if the cause was indeed recurrent tumor but may also provide relief for symptomatic radiation treatment effects by removing the tissue causing the progressive inflammatory process.
Table 1.
Imaging changes after radiation therapy.
| Parameter | Pseudoprogression | Treatment effect after radiosurgery | Radionecrosis | Tumor progression |
|---|---|---|---|---|
| Time to onset | 2–6 months after RT | 3–12 months after RT | 6–12 months after RT (but can be decades) | Any |
| Characteristic imaging appearance | Contrast enhancement with FLAIR hyperintensity | Contrast enhancement with FLAIR hyperintensity | Contrast enhancement with FLAIR hyperintensity; ‘soap bubble’ or ‘Swiss cheese’ sign | Contrast enhancement with FLAIR hyperintensity |
| Treatment | Observation with close follow-up | Observation with close follow-up | Steroids, bevacizumab, surgical resection | Tumor-directed therapy |
| Usually symptomatic? | No | No | Yes | Yes |
| MRI spectroscopy | Not clearly radionecrosis or tumor progression | Not clearly radionecrosis or tumor progression | Relatively less choline | Relatively less NAA and creatine; more choline and lactate |
| Diffusion weighted imaging | Not clearly radionecrosis or tumor progression | Not clearly radionecrosis or tumor progression | Less restricted diffusion (lower signal) | More restricted diffusion (higher signal) |
| Dynamic contrast imaging | Relatively low mean cerebral blood volume compared with true tumor progression | Relatively low mean cerebral blood volume compared with true tumor progression | Relatively low mean cerebral blood volume compared with true tumor progression | Relatively high mean cerebral blood volume |
FLAIR: Fluid attenuated inversion recovery; RT: Radiation therapy.
Advanced imaging techniques, despite great promise, should not be solely relied upon to distinguish treatment effect from growing tumor. When performed properly, diffusion and perfusion weighted imaging can be helpful in certain situations, but they must be considered within the clinical context. The images should be reviewed carefully by neuroradiologists with experience in such imaging techniques and preferably in the setting of a multidisciplinary tumor board. Dynamic contrast imaging and spectroscopy are promising tools as well, but still require additional prospective validation.
Future perspective
Being able to successfully differentiate between true disease progression and radiation effect in the CNS would improve the management of patients after RT to the brain. The imaging techniques under investigation described in this review hold great promise; however, further rigorous investigation into these treatment modalities as well as other novel imaging techniques to noninvasively assess growing lesions after stereotactic and conventional radiotherapy is warranted. One barrier to the successful evaluation of advanced imaging techniques is the lack of funding to routinely scan a cohort of patients prior to treatment and at regular intervals over time, rather than scanning patients at the isolated time point of possible recurrence without prior imaging that allows assessment of changes. Such disciplined prospective studies are greatly needed. Without such funding limitations, studies might even utilize a combination of imaging techniques at regular intervals, rather than focusing on a single modality. We anticipate continued improvements in the treatment and prevention of radiation neurotoxicity as we gain a further understanding of the cytokine cascades and intracellular molecular signaling pathways involved in this response. In addition to information obtained from imaging, identification of a molecular signature (or ‘fingerprint’) at the time of diagnosis could be used to risk stratify patients as being more or less likely to demonstrate treatment effect, particularly in primary CNS malignancies. Research needs to continue on these fronts, and the optimum management of patients in the future may include a series of imaging modalities performed at regular intervals, molecular characterization of malignancies at the time of diagnosis and prevention of neurotoxicity.
EXECUTIVE SUMMARY.
Background
Radiation treatment effects and injury may appear similar to tumor progression and can develop during treatment or months to years later. Caution is required in evaluating the meaning of postradiation imaging.
Radiation injury
Acute radiation effects occur during or shortly after radiation therapy (RT) and symptoms almost always improve over time. Diffuse brain swelling may be visualized on MRI.
Subacute/early-delayed effects occur within the first few months after RT. Symptoms are usually mild and stabilize or diminish over time. Imaging findings vary from diffuse edema to increased contrast enhancement.
Late effects occur a few months to years after RT. Radionecrosis is typically an irreversible late complication that often mimics disease progression. The incidence of radionecrosis is <5% when 60 Gy of RT is delivered to the brain in 2 Gy fractions. Treatment options include dexamethasone, bevacizumab and surgical resection.
Radiation treatment effect in various radiation modalities
Treatment effects that mimic radiographic or clinical treatment failure occur with all radiation modalities.
Intensity-modulated RT: pseudoprogression is a temporary and self-limiting treatment related phenomenon that mimics tumor progression after chemo-RT for glioblastoma multiforme (GBM) that may or may not be associated with clinical symptoms. Typical onset is within the first 2–3 months after RT.
Stereotactic radiosurgery (SRS): up to a third to a half of brain metastases treated with SRS will experience treatment effect and transient growth up to 2 years following treatment. A similar phenomenon is seen after SRS for meningiomas and vestibular schwannomas.
Brachytherapy: after GliaSite for GBM, there is a very high incidence of contrast enhancement, which is more likely to represent treatment effect with T1 contrast enhancement alone (without T2 hyperintensity). Intensified local therapies, including local chemotherapy with Gliadel® wafers (Eisai Inc., Tokyo, Japan), leads to an increased rate of treatment-related contrast enhancement. In the treatment of meningiomas, radionecrosis is not an uncommon event after iodine-125 brachytherapy.
Investigational imaging techniques for distinguishing progression from treatment effects
There is not yet a validated imaging technique that distinguishes treatment effect from true treatment failure, and clinical follow-up and/or pathologic confirmation are currently utilized to distinguish these processes. Several currently available imaging techniques may potentially be helpful, but rigorous evaluation testing the clinical usefulness has not occurred.
- Alternative MRI sequences
-
–Dynamic contrast imaging takes advantage of increased vascularity of the tumor compared with the normal or necrotic tissue. The amount of contrast flowing through a given volume is measured by assessment of T2* alteration, and higher flow of contrast through the tissue is indicative of more vascularization. This method may be more sensitive and specific that delayed contrast enhancement imaging alone.
-
–Diffusion weighted imaging is a reflection of the local motion of water molecules in the microenvironment. The more restricted the motion of water molecules, the higher signal obtained on diffusion weighted images. In general, cancer cells are tightly packed with high nucleolus to cytoplasm ratios, hence, more restricted environment and higher signal.
-
–Spectroscopy can yield valuable information regarding the composition of major metabolites within the tissue. Choline has been shown to increase in cases of tumor progression while decreasing in radiation-induced necrosis. The downside of spectroscopy is long acquisitions and volume averaging.
-
–
PET and single-photon emission computed tomography are based on metabolic activity of the tissue and rate of uptake of the radiopharmaceutical. While currently not the mainstream modalities to differentiate tumor progression versus postradiation changes – mainly marred by low specificity and low special resolution – they hold a promising future, given ongoing development of tumor-specific radiotracers and molecular imaging.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Sheline GE. Radiation therapy of brain tumors. Cancer. 1977;39(2 Suppl):873–881. doi: 10.1002/1097-0142(197702)39:2+<873::aid-cncr2820390725>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corn BW, Yousem DM, Scott CB, et al. White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83–02) Cancer. 1994;74(10):2828–2835. doi: 10.1002/1097-0142(19941115)74:10<2828::aid-cncr2820741014>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 5.Glass JP, Hwang TL, Leavens ME, Libshitz HI. Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer. 1984;54(9):1966–1972. doi: 10.1002/1097-0142(19841101)54:9<1966::aid-cncr2820540930>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Ruben JD, Dally M, Bailey M, Smith R, Mclean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7••.Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7(2):243–252. doi: 10.1016/0360-3016(81)90443-0. Review of published data regarding radiation dose-volume effects in the CNS. [DOI] [PubMed] [Google Scholar]
- 8••.Lawrence YR, Li XA, El Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 76(3 Suppl):S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. Provides a detailed review of pseudoprogression in malignant gliomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandsma D, Stalpers L, Taal W, Sminia P, Van Den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 10.Kramer S, Hendrickson F, Zelen M, Schotz W. Therapeutic trials in the management of metastatic brain tumors by different time/dose fraction schemes of radiation therapy. Natl Cancer Inst Monogr. 1977;46:213–221. [PubMed] [Google Scholar]
- 11.Hindo WA, Detrana FA, 3rd, Lee MS, Hendrickson FR. Large dose increment irradiation in treatment of cerebral metastases. Cancer. 1970;26(1):138–141. doi: 10.1002/1097-0142(197007)26:1<138::aid-cncr2820260117>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Young DF, Posner JB, Chu F, Nisce L. Rapid-course radiation therapy of cerebral metastases: results and complications. Cancer. 1974;34(4):1069–1076. doi: 10.1002/1097-0142(197410)34:4<1069::aid-cncr2820340416>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13•.Freeman JE, Johnston PG, Voke JM. Somnolence after prophylactic cranial irradiation in children with acute lymphoblastic leukaemia. Br Med J. 1973;4(5891):523–525. doi: 10.1136/bmj.4.5891.523. Provides a detailed historical account of the use of radiation therapy for primary CNS neoplasms, including imaging changes after treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leibel SA, Sheline GE. Radiation therapy for neoplasms of the brain. J Neurosurg. 1987;66(1):1–22. doi: 10.3171/jns.1987.66.1.0001. [DOI] [PubMed] [Google Scholar]
- 15.Rane N, Quaghebeur G. CNS effects following the treatment of malignancy. Clin Radiol. 2012;67(1):61–68. doi: 10.1016/j.crad.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6(9):1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 17.Brown MS, Stemmer SM, Simon JH, et al. White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. Am J Neuroradiol. 1998;19(2):217–221. [PMC free article] [PubMed] [Google Scholar]
- 18.Hertzberg H, Huk WJ, Ueberall MA, et al. CNS late effects after ALL therapy in childhood. Part I Neuroradiological findings in long-term survivors of childhood ALL – an evaluation of the interferences between morphology and neuropsychological performance The German Late Effects Working Group. Med Pediatr Oncol. 1997;28(6):387–400. doi: 10.1002/(sici)1096-911x(199706)28:6<387::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008;25(2):51–58. doi: 10.1007/s10014-008-0233-9. [DOI] [PubMed] [Google Scholar]
- 20.Gutin PH, McDermott MW, Ross G, et al. Polyamine accumulation and vasogenic oedema in the genesis of late delayed radiation injury of the central nervous system (CNS) Acta Neurochir Suppl (Wien) 1990;51:372–374. doi: 10.1007/978-3-7091-9115-6_125. [DOI] [PubMed] [Google Scholar]
- 21.Leibel SA, Sheline GE. Tolerance of the brain and spinal cord to conventional irradiation. In: Gutin PH, Leibel SA, Sheline GE, editors. Radiation Injury to the Nervous System. Raven Press; NY, USA: 1991. pp. 211–239. [Google Scholar]
- 22.Lee AW, Kwong DL, Leung SF, et al. Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys. 2002;53(1):75–85. doi: 10.1016/s0360-3016(02)02711-6. [DOI] [PubMed] [Google Scholar]
- 23.Leibel SA, Sheline GE. Tolerance of the Brain and Spinal Cord to Conventional Irradiation. Raven Press; NY, USA: 1991. pp. 211–239. [Google Scholar]
- 24•.McDonald LW, Hayes TL. The role of capillaries in the pathogenesis of delayed radionecrosis of brain. Am J Pathol. 1967;50(5):745–764. Retrospective review of MRI changes and pathologic correlates after chemoradiation for glioblastoma multiforme. [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 26.Fike JR, Sheline GE, Cann CE, Davis RL. Radiation necrosis. Prog Exp Tumor Res. 1984;28:136–151. doi: 10.1159/000408242. [DOI] [PubMed] [Google Scholar]
- 27.Burger PC, Boyko OB. The pathology of central nervous system radiation injury. In: Gutin PH, Leibel SA, Sheline GE, editors. Radiation Injury to the Nervous System. Raven; NY, USA: 1991. pp. 191–208. [Google Scholar]
- 28.Kurita H, Kawahara N, Asai A, Ueki K, Shin M, Kirino T. Radiation-induced apoptosis of oligodendrocytes in the adult rat brain. Neurol Res. 2001;23(8):869–874. doi: 10.1179/016164101101199324. [DOI] [PubMed] [Google Scholar]
- 29.Castel JC, Caille JM. Imaging of irradiated brain tumours. Value of magnetic resonance imaging. J Neuroradiol. 1989;16(2):81–132. [PubMed] [Google Scholar]
- 30•.Sawaya RE. The fibrinolytic enzymes in the biology. In: Sawaya RE, editor. Fibrinolysis and the Central Nervous System. Hanley & Belfus; PA, USA: 1990. pp. 106–126. Provides detailed historical account of CNS toxicity after radiation. [Google Scholar]
- 31.New P. Radiation injury to the nervous system. Curr Opin Neurol. 2001;14(6):725–734. doi: 10.1097/00019052-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Kawamoto H, Kiya K, Yuki K, Uozumi T. Delayed cerebral radiation necrosis occurring twice at different regions and times in a patient with a metastatic brain tumour. Acta Neurochir (Wien) 2000;142(5):599–600. doi: 10.1007/s007010050477. [DOI] [PubMed] [Google Scholar]
- 33.Lampert PW, Davis RL. Delayed effects of radiation on the human central nervous system; ‘early’ and ‘late’ delayed reactions. Neurology. 1964;14:912–917. doi: 10.1212/wnl.14.10.912. [DOI] [PubMed] [Google Scholar]
- 34.Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC., Jr Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44(11):2020–2027. doi: 10.1212/wnl.44.11.2020. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94(1):63–68. doi: 10.1007/s11060-009-9801-z. [DOI] [PubMed] [Google Scholar]
- 37.Liu AK, Macy ME, Foreman NK. Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys. 2009;75(4):1148–1154. doi: 10.1016/j.ijrobp.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuba PJ, Aronin P, Bhambhani K, et al. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer. 1997;80(10):2005–2012. doi: 10.1002/(sici)1097-0142(19971115)80:10<2005::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 40.Leber KA, Eder HG, Kovac H, Anegg U, Pendl G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg. 1998;70(Suppl. 1):229–236. doi: 10.1159/000056426. [DOI] [PubMed] [Google Scholar]
- 41.Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192–200. doi: 10.1159/000338251. [DOI] [PubMed] [Google Scholar]
- 42.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl. 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 44.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 45.Fiegler W, Langer M, Scheer M, Kazner E. Reversible computed tomographic changes following brain tumor irradiation induced by the ‘early-delayed reaction’ after radiation. Radiologe. 1986;26(4):206–209. [PubMed] [Google Scholar]
- 46.Watne K, Hager B, Heier M, Hirschberg H. Reversible oedema and necrosis after irradiation of the brain. Diagnostic procedures and clinical manifestations. Acta Oncol. 1990;29(7):891–895. doi: 10.3109/02841869009096385. [DOI] [PubMed] [Google Scholar]
- 47.Griebel M, Friedman HS, Halperin EC, et al. Reversible neurotoxicity following hyperfractionated radiation therapy of brain stem glioma. Med Pediatr Oncol. 1991;19(3):182–186. doi: 10.1002/mpo.2950190307. [DOI] [PubMed] [Google Scholar]
- 48.De Wit MC, De Bruin HG, Eijkenboom W, Sillevis Smitt PA, Van Den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 49.Taal W, Brandsma D, De Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 50.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 51.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 52•.Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26(25):4189–4199. doi: 10.1200/JCO.2007.11.5964. Provides an update on theresponse criteria in GBM. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for Phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 54.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. Describes imaging changes after stereotactic radiosurgery for brain metastases. [DOI] [PubMed] [Google Scholar]
- 56.Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. Am J Neuroradiol. 2011;32(10):1885–1892. doi: 10.3174/ajnr.A2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stockham AL, Tievsky AL, Koyfman SA, et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012;109(1):149–158. doi: 10.1007/s11060-012-0881-9. [DOI] [PubMed] [Google Scholar]
- 58.Cai R, Barnett GH, Novak E, Chao ST, Suh JH. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010;66(3):513–522. doi: 10.1227/01.NEU.0000365366.53337.88. [DOI] [PubMed] [Google Scholar]
- 59.Ross DA, Sandler HM, Balter JM, Hayman JA, Archer PG, Auer DL. Imaging changes after stereotactic radiosurgery of primary and secondary malignant brain tumors. J Neurooncol. 2002;56(2):175–181. doi: 10.1023/a:1014571900854. [DOI] [PubMed] [Google Scholar]
- 60.Bitzer M, Wockel L, Luft AR, et al. The importance of pial blood supply to the development of peritumoral brain edema in meningiomas. J Neurosurg. 1997;87(3):368–373. doi: 10.3171/jns.1997.87.3.0368. [DOI] [PubMed] [Google Scholar]
- 61.Novotny J, Jr, Kollova A, Liscak R. Prediction of intracranial edema after radiosurgery of meningiomas. J Neurosurg. 2006;105(Suppl):120–126. doi: 10.3171/sup.2006.105.7.120. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura H, Jokura H, Takahashi K, Boku N, Akabane A, Yoshimoto T. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. Am J Neuroradiol. 2000;21(8):1540–1546. [PMC free article] [PubMed] [Google Scholar]
- 63.Flickinger JC, Lunsford LD, Linskey ME, Duma CM, Kondziolka D. Gamma knife radiosurgery for acoustic tumors: multivariate analysis of four year results. Radiother Oncol. 1993;27(2):91–98. doi: 10.1016/0167-8140(93)90127-t. [DOI] [PubMed] [Google Scholar]
- 64.Juratli TA, Schackert G, Krex D. Current status of local therapy in malignant gliomas – a clinical review of three selected approaches. Pharmacol Ther. 2013;139(3):341–358. doi: 10.1016/j.pharmthera.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Chan TA, Weingart JD, Parisi M, et al. Treatment of recurrent glioblastoma multiforme with GliaSite brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62(4):1133–1139. doi: 10.1016/j.ijrobp.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 66.Kleinberg L, Yoon G, Weingart JD, et al. Imaging after GliaSite brachytherapy: prognostic MRI indicators of disease control and recurrence. Int J Radiat Oncol Biol Phys. 2009;75(5):1385–1391. doi: 10.1016/j.ijrobp.2008.12.074. [DOI] [PubMed] [Google Scholar]
- 67.Dixit S, Hingorani M, Achawal S, Scott I. The sequential use of carmustine wafers (Gliadel®) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. Br J Neurosurg. 2011;25(4):459–469. doi: 10.3109/02688697.2010.550342. [DOI] [PubMed] [Google Scholar]
- 68.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 69.Kleinberg LR, Weingart J, Burger P, et al. Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: implications for patient management. Cancer Invest. 2004;22(1):1–9. doi: 10.1081/cnv-120027575. [DOI] [PubMed] [Google Scholar]
- 70.Giese A, Kucinski T, Knopp U, et al. Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol. 2004;66(3):351–360. doi: 10.1023/b:neon.0000014539.90077.db. [DOI] [PubMed] [Google Scholar]
- 71.Van Tassel P, Bruner JM, Maor MH, et al. MR of toxic effects of accelerated fractionation radiation therapy and carboplatin chemotherapy for malignant gliomas. Am J Neuroradiol. 1995;16(4):715–726. [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar PP, Patil AA, Leibrock LG, et al. Continuous low dose rate brachytherapy with high activity iodine-125 seeds in the management of meningiomas. Int J Radiat Oncol Biol Phys. 1993;25(2):325–328. doi: 10.1016/0360-3016(93)90355-y. [DOI] [PubMed] [Google Scholar]
- 73.Suh JH, Barnett GH. Brachytherapy for brain tumor. Hematol Oncol Clin North Am. 1999;13(3):635–650. viii–ix. doi: 10.1016/s0889-8588(05)70080-0. [DOI] [PubMed] [Google Scholar]
- 74.Obasi PC, Barnett GH, Suh JH. Brachytherapy for intracranial meningioma using a permanently implanted iodine-125 seed. Stereotact Funct Neurosurg. 2002;79(1):33–43. doi: 10.1159/000069502. [DOI] [PubMed] [Google Scholar]
- 75.Ware ML, Larson DA, Sneed PK, Wara WW, McDermott MW. Surgical resection and permanent brachytherapy for recurrent atypical and malignant meningioma. Neurosurgery. 2004;54(1):55–63. doi: 10.1227/01.neu.0000097199.26412.2a. [DOI] [PubMed] [Google Scholar]
- 76.Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63(5):898–903. doi: 10.1227/01.NEU.0000333263.31870.31. [DOI] [PubMed] [Google Scholar]
- 77.Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery. 2010;66(3):486–491. doi: 10.1227/01.NEU.0000360391.35749.A5. [DOI] [PubMed] [Google Scholar]
- 78.Dhermain FG, Hau P, Lanfermann H, Jacobs Ah, Van Den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 79.Gasparetto EL, Pawlak MA, Patel SH, et al. Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology. 2009;250(3):887–896. doi: 10.1148/radiol.2502071444. [DOI] [PubMed] [Google Scholar]
- 80.Essig M, Waschkies M, Wenz F, Debus J, Hentrich HR, Knopp MV. Assessment of brain metastases with dynamic susceptibility-weighted contrast-enhanced MR imaging: initial results. Radiology. 2003;228(1):193–199. doi: 10.1148/radiol.2281020298. [DOI] [PubMed] [Google Scholar]
- 81.Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010;99(1):81–88. doi: 10.1007/s11060-009-0106-z. [DOI] [PubMed] [Google Scholar]
- 82.Galban CJ, Chenevert TL, Meyer CR, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15(5):572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hazle JD, Jackson EF, Schomer DF, Leeds NE. Dynamic imaging of intracranial lesions using fast spin-echo imaging: differentiation of brain tumors and treatment effects. J Magn Reson Imaging. 1997;7(6):1084–1093. doi: 10.1002/jmri.1880070622. [DOI] [PubMed] [Google Scholar]
- 84.Bisdas S, Naegele T, Ritz R, et al. Distinguishing recurrent high-grade gliomas from radiation injury: a pilot study using dynamic contrast-enhanced MR imaging. Acad Radiol. 2011;18(5):575–583. doi: 10.1016/j.acra.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 85.Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79(2):514–523. doi: 10.1016/j.ijrobp.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malayeri AA, El Khouli RH, Zaheer A, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31(6):1773–1791. doi: 10.1148/rg.316115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. Am J Neuroradiol. 2004;25(2):201–209. [PMC free article] [PubMed] [Google Scholar]
- 88.Cha J, Kim ST, Kim HJ, et al. Analysis of the layering pattern of the apparent diffusion coefficient (ADC) for differentiation of radiation necrosis from tumour progression. Eur Radiol. 2013;23(3):879–886. doi: 10.1007/s00330-012-2638-4. [DOI] [PubMed] [Google Scholar]
- 89.Sundgren PC. MR spectroscopy in radiation injury. Am J Neuroradiol. 2009;30(8):1469–1476. doi: 10.3174/ajnr.A1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sundgren PC, Nagesh V, Elias A, et al. Metabolic alterations: a biomarker for radiation-induced normal brain injury-an MR spectroscopy study. J Magn Reson Imaging. 2009;29(2):291–297. doi: 10.1002/jmri.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herminghaus S, Pilatus U, Moller-Hartmann W, et al. Increased choline levels coincide with enhanced proliferative activity of human neuroepithelial brain tumors. NMR Biomed. 2002;15(6):385–392. doi: 10.1002/nbm.793. [DOI] [PubMed] [Google Scholar]
- 92.Alexander A, Murtha A, Abdulkarim B, et al. Prognostic significance of serial magnetic resonance spectroscopies over the course of radiation therapy for patients with malignant glioma. Clin Invest Med. 2006;29(5):301–311. [PubMed] [Google Scholar]
- 93.Weybright P, Sundgren PC, Maly P, et al. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. Am J Roentgenol. 2005;185(6):1471–1476. doi: 10.2214/AJR.04.0933. [DOI] [PubMed] [Google Scholar]
- 94.Elias AE, Carlos RC, Smith EA, et al. MR spectroscopy using normalized and non-normalized metabolite ratios for differentiating recurrent brain tumor from radiation injury. Acad Radiol. 2011;18(9):1101–1108. doi: 10.1016/j.acra.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Schwartz RB, Carvalho PA, Alexander E, 3rd, Loeffler JS, Folkerth R, Holman BL. Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. Am J Neuroradiol. 1991;12(6):1187–1192. [PMC free article] [PubMed] [Google Scholar]
- 96.Schwartz RB, Holman BL, Polak JF, et al. Dual-isotope single-photon emission computerized tomography scanning in patients with glioblastoma multiforme: association with patient survival and histopathological characteristics of tumor after high-dose radiotherapy. J Neurosurg. 1998;89(1):60–68. doi: 10.3171/jns.1998.89.1.0060. [DOI] [PubMed] [Google Scholar]
- 97.Carvalho PA, Schwartz RB, Alexander E, 3rd, et al. Detection of recurrent gliomas with quantitative thallium-201/technetium-99m HMPAO single-photon emission computerized tomography. J Neurosurg. 1992;77(4):565–570. doi: 10.3171/jns.1992.77.4.0565. [DOI] [PubMed] [Google Scholar]
- 98.Horky LL, Treves ST. PET and SPECT in brain tumors and epilepsy. Neurosurg Clin N Am. 2011;22(2):169–184. viii. doi: 10.1016/j.nec.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Chen W. Clinical applications of PET in brain tumors. J Nucl Med. 2007;48(9):1468–1481. doi: 10.2967/jnumed.106.037689. [DOI] [PubMed] [Google Scholar]
- 100.Padma MV, Said S, Jacobs M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003;64(3):227–237. doi: 10.1023/a:1025665820001. [DOI] [PubMed] [Google Scholar]
- 101.Hustinx R, Pourdehnad M, Kaschten B, Alavi A. PET imaging for differentiating recurrent brain tumor from radiation necrosis. Radiol Clin North Am. 2005;43(1):35–47. doi: 10.1016/j.rcl.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med. 2000;41(11):1861–1867. [PubMed] [Google Scholar]
- 103.Spence AM, Muzi M, Mankoff DA, et al. 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med. 2004;45(10):1653–1659. [PubMed] [Google Scholar]
- 104.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46(6):945–952. [PubMed] [Google Scholar]
- 105.Muzi M, Spence AM, O’Sullivan F, et al. Kinetic analysis of 3′-deoxy-3′-18F-fluorothymidine in patients with gliomas. J Nucl Med. 2006;47(10):1612–1621. [PubMed] [Google Scholar]
- 106.Rachinger W, Goetz C, Popperl G, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57(3):505–511. doi: 10.1227/01.neu.0000171642.49553.b0. [DOI] [PubMed] [Google Scholar]
- 107.Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–911. [PubMed] [Google Scholar]


