Abstract
The endocrine system mediates long-range peptide hormone signaling to broadcast changes in metabolic status to distant target tissues via the circulatory system. In many animals, the diffuse endocrine system of the gut is the largest endocrine tissue, with the full spectrum of endocrine cell subtypes not yet fully characterized. Here, we combine molecular mapping, lineage tracing and genetic analysis in the adult fruit fly to gain new insight into the cellular and molecular mechanisms governing enteroendocrine cell diversity. Neuropeptide hormone distribution was used as a basis to generate a high-resolution cellular map of the diffuse endocrine system. Our studies show that cell diversity is seen at two distinct levels: regional and local. We find that class I and class II enteroendocrine cells can be distinguished locally by combinatorial expression of secreted neuropeptide hormones. Cell lineage tracing studies demonstrate that class I and class II cells arise from a common stem cell lineage and that peptide profiles are a stable feature of enteroendocrine cell identity during homeostasis and following challenge with the enteric pathogen Pseudomonas entomophila. Genetic analysis shows that Notch signaling controls the establishment of class II cells in the lineage, but is insufficient to reprogram extant class I cells into class II enteroendocrine cells. Thus, one mechanism by which secretory cell diversity is achieved in the diffuse endocrine system is through cell-cell signaling interactions within individual adult stem cell lineages.
Keywords: Drosophila, Diffuse endocrine system, Neuropeptide, Stem cell, Notch
Summary: Molecular mapping, lineage tracing and genetic analyses provide insight into the mechanisms governing enteroendocrine cell diversity in the adult Drosphila gastrointestinal tract.
INTRODUCTION
On 16 January 1902, Ernest Starling observed a striking increase in pancreatic secretion when he prepared lysate from a segment of freshly excised intestinal mucosa rubbed with sand and weak HCl, filtered it through cotton wool and injected the extract into the jugular vein of an anesthetized dog (Bayliss and Starling, 1902). Documentation of this novel ‘reflex’ marked the inception of endocrinology as a field and implicated the intestinal epithelium as a potent source of peptides capable of acting over a great distance via the circulatory system to profoundly alter physiology. We now know that the gut epithelium is a rich source of secreted hormones that originate from specialized enteroendocrine cells and comprise a diffuse endocrine system distinct from the well-studied glandular endocrine system (Engelstoft et al., 2013; Schonhoff et al., 2004). Gut endocrine cells are embedded in the barrier epithelium and mediate luminal chemosensitivity (Furness et al., 2013; Reimann et al., 2012). In response to environmental stimuli, peptides secreted from the gut epithelium act broadly on endocrine, nervous and immune systems to encode adaptive physiologic responses. Such peptides (also called neuropeptides) belong to distinct families, such as allatostatins, diuretic hormones and tachykinins (Bendena et al., 1999; Gäde, 2004; Nässel and Wegener, 2011; Van Loy et al., 2010). Knowledge differs widely with respect to each peptide family member and their experimentally defined roles within a given species. However, neuropeptide hormones have been implicated in processes such as growth control, muscle activity, feeding behavior, osmotic balance, reproduction and epithelial secretion. The prodigious number of enteroendocrine cells and their varied neuropeptide expression combine to make the gut the largest endocrine organ in the body. And yet, the mechanisms underlying endocrine diversity in this central endocrine tissue have not been fully understood.
The invertebrate gastrointestinal (GI) tract, like its mammalian counterpart, contains a diffuse endocrine system providing a tractable experimental model to investigate the basis of enteroendocrine diversity. This population of cells can be easily detected along the entire anterior-posterior (A/P) axis of the adult midgut with the pan-enteroendocrine marker Prospero (Pros). Adult enteroendocrine cells are continuously maintained by stem cell divisions that occur in physiologically and functionally distinct regions of the midgut (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Strand and Micchelli, 2011). Genomic analysis at the organ level has shown that a wide variety of neuropeptides and their cognate receptors, many of which are conserved, are expressed both within the gut and in the periphery (Marianes and Spradling, 2013; Buchon et al., 2013; Veenstra et al., 2008; Veenstra, 2009; Veenstra and Ida, 2014). It is also now possible to specifically isolate enteroendocrine cells and to obtain expression profiles at the cellular level (Dutta et al., 2013). Moreover, experimental means to challenge the barrier epithelium with pathogenic or nutritional insults are now available and combine readily with existing molecular genetic tools (Vodovar et al., 2005; Lee and Micchelli, 2013; Piper et al., 2014). Thus, the GI tract of Drosophila provides an unsurpassed experimental model to investigate aspects of diffuse endocrine system biology.
Studies of adult Drosophila enteroendocrine cells have focused on the molecular events that determine whether a newly formed intestinal progenitor, called enteroblast, will adopt the secretory (endocrine) or absorptive (enterocyte) cell fate (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). The idea that Notch signaling might control this decision was first suggested following the observation that high levels of a transcriptional reporter for Notch could be detected in the enteroblast (Micchelli and Perrimon, 2006). Subsequent functional studies showed that the Notch ligand Delta can be detected at high levels in the neighboring intestinal stem cell and that Delta is both necessary and sufficient to distinguish the endocrine and enterocyte fate (Beebe et al., 2010; Ohlstein and Spradling, 2007). Together, these observations led to a simple model in which stem cells signal to enteroblasts to influence cell fate: low levels of Notch transduction in the enteroblast promote the enteroendocrine differentiation and high levels of Notch transduction in the enteroblast promote the enterocyte differentiation. This asymmetry between stem cell and daughter cell is reinforced by segregation of Sara endosomes into the newly formed enteroblast (Montagne and Gonzalez-Gaitan, 2014). However, precisely when the specifying Notch signaling event occurs remains an open question (Perdigoto et al., 2011). Transcription factors of the Enhancer of split and achaete-scute complexes have both been shown to control endocrine differentiation (Bardin et al., 2010). More recently, Slit/Robo signaling has been implicated in enteroendocrine specification (Biteau and Jasper, 2014). Possible interactions between Notch and Robo signaling pathways have not yet been fully explored.
The goal of the current study is to characterize the extent of cellular diversity in the diffuse endocrine system of adult Drosophila and to gain insight into the molecular mechanisms underlying this process. Here, we focus on the posterior midgut and use differential neuropeptide expression as a criterion to map enteroendocrine cell types in situ with great spatial resolution. We show that even immediately adjacent endocrine cells along the midgut express different combinations of neuropeptides, dividing the diffuse endocrine system into class I and class II endocrine cells. Directed cell lineage-tracing studies show that, once an enteroendocrine cell subtype is established, it is stably maintained in the adult, even following environmental challenge. Thus, diversity of endocrine cells is seen at two levels: regional and local. We demonstrate that individual gut stem cells generate pairs of class I and class II enteroendocrine cells displaying distinct neuropeptide profiles. Moreover, the precise combination of neuropeptide expression depends on stem cell position within the midgut. Finally, our analysis shows that Notch signaling is initially required to generate class II enteroendocrine cell diversity in posterior midgut stem cell lineages, but is dispensable for both underlying regional identity and neuropeptide expression in extant endocrine cells. We conclude that local endocrine diversity is generated in the diffuse endocrine system during adult homeostasis through cell-cell signaling interactions within individual stem cell lineages.
RESULTS
Neuropeptide expression distinguishes adult enteroendocrine cells in the posterior midgut
The adult Drosophila midgut epithelium is a rich source of secretory neuropeptide hormones, showing elevated levels of Allatostatin A (AstA), Allatostatin B (AstB), Allatostatin C (AstC), Neuropeptide F (Npf), Diuretic hormone 31 (DH31) and Tachykinin (Tk) (Veenstra et al., 2008; Reiher et al., 2011; Veenstra and Ida, 2014). Neuropeptide expression subdivides the gut into broad regions along the A/P axis, which are evident with regards to both anatomical and molecular markers (supplementary material Fig. S1A-N; Table S1) (Veenstra et al., 2008; Veenstra and Ida, 2014). For example, the posterior midgut is divided into two primary neuropeptide-expressing regions that we refer to as region 1 and region 2, respectively (Fig. 1A; supplementary material Fig. S1A,B, Fig. S2A-F; Table S1). A panel of transgenic molecular markers have been used to subdivide the posterior midgut into regions (Marianes and Spradling, 2013; Buchon et al., 2013). However, careful comparison of relevant marker strains with neuropeptide antisera revealed segmental boundaries that were distinct (supplementary material Fig. S2A-F). Therefore, for the purpose of this study, which focuses primarily on the peptide-rich posterior midgut, we use the ‘region 1’ and ‘region 2’ designations to refer directly to native domains of peptide expression in the adult midgut.
Fig. 1.
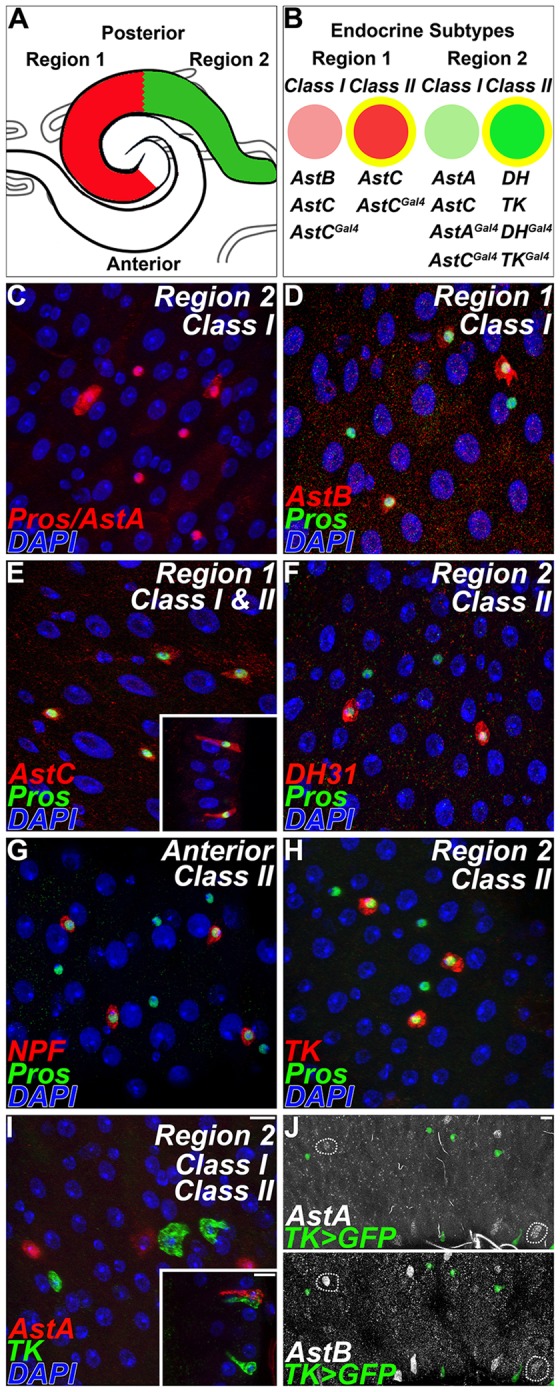
Diversity of adult enteroendocrine cells is both regional and local. (A) Regional differences in peptide hormone expression distinguish segments of the adult posterior midgut (shading). (B) Within each region, local differences in peptide hormones are detected among endocrine cell pairs. Class I and II enteroendocrine cells are distinguished by peptide antisera and Gal4 lines. Yellow indicates Notch-dependent cell fates. DH, DH31. (C-H) Peptides are differentially detected in Pros+ cell pairs. (C) Anti-AstA, red; anti-Pros, red. (D) Anti-AstB, red; anti-Pros, green. (E) Anti-AstC, red; anti-Pros, green. (F) Anti-DH31, red; anti-Pros, green. (G) Anti-Npf, red; anti-Pros, green. (H) Anti-Tk, red; anti-Pros, green. (I) Pairs of endocrine cells express different peptides. Anti-AstA, red; anti-Tk, green. (J) At the transition between region 1 and 2, AstA increases posteriorly, whereas AstB decreases posteriorly. Anti-AstA and anti-AstB, white; Tk>GFP, green. Scale bars: 10 µm.
Adult stem cells maintain the differentiated epithelial lining of the midgut (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). This monolayer consists primarily of large absorptive enterocyes, but also includes ∼1000 (∼10%) secretory enteroendocrine cells that can be identified by the pan-endocrine marker Pros. However, it has been unclear whether neuropeptide expression in the midgut is restricted only to endocrine cells, or the extent to which individual cells might express specific combinations of particular neuropeptides. To determine whether secretory neuropeptides are expressed in Pros+ cells, we conducted a series of double-labeling experiments on five-day-old adult midguts. Our studies show that distinct subsets of Pros+ cells also stain positive for AstA, AstB, AstC, DH31, Npf and Tk when assayed in a pairwise manner (Fig. 1C-H). Initial characterization indicated that, with the exception of AstC in region 1, only one of the two adjacent Pros+ cells stained positive for the peptide assayed. This impression was confirmed by scoring the percentage of Pros+ cells within a given region that also expressed a particular neuropeptide (Table 1). Values ranged from 48 to 52%, or approximately half of the Pros+ cells. Neuropeptide expression was specific to endocrine cells, with no obvious expression detected above background levels elsewhere in the gut epithelium. These results were independently confirmed using specific neuropeptide Gal4 driver lines, which we found to be expressed in the adult midgut (Materials and Methods; supplementary material Fig. S1F,J,N and Fig. S3; Table S1). Thus, neuropeptide expression in the gut is limited to endocrine cells, and in all but one case, labels half of the Pros+ cells in a particular midgut region.
Table 1.
Distribution of neuropeptides in Prospero+ cells
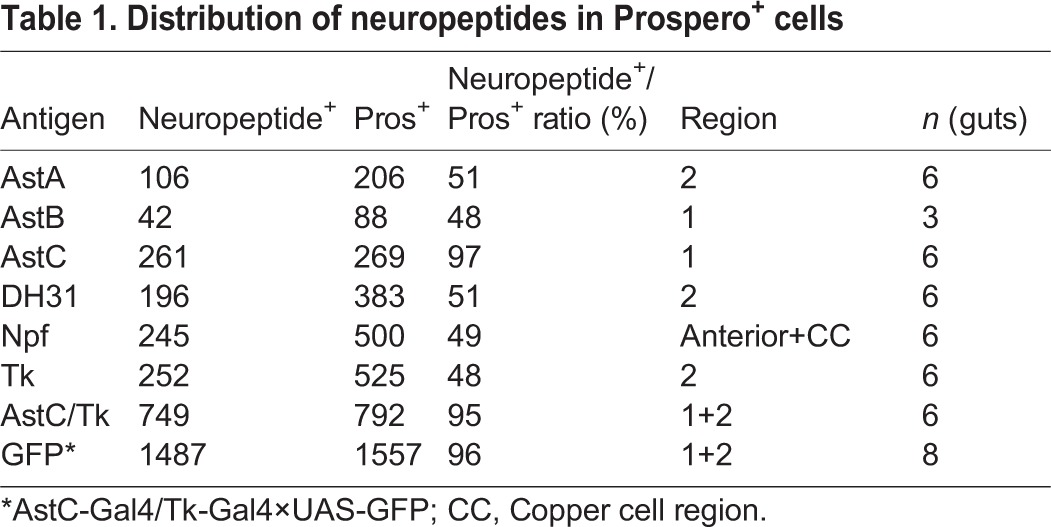
Adjacent Pros+ enteroendocrine cells can be unambiguously distinguished by unique neuropeptide expression profiles (Fig. 1B,I; supplementary material Fig. S1A-N and Fig. S3A-N; Table S1). A comprehensive series of double- and triple-staining studies, employing both antibodies and Gal4 lines, was performed. This analysis showed that in region 1, for example, class I enteroendocrine cells are AstB+, AstC+, whereas class II enteroendocrine cells are AstC+ (supplementary material Fig. S3E,F). In region 2, class I enteroendocrine cells are AstA+, AstC+ (supplementary material Fig. S3C,D,G,H), whereas class II enteroendocrine cells are DH31+, Tk+ (supplementary material Fig. S3I,J,M,N). To determine which fraction of Pros+ cells express either class I or class II neuropeptide markers we simultaneously labeled AstC+, Tk+ and Pros+ cells (Table 1). Using combinations of either antisera or Gal4 lines revealed that the vast majority (>95%) of Pros+ cells also express neuropeptides. A transition zone between region 1 and region 2 was identified, where neuropeptide protein is detected in a graded manner within class I endocrine cells (Fig. 1J; supplementary material Table S1). We note that pairs of class I and class II enteroendocrine cells were also detected in the anterior and middle regions of the adult midgut (Fig. 1G; supplementary material Fig. S1; Table S1). Thus, precise combinations of secretory neuropeptides can be used to distinguish each cell within an enteroendocrine pair 5 days after eclosion.
Neuropeptide expression is a fixed characteristic of enteroendocrine cell subtypes
Neuropeptides actively modulate organismal physiology. It is therefore possible that established cellular profiles of peptide expression associated with a class of enteroendocrine cells can change during adulthood. Alternatively, peptide expression might be a fixed characteristic of endocrine cell subtypes. To test these possibilities, we conducted directed cell lineage-tracing experiments using specific neuropeptide Gal4 driver lines (Fig. 2). Class I enteroendocrine cells in region 2 of the posterior midgut were genetically labeled by crossing the AstA-Gal4 to the actin5C FRT stop FRT lacZnuc; UAS-flp lineage tracer (AstA>Trace; Fig. 2A,C,E,G). The AstA-Gal4 driver line was first detected in the adult midgut 4 days following eclosion (Fig. 2A,C). Ten days following eclosion, midguts were stained to identify DH31+ cells, a marker of class II enteroendocrine cells in region 2 of the posterior midgut. We observed that cells labeled by AstA-Gal4 were DH31−, although DH31 staining was readily detected in adjacent endocrine cells (Fig. 2E). In a complementary experiment, we labeled class II enteroendocrine cells in region 2 using Tk-Gal4 (Fig. 2B,D,F,H). The Tk-Gal4 driver line was also first detected in the adult midgut 4 days following eclosion (Fig. 2B,D). Ten days following eclosion, we stained for AstA+, a neuropeptide that marks class I enteroendocrine cells in region 2. We observed that cells labeled by Tk-Gal4 were AstA−, although AstA+ cells were readily detected in adjacent endocrine cells (Fig. 2F). To further document these observations we counted the number of lacZ+, DH31+ and lacZ+, AstA+ cells present in adult midguts at 0, 4 and 10 days after eclosion, and again 24 h following challenge with the Gram-negative enteric pathogen Pseudomonas entomophila (Pe) (Fig. 2G,H; Table 2). This analysis shows that AstA-Gal4 and Tk-Gal4 driver lines mark endocrine cell subtypes that remain DH31− or AstA− during adult homeostasis, as well as following pathogenic challenge. Taken together, these experiments suggest that the class I and class II neuropeptide profiles remain stable in enteroendocrine cells during adult gastrointestinal homeostasis as well as following pathogenic challenge. Thus, neuropeptide expression provides a reliable means of distinguishing distinct enteroendocrine cell subtypes in the adult midgut.
Fig. 2.
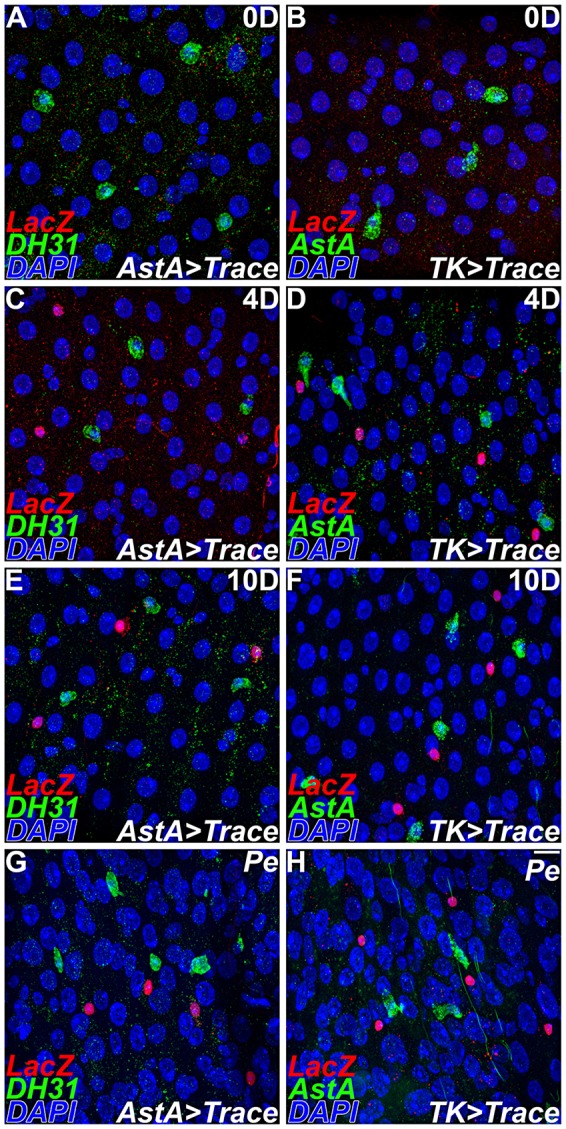
Peptide expression stably distinguishes class I and II enteroendocrine cells. (A-H) Directed lineage tracing of class I and II enteroendocrine cells using actin5C>lacZ; UAS-flp. Anti-β-Gal, red; anti-AstA/anti-DH31, green. (A,C,E,G) AstA-Gal4. (B,D,F,H) Tk-Gal4. (A,B) Gal4 lines are not detected in newly eclosed adult midguts. (C,D) 4 days following eclosion Gal4 drivers mark endocrine subtypes. Marked cells are distinguished from their neighbors with neuropeptide antisera. (E,F) 10 days following eclosion. (G,H) Challenge with Pe. Scale bar: 10 µm.
Table 2.
Directed cell lineage tracing of enteroendocrine cell subtypes
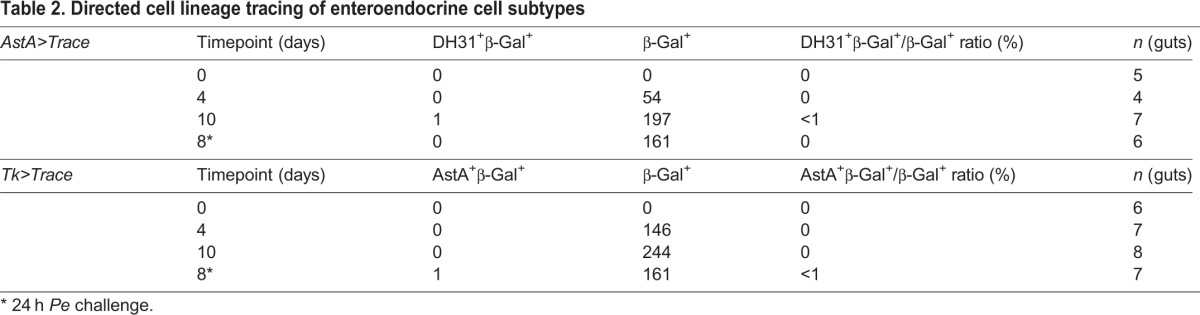
Class I and class II enteroendocrine cells are lineally related
The presence of enteroendocrine cells with distinct neuropeptide expression profiles raised the question whether such cells might arise from a common cell lineage or whether they are lineally distinct (Fig. 3A). To distinguish these possibilities, we conducted cell lineage-tracing studies. Wild-type lineages were labeled using the MARCM system (Lee and Luo, 1999) and stained to identify enteroendocrine subtypes. We focused first on region 2 of the posterior midgut, where simultaneous detection of class I and II enteroendocrine cells was possible. Here, marked lineages were found to span both class I and II endocrine cell subtypes (Fig. 3B-E). To further characterize the distribution of endocrine cell subtypes, the number of AstA+ and Tk+ cells per clone was scored for each marked lineage recovered (Table 3). The total number of AstA+ and Tk+ cells per clone ranged from 0 to 4. In every case in which a marked lineage contained two or more endocrine cells, we scored the presence of both AstA+ and Tk+ enteroendocrine cells. We conclude that in region 2 of the posterior midgut, class I and II enteroendocrine cell pairs arise from a common cell lineage.
Fig. 3.
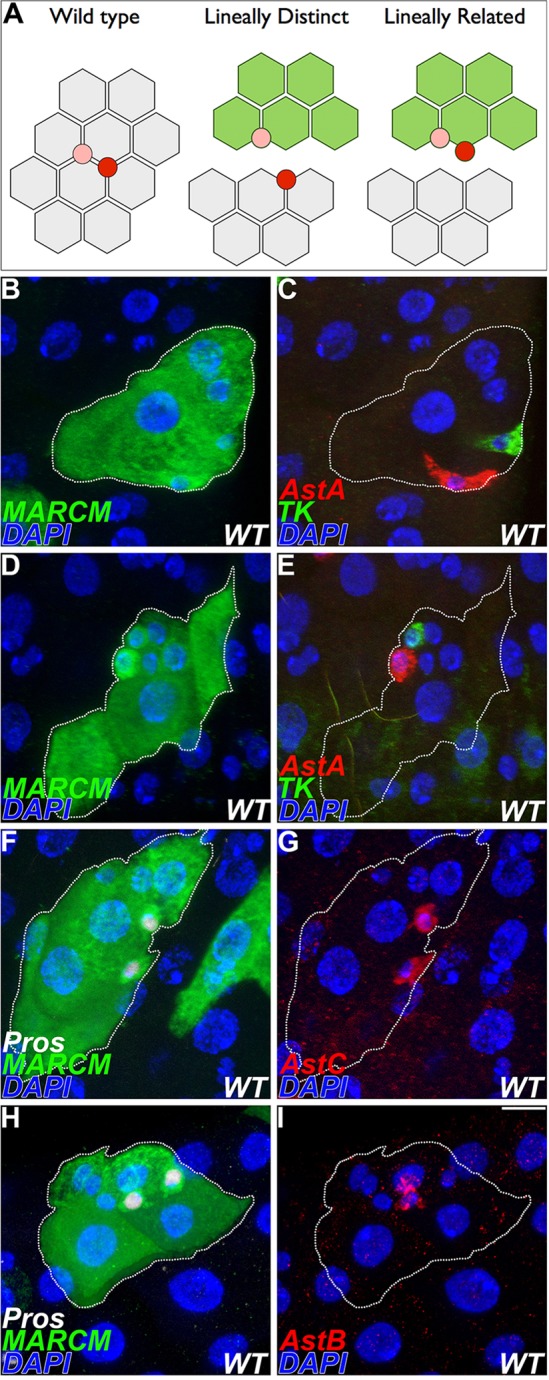
Class I and II enteroendocrine cells arise from a common lineage. (A) Wild-type class I (pink) and class II (red) endocrine cells are present in pairs distributed among enterocytes (gray). Alternate lineage models are distinguished by cell-labeling studies (green). (B-I) Wild-type MARCM lineages 5 days after induction, dashed line. (B-E) Lineages spanning class I and II entoendocrine cells, region 2. Anti-AstA, red; anti-Tk, green. Distinct endocrine subtypes arise from a common lineage. (F-I) Marked lineages, region 1. Anti-Pros, white. (G) Anti-AstC, red. (I) Anti-AstB, red. Scale bar: 10 µm.
Table 3.
Analysis of neuropeptides in region 2 cell lineages
Characterization of wild-type lineages was also performed in region 1 of the posterior midgut, yielding similar results. However, analysis of the region 1 was limited by the lack of markers to simultaneously distinguish class I and class II enteroendocrine cells (e.g. Fig. 1B and Fig. 3F,G; supplementary material Table S1). Thus, we scored the fraction of Pros+ cells that were also AstB+ (Fig. 3H,I; Table 4). We predicted that half of the Pros+ enteroendocrine cells present in each clone would be AstB+. We found that marked lineages recovered in region 1 contained between 0 and 3 Pros+ cells. However, among the clones that contained two Pros+ cells, 48% fit this criterion (Table 4). In summary, nearly half of the wild-type clones observed in region 1 were consistent with a model in which class I and II cells arise from a common lineage, as shown for region 2. It is likely that the method of clone scoring used for analysis in region 1 enriched for a set of early clones containing less differentiated cells (i.e. Pros+ only). By contrast, the analysis in region 2 was biased toward late clones identified by the presence of highly differentiated endocrine cells (i.e. AstA+, Tk+). Taken together, analysis of wild-type cell lineages supports a general model in which class I and class II endocrine cell pairs of the posterior midgut arise from a common stem cell lineage.
Table 4.
Analysis of neuropeptides in region 1 cell lineages
Class II enteroendocrine cells arise from a Su(H)-Gal4 expressing progenitor
The choice of cell fate between absorptive enterocytes and secretory endocrine cells in the midgut is controlled by the Notch signaling pathway (Bardin et al., 2010; Beebe et al., 2010; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007; Perdigoto et al., 2011). Activation of Notch signaling in stem cell daughters, called enteroblasts, promotes enterocyte formation, whereas reduced Notch signaling leads to the production of ectopic enteroendocrine cells. This model predicts that directed cell lineage-tracing of progenitors transducing high levels of Notch signaling should label only enterocytes. We tested this prediction using a pulse/chase experiment and the conditional Su(H)-Gal4, tub-Gal80TS strain to label a fraction of adult cell lineages with actin5C FRT stop FRT lacZnuc; UAS-flp under both baseline conditions and following environmental challenge (Table 5; Fig. 4A-D). As predicted, enterocytes were readily labeled by this procedure when cell marking was initiated, following a shift to the non-permissive temperature (Fig. 4A-D). However, quite unexpectedly, we also recovered labeled Tk+ class II endocrine cells at a frequency of 0.65% under baseline conditions (Table 5; Fig. 4A). Similarly, we observed labeled DH31+ cells, a second marker correlated with class II endocrine fate, following environmental challenge (Table 5; Fig. 4C). By contrast, Tk+ class II cells were never labeled in unshifted control experiments; nor were AstA+ class I cells labeled at the non-permissive experimental temperature (Table 5). This suggested that Notch signaling might be active specifically in the class II enteroendocrine cell lineage. Consistently, Notch pathway reporters were also detectable in Tk+ cells (Fig. 4E-H). From this, we conclude that DH31+ and Tk+ class II enteroendocrine cells arise from a Su(H)-Gal4-expressing progenitor. These findings prompted a series of experiments to directly test the function of Notch signaling in the generation of regional diversity among midgut endocrine cell subtypes.
Table 5.
Analysis of Su(H)>Trace
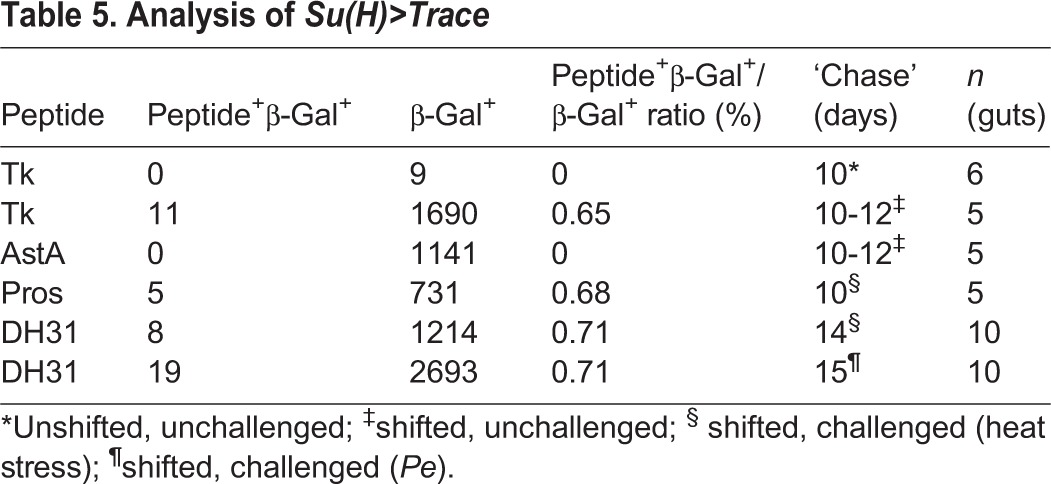
Fig. 4.
Class II endocrine cells arise from a Su(H)-Gal4 progenitor. (A-D) Conditional lineage tracing of Su(H)-Gal4TS cells using actin5C>lacZ; UAS-flp, 12 days after induction. (A,B) Cellular products of Su(H)-Gal4TS labeling, red/white; anti-Tk, green. Arrows indicate Tk+ cells derived from Su(H)-Gal4TS cells. Asterisks show unlabeled Tk+ cells. (B) Single channel. (C,D) Cellular products of Su(H)-Gal4TS, 7 days following exposure to Pe, red/white; anti-DH31, green. Arrows indicate DH31+ cells derived from Su(H)-Gal4TS cells. Asterisks show unlabeled DH31+ cells. (D) Single channel. (E-H) Markers of Notch signaling in Tk+ cells, red. Arrows indicate Tk+ cells. Asterisks show unlabeled Tk+ cells. (E,F) Su(H)GBE-lacZ reporter. Note lower levels of the reporter in the Tk+ cells. (E) esg>GFP, green; anti-Tk, red. (F) Su(H)GBE-lacZ, white. (G,H) Dl-lacZ labeling, red/white; anti-Tk, green. (H) Single channel. Scale bars: 10 µm.
Class II enteroendocrine cells are established by Notch/Delta signaling
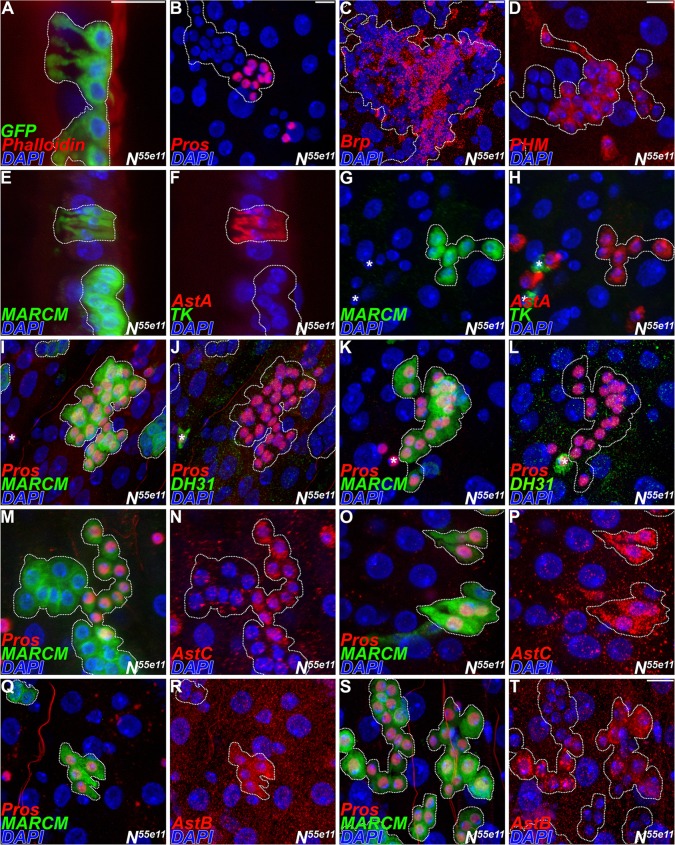
Notch loss in the intestinal stem cell lineage leads to the production of ectopic Pros+ cells, suggesting the formation of enteroendocrine tumors (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Yet, the full extent of endocrine cell differentiation in these tumors is unclear. Therefore, we first confirmed and extended previous studies by generating null Notch clones and monitoring the endocrine fate using an expanded panel of endocrine cell markers, including the pan-endocrine marker Pros; the ELKS family protein Bruchpilot (Brp), which supports calcium-dependent secretory vesicle release; and cell morphology. In addition, we examined the levels of Peptidylglycine α-hydroxylating monoxygenase (Phm). Phm is an enzyme that catalyzes the rate-limiting step in C-terminal α-amidation, a necessary step for the production of active neurosecretory peptides from pro-forms of the peptides. We found that Notch loss led to ectopic cells displaying the characteristic morphology of mature enteroendocrine cells with polarized cellular processes projecting toward the gut lumen (Fig. 5A). In addition, these cells were Pros+, Brp+ and Phm+ (Fig. 5B-D). Thus, we conclude that the ectopic cells that arise in Notch mutant lineages exhibit multiple features of well-differentiated endocrine tumors.
Fig. 5.
Notch is necessary to establish class II endocrine cells. (A-T) Mosaic analysis of Notch using MARCM; representative clones are highlighted by dotted lines. (A-D) General endocrine markers, 7 days after induction. (A) Anti-GFP, green; phalloidin, red, staining F-actin (muscle). (B) Anti-Pros, red. (C) Anti-Brp, red. (D) Anti-Phm, red. (E-T) Distribution of neuropeptides in Notch clones, 5 days after induction. Clones on left in paired micrographs, green. Asterisks, internal controls. (E-L) Region 2. (F,H) Anti-AstA, red; anti-Tk, green. Endocrine cell morphology is evident in cross-section. (I-M,O,Q,S) Anti-Pros, red. (J,L) Anti-DH31, green. Note the absence of DH31+ cells within the clone. (M-T) Region 1. (N,P) Anti-AstC, red. (R,T) Anti-AstB, red. Scale bars: 10 µm.
By contrast, Notch loss was found to differentially affect the distribution of specific secretory neuropeptides. We examined distinguishing markers for both class I and class II enteroendocrine cells in region 2 of the posterior midgut (Fig. 5E-T). Notch clones in region 2 led to loss of both DH31+ and Tk+, whereas AstA+ cells remained present in the lineage (Fig. 5E-L). We quantified this phenotype and found that, in every case in which two or more endocrine cells were present, clones retained AstA+ cells but lost Tk+ cells (Table 3). Similar results were observed using additional class I and class II markers in the anterior midgut, copper cell region and region 1 of the posterior midgut (Fig. 5M-T; supplementary material Fig. S4; Table S4). Importantly, these findings were independently confirmed using conditional Notch knockdown (supplementary material Fig. S5A-J). We note that, whereas Notch loss affected local neuropeptide profiles among endocrine pairs, it was not associated with changes in underlying regional identity in neuropeptide expression (supplementary material Fig. S5K). Thus, Notch signaling is necessary to specify the class II endocrine cell subtype in the adult midgut, without affecting neuropeptide processing enzymes or the cellular machinery implicated in secretory vesicle release.
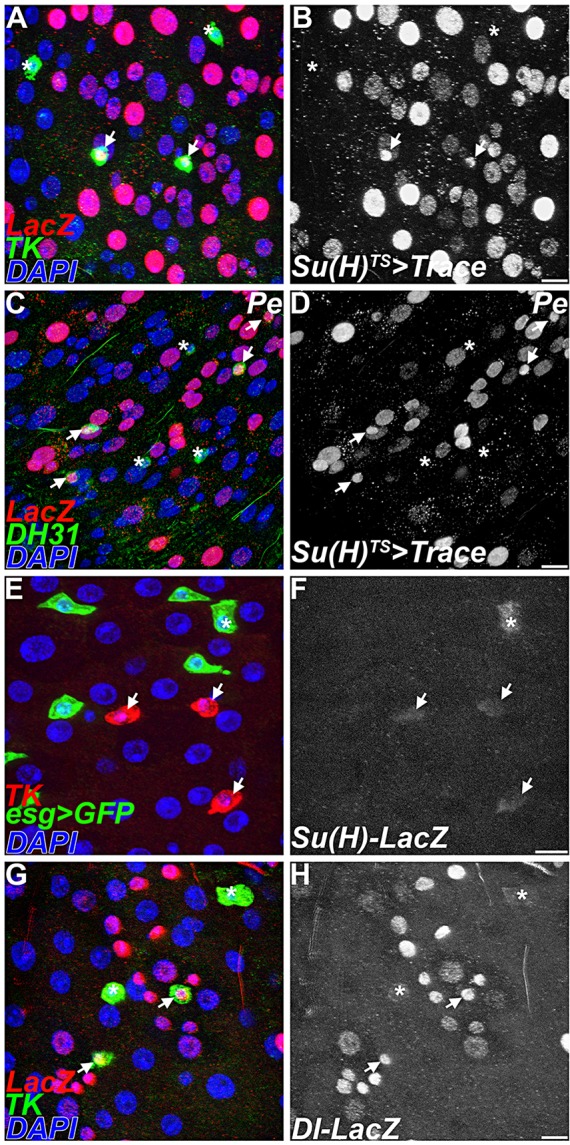
The Notch pathway can be activated by Delta and Serrate ligands. As in the case of wild type, marked lineages lacking Serrate produced both AstA+ and Tk+ endocrine cells (Fig. 6A,B). However, loss of Delta was necessary for the production of Tk+ enteroendocrine cells (Fig. 6C-F). We note that Delta clones exhibited greater heterogeneity in the progenitor marker esg-lacZ than Notch, which correlated with heterogeneity of the neuropeptide phenotype (Fig. 6G,H; supplementary material Fig. S6). Thus, Delta, but not Serrate, is necessary for establishing class II enteroendocrine cells during adult midgut homeostasis.
Fig. 6.
Delta is necessary to establish class II endocrine cells. (A-H) Analysis of Delta and Serrate lineages using MARCM, dotted line. (A,B) Serrate clone, 5 days after induction. (B) Anti-AstA, red; anti-Tk, green. (C,D) Delta clone, 5 days after induction. (D) Anti-AstA, red; anti-Tk, green. Asterisk shows Tk+ cell outside clone. (E,F) Delta clone, 10 days after induction. (F) Anti-AstA, red. (G,H) Delta clone, 10 days after induction. (H) Anti-AstA, red; esg-lacZ, white. Scale bars: 10 µm.
Notch function is dispensable for endocrine cell maintenance
Notch might function only in the establishment of endocrine diversity. Alternatively, Notch might function in both the establishment and maintenance of endocrine diversity. To distinguish these possibilities, we pursued a conditional, cell type-specific genetic loss-of-function approach. Conditional Notch knockdown was achieved in extant class II enteroendocrine cells using the UAS-GFP, tub-Gal80TS; Tk-Gal4 strain. However, despite strong Notch knockdown with this construct (e.g. supplementary material Fig. S5), no change in either DH31+ or AstA+ cells was detected (supplementary material Fig. S7A-D). This suggests that Notch signaling is dispensable for maintenance of neuropeptide expression in enteroendocrine cells.
Notch activation is sufficient to establish class II enteroendocrine cells
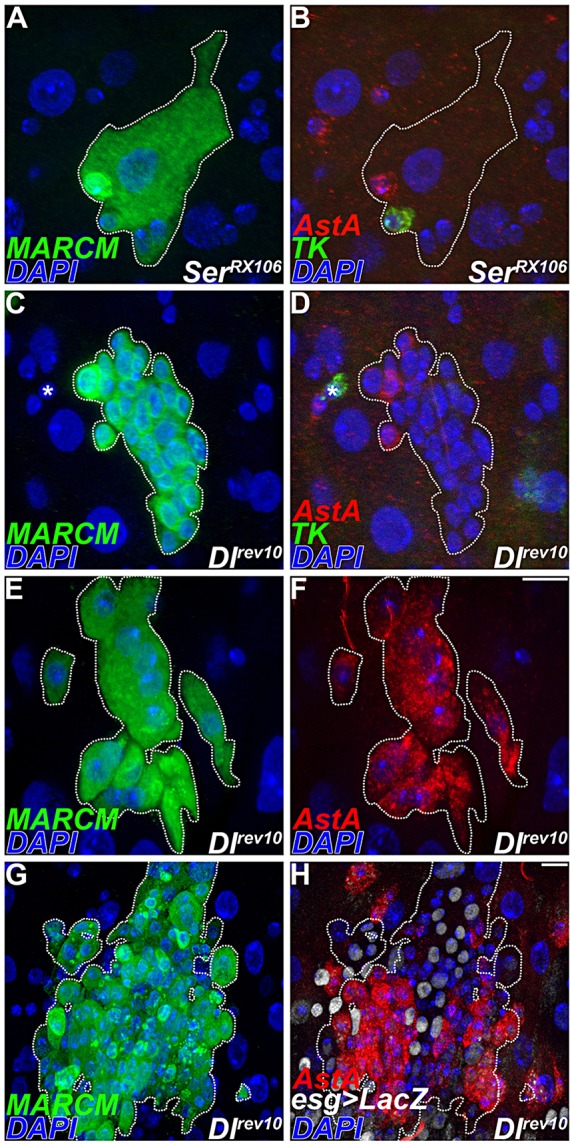
We tested whether Notch signaling is also sufficient to establish local differences in endocrine cell subtype. In a first test, we examined the consequences of global Notch activation on endocrine cell identity by monitoring DH31+ cells at 6 and 24 h following induction of an hs-Notchintra transgene (supplementary material Fig. S8). To distinguish newly formed cells from previously differentiated cells, we performed the experiment in an esg>GFP background. As previously reported, we readily observed that Notch pathway activation promoted differentiation of esg>GFP+ into large enterocytes 24 h following induction (supplementary material Fig. S8A,C). We also observed a low frequency of DH31+ doublets under these conditions, which are not typically seen in wild-type cell lineages (supplementary material Fig. S8C,D; Table 3). This observation is consistent with the idea that Notch activation is sufficient to promote class II endocrine cell fate. The low frequency phenotype is predicted because endocrine progenitors are an order of magnitude rarer than epithelial progenitors and because, under baseline conditions, proliferation of all progenitors is infrequent. We reasoned that, if progenitor pools are first increased, using genetic means, and then Notch is ectopically activated, the phenotype should be detected with greater frequency. Therefore, in a second test of Notch sufficiency, conditional Presenillin knockdown with esg-Gal4; UAS-GFP, tub-Gal80TS was followed by induction of the hs-Notchintra transgene. Under these conditions we observed a rapid, robust and significant increase in the number of Pros+, DH31+ cells (Fig. 7A-F). Finally, we tested whether Notch activation is sufficient to reprogram fully differentiated enteroendocrine cell fate from class I to class II. However, conditional activation of Notchintra in AstA+ cells was insufficient to induce DH31 peptide in these cells (Fig. 7G,H). Altogether, these results suggest that Notch signaling is both necessary and sufficient to specify the class II endocrine cell subtype during adult midgut homeostasis. However, once a class I endocrine cell is established, ectopic Notch activation is insufficient to reprogram subtype identity.
Fig. 7.
Notch activation is sufficient to establish class II enteroendocrine cells. (A-F) Analysis of Notch pathway activation on endocrine cell fate, 8 days following conditional Presenilin knockdown using esgTS. Adults were shifted to the non-permissive temperature for 7 days to induce Presenilin knockdown and then stained against DH31+ cells 24 h later, in the presence or absence of global Notch pathway activation. (A,C,E) Anti-Pros, red. (A-D) Anti-DH31, green. (A,B) Presenilin knockdown leads to endocrine hyperplasia and a reduction in DH31+ cells. (C,D) Presenilin knockdown leads to endocrine hyperplasia and a rapid increase in DH31+ cells following global Notch activation. Inset, DH31+ cells exhibit endocrine morphology. (E) Notch activation is sufficient to promote differentiation of esg+ cells to large enterocytes, green. (F) Quantitation. The average number of DH31+ cells per gut scored for each genotype (n=7-12 guts per genotype, error bars represent s.e.m.; ***P<0.0001). (G-H) Analysis of Notch pathway activation in fully differentiated class I endocrine cells using AstA-Gal4TS. Adults were shifted to the non-permissive temperature for 7 days. AstA>GFP, green; Anti-DH31, red. (G) Controls shifted to non-permissive temperature show conditional expression of GPF. (H) Notch overexpression in AstA+ cells does not cause expression of the class II marker DH31. (I) Schematic cell lineage diagram. Undifferentiated stem cell daughters (enteroblasts) give rise to either the secretory (enteroendocrine) or absorptive (enterocyte) cell fate. Branch points indicate Notch-dependent cell fate decisions. Progenitors, green; differentiated cells, pink, red and blue; Notch-dependent cell fates, yellow. ISC, intestinal stem cell; EB, enteroblast. Scale bars: 20 µm.
DISCUSSION
The barrier epithelium of the gut is a unique biological interface. It is here that a complex stream of continuously changing luminal stimuli are presented to the organism and must be converted into appropriate physiologic and neuromodulatory states. The cells of the diffuse endocrine system represent an early, if not the primary, level at which these changes are sensed and broadcast. We have used Drosophila as a model system to address the molecular mechanisms by which cellular diversity in the diffuse endocrine system is homeostatically maintained.
Organization of the adult enteroendocrine system
Endocrine cells can be characterized by the neuropeptides they secrete. From this perspective, the adult diffuse endocrine system exhibits both regional and local organization. We show that neuropeptide expression in the adult midgut is restricted to cells positive for Pros, a pan-endocrine marker defining the extent of the diffuse endocrine system. Among Pros+ cells, distinct segmental regions of neuropeptide expression along the A/P axis are evident. Within each region, local differences in combinatorial neuropeptide distribution distinguish class I and II endocrine cells. Our analysis indicates that >95% of Pros+ cells express one or more distinct neuropeptides. We predict that the remaining ∼5% of Pros+ cells correspond either to newly formed endocrine cells in the process of differentiating, dividing progenitors (see Biteau and Jasper, 2014; Zielke et al., 2014) or differentiated endocrine cells expressing peptides other than those tested.
The mapping data presented here confirm and extend a rapidly growing body of work, which has provided essential insight into the distribution of regulatory neuropeptides in Drosophila (Buchon et al., 2013; Chintapalli et al., 2007; Marianes and Spradling, 2013; Reiher et al., 2011; Veenstra et al., 2008; Veenstra and Ida, 2014). Overall, these reports are in general agreement, despite significant differences in approach, methodology and scope.
Our systematic, combinatorial mapping strategy provides high cellular and temporal resolution of neuropeptide distribution in vivo under baseline conditions. A pairwise comparison of available antisera with Gal4 driver lines suggests that patterns of neuropeptide expression and protein distribution are similar, if not identical, in the adult midgut. Importantly, these validated reagents provide powerful and specific new tools for manipulating the endocrine system, as we demonstrate. Finally, directed cell lineage analysis shows that neuropeptide profiles are stable features of endocrine cells during both normal homeostasis and following challenge with the enteric Gram-negative pathogen Pe.
In mammals, the classical view of the diffuse endocrine system is the ‘one cell-one hormone’ dogma (Engelstoft et al., 2013; Schonhoff et al., 2004). However, recent studies in mouse and human have challenged this perspective (Egerod et al., 2012; Habib et al., 2012; Sei et al., 2011). It is now clear that the mammalian GI tract exhibits not only regional diversity but also local diversity in the combinatorial expression of neuropeptides in single endocrine cells of the diffuse endocrine system. For example, in the murine intestine, endocrine cells in the crypt express multiple endocrine hormones, but endocrine cells on the villus do not (Sei et al., 2011). Interestingly, in mammals, peptides that are coexpressed also appear to have overlapping or coordinated functions. Thus, broad coexpression of secretory neuropeptides is a conserved feature of the diffuse endocrine system among metazoans.
Class I and class II endocrine subtypes arise from a common stem cell lineage
The diffuse endocrine system differs from the glandular endocrine system in that it must be continuously renewed. The combinatorial differences among endocrine neuropeptides described here provide an experimental basis to address how diversity is maintained in the diffuse endocrine system. We distinguish two possible models and directly demonstrate that endodermal stem cell lineages give rise to both class I and class II endocrine cells from a single lineage. Thus, local diversity within the diffuse endocrine system is controlled by individual stem cell lineages situated at distinct positions along the length of the GI tract. Developmental studies have characterized the stepwise process of endocrine cell development (Micchelli et al., 2011; Takashima et al., 2011). Cell divisions occurring during late pupal stages initially establish pairs of gut enteroendocrine cells. Moreover, recent studies have implicated the gut neurosecretory peptide Bursicon as a key peptide in the complex behavioral process of pupal eclosion (Scopelliti et al., 2014). The fact that cellular diversity in the diffuse endocrine system is actively maintained by adult stem cell lineages suggests that endocrine cells have broad functional significance in maintaining many aspects of metabolic homeostasis throughout the course of adulthood. These crucial functions remain to be fully characterized.
Controlling cell type in the endocrine lineage
Simultaneous detection of class I and II endocrine cells in marked endodermal lineages suggested that endocrine cell fate is controlled by signaling events within individual stem cell lineages. Su(H)-Gal4 progenitors were found to give rise to class II, but not class I, endocrine cells, under a wide variety of experimental conditions that included baseline homeostasis and different environmental challenges, suggesting a key role for the Notch signaling pathway in generating enteroendocrine diversity. We note that this finding differs significantly from another report, which concluded that endocrine cells do not arise from a Su(H)Gal4+ progenitor (Biteau and Jasper, 2014). For example, in one experiment, we detected five Pros+ cells among a total of 731 labeled cells (10 days following induction, five posterior midguts), whereas Biteau and Jasper report no Pros+ cells among 418 (4 days following induction, eight guts). This disparity is therefore most likely explicable by the short chase period used and the relative rarity of endocrine progenitors in the gut.
Our subsequent functional characterization clearly showed that Notch signaling is both necessary and sufficient to control cell diversity in the diffuse endocrine system. Although Notch loss leads to well-differentiated endocrine tumors, they exhibit specific changes in the type of endocrine cells produced. By contrast, Notch function was dispensable in controlling underlying regional identity along the length of the gut. Gaining insight into the process underlying regional specification represents an interesting line of future investigation. Although our analysis focused on the posterior midgut, where peptide diversity is greatest, our studies also indicate that similar requirements for Notch in class II endocrine cells exist along the entire length of the GI tract. Finally, targeted manipulation of Notch signaling in differentiated endocrine cells failed to alter endocrine identity, consistent with a model in which Notch signaling functions specifically in endocrine progenitors. Thus, Notch signaling plays at least two separable roles in controlling cell fate within the gut stem cell lineages: a previously known requirement in specifying absorptive enterocytes, and the newly described role in controlling the diversity of secretory enteroendocrine cells along the length of the adult midgut demonstrated here (Fig. 7I, yellow).
Non-autonomous consequences of endocrine tumors are implicit by the very nature of the peptides these cells secrete. Indeed, carcinoid tumors are neuroendocrine malignancies that secret hormones, which cause debilitating symptoms in patients. In addition, loss of endocrine cell subtypes has been associated with metabolic disruptions, including lipid absorption and glucose homeostasis (Mellitzer et al., 2010). Our study suggests that these effects might result from either the loss of specific peptides or overabundance of others in the diffuse endocrine system. Thus, further insight into how endocrine peptides are produced and how secretion is controlled might have important consequences for understanding tumor pathophysiology and for developing targeted treatments for metabolic disease.
MATERIALS AND METHODS
Fly stocks
The following fly stocks were used: from Bloomington Stock Center: w1118 (#39352; wild-type control); Canton-S (#1; wild-type control); w1118; GMR65D06-Gal4 (#39352; AstA-Gal4, this study); w1118; GMR61H08-Gal4 (#39283; AstC-Gal4, this study); w1118; GMR57F07-Gal4 (#46389; DH31-Gal4, this study); w1118; GMR61H07-Gal4 (#39282; called Tk-Gal4, this study); w1118; GMR50A12-Gal4/TM3 (#47618); w1118; GMR42C06-Gal4 (#50150); w1118; GMR46B08-Gal4 (#47361); pros10419, ry506 (#11744; pros-lacZ); y,w; UAS-GFP.nls (#4775); w; FRT82B, hsπM (#2004; wild type); UAS-dicer 2 (#24648); tub-Gal80TS (#7108); tub-Gal80TS (#7017); ry506 P{PZ} Dl05151/TM3 (#11651; Dl-lacZ); w, hsflp, tub-Gal80, FRT19A (#5133); y,w; esgk606 (#10359); UAS-NRNAi (#7078); UAS-psnRNAi (#27681); UAS-Nintra (#52008). w1118; npf-Gal4 (Wu et al., 2003); Fer1-GFP (Flytrap #G00188); w; actin5C FRT stop FRT lacZnuc; UAS-flp (actin5C>lacZ; Struhl and Basler, 1993); y,w, UAS-GFP, hsflp; tub-Gal4, FRT82B, tub-Gal80/TM6B; Su(H)GBE-Gal4 (Zeng et al., 2010); Su(H)GBE-lacZ (Furriols and Bray, 2001); esg-Gal4, UAS-GFP (Micchelli and Perrimon, 2006); N55e11 FRT19A/FM7 (Kidd et al., 1983); w, hsflp, tub-Gal80, FRT19A; UAS-GFP, UAS-lacZ; tub-Gal4; w; esg-Gal4, UAS-GFP, tub-Gal80TS (Micchelli and Perrimon, 2006; esgTS); Dlrev10 FRT82B (Haenelin et al., 1990); Serrx106 FRT82B (Thomas et al., 1991); y,w, UAS-GFP, hsflp; esgk606; tub-Gal4, FRT82B, tub-Gal80/TM6B; hs-Nintra (Struhl et al., 1993). For additional information, see http://flybase.org.
Characterization of neuropeptide distribution
Characterization of adult midgut neuropeptide distribution was carried out in wild-type flies (w1118 or Canton-S). We present the results of neuropeptide distribution in newly eclosed flies selected from low-density cultures, aged for 5 days at 25°C and dissected directly in fixative.
Directed cell lineage tracing
Directed tracing of adult endocrine cell lineages was performed by crossing specific Gal4 driver lines to the w; actin5C FRT stop FRT lacZ; UAS-flp lineage tracer and culturing at 25°C. Neuropeptide Gal4 lineage tracing experiments were analyzed as newly eclosed virgins (day 0) and then 4 and 10 days after eclosion under baseline conditions; they were also analyzed 8 days after eclosion following a 24 h Pe (OD5) infection. Conditional lineage tracing was performed by combining either neuropeptide Gal4 or Su(H)-Gal4 lines with tub-Gal80TS. The resultant flies were then crossed to the w; actin5C FRT stop FRT lacZnuc; UAS-flp lineage tracer. Crosses were established and cultured at 18°C until adulthood. F1 female progeny were aged 3-5 days at 18°C and were then shifted to 29°C for defined periods. Su(H)-Gal4TS lineage-tracing experiments were analyzed 10-12 days following the shift to 29°C and compared with unshifted controls grown at 18°C for 10 days. Su(H)-Gal4TS lineage-tracing experiments were also analyzed following challenge by heat stress or ad libitum exposure to Pe. For heat challenge, adults were grown at 29°C for 5 days, heat-shocked (37°C water bath, 45 min in the morning, plus 45 min at night) and cultured for an additional 5 days (10 day ‘chase’). An additional heat challenge protocol was also tested. Adults were grown at 29°C for 7 days, heat-shocked (37°C water bath, 45 min in the morning, plus 45 min at night) and cultured for an additional 7 days (14 day ‘chase’). For bacterial challenge, adults were grown at 29°C 7 days, exposed to Pe (24 h on Pe OD20-laced food vials at 29°C) and cultured for an additional 7 days (15 day ‘chase’).
Mosaic analysis
Positively marked cell lineages were generated in female midguts using the MARCM system. Fly crosses were cultured on standard media supplemented with yeast paste at 25°C. Newly eclosed females of the appropriate genotype were aged 3-5 days prior to clone induction. Clones were induced by placing vials in a 37°C water bath for 30-40 min. Flies were heat-shocked 2-3 times within a 24 h period.
Pe infection
Flies were infected ad libitum with Pe. Infected flies were fed on food supplemented with 0.5 ml of Pe at OD5-OD20 in 5% sucrose. Mock-infected flies were placed on food supplemented with 0.5 ml 5% sucrose. Flies were infected with Pe for 24 h and subsequently maintained at 29°C throughout the course of the experiment.
Conditional manipulation of Notch pathway
To conditionally target progenitor cells or differentiated endocrine cell populations in the adult midgut, esg-Gal4, Tk-Gal4 or AstA-Gal4 was first combined with tub-Gal80TS and then crossed to UAS-NotchRNAi, UAS-PresenilinRNAi or UAS-Nintra. Crosses were established and cultured at 18°C until adulthood. F1 female progeny of the appropriate genotype were aged 3-5 days at 18°C and then shifted to 29°C for 7 days. Global activation of the Notch pathway using the hs-Nintra transgene was accomplished by providing two 30-min heat shocks at 37°C, one in the morning and the second at night.
Histology
Generation of whole-mount samples for immunostaining was performed as previously described (Micchelli, 2014). Briefly, adult females were in fixed 0.5× PBS and 4% EM-grade formaldehyde (Polysciences) overnight, washed in 1× PBS, 0.1% Triton X-100 (PBST) for a minimum of 2 h, and incubated in primary antisera (see below) overnight. Samples were washed and incubated in secondary antibodies for 3 h, washed again in PBST and mounted in Vectashield+DAPI. All steps were completed at 4°C.
Antisera
Chicken anti-GFP (Abcam, #ab13970; 1:10,000); mouse anti-β-Gal (DSHB, #40-1a; 1:100); mouse anti-Pros (DSHB, #MR1A; 1:100); mouse anti-Brp (DSHB, #nc82; 1:10); mouse anti-Cut (DSHB, #2B10; 1:100); mouse anti-AstA (DSHB, #5F10; 1:20); rabbit anti-β-Gal (Cappel, #08559761; 1:4000); rabbit anti-Tk (1:500, a generous gift from Dr Dick Nässel, Stockholm University, Sweden) rabbit anti-Npf (1:750, a generous gift from Dr Paul Taghert, Washington University, USA); rabbit anti-Phm (1:250, a generous gift from Dr Paul Taghert); rabbit anti-AstB (1:500, a generous gift from Drs Paul Taghert and Jan Veenstra, Université de Bordeaux, France); rabbit anti-AstC (1:500, a generous gift from Dr Jan Veenstra); rabbit anti-DH31 (1:500, a generous gift from Dr Jan Veenstra). Alexa Fluor-conjugated secondary antibodies (Molecular Probes, #A-11039, #A-11029, #A-11031, #A-21236, #A-11034, #A-11036, #A-21245; 1:2000).
Microscopy and imaging
Samples were analyzed on a Leica DM5000 or Leica TCS SP5 confocal microscope. Images were processed for brightness and contrast in Photoshop CS (Adobe).
Supplementary Material
Acknowledgements
We wish to acknowledge the collegiality of members of the fly community who freely shared reagents. We are indebted to Drs D. Nässel, P. Taghert and J. Veenstra for generous gifts of very limited antibodies, without which this study would simply not have been possible. We also acknowledge Joseph Burclaff for initial screening and characterization of neuropeptide Gal4 lines. Finally, we thank members of the Micchelli lab for constructive discussions and comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
R.B.-E. performed the experiments. R.B.-E. and C.A.M. wrote the manuscript.
Funding
This work was supported by grants from the American Cancer Society and the National Institutes of Health [NIH RO1 DK095871 to C.A.M.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.114959/-/DC1
References
- Bardin A. J., Perdigoto C. N., Southall T. D., Brand A. H. and Schweisguth F. (2010). Transcriptional control of stem cell maintenance in the Drosophila intestine. Development 137, 705-714 10.1242/dev.039404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss W. M. and Starling E. H. (1902). The mechanism of pancreatic secretion. J. Physiol. 28, 325-353 10.1113/jphysiol.1902.sp000920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe K., Lee W.-C. and Micchelli C. A. (2010). JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev. Biol. 338, 28-37 10.1016/j.ydbio.2009.10.045 [DOI] [PubMed] [Google Scholar]
- Bendena W. G., Donly B. C. and Tobe S. S. (1999). Allatostatins: a growing family of neuropeptides with structural and functional diversity. Ann. N. Y. Acad. Sci. 897, 311-329 10.1111/j.1749-6632.1999.tb07902.x [DOI] [PubMed] [Google Scholar]
- Biteau B. and Jasper H. (2014). Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 7, 1867-1875 10.1016/j.celrep.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Osman D., David F. P. A., Fang H. Y., Boquete J.-P., Deplancke B. and Lemaitre B. (2013). Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 3, 1725-1738 10.1016/j.celrep.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J. and Dow J. A. T. (2007). Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, 715-720 10.1038/ng2049 [DOI] [PubMed] [Google Scholar]
- Dutta D., Xiang J. and Edgar B. A. (2013). RNA expression profiling from FACS-isolated cells of the Drosophila intestine. Curr. Protocol Stem Cell Biol. 27, 2F.2.1-2F.2.12 10.1002/9780470151808.sc02f02s27 [DOI] [PubMed] [Google Scholar]
- Egerod K. L., Engelstoft M. S., Grunddal K. V., Nøhr M. K., Secher A., Sakata I., Pedersen J., Windeløv J. A., Füchtbauer E.-M., Olsen J. et al. (2012). A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153, 5782-5795 10.1210/en.2012-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft M. S., Egerod K. L., Lund M. L. and Schwartz T. W. (2013). Enteroendocrine cell types revisited. Curr. Opin. Pharmacol. 13, 912-921 10.1016/j.coph.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Furness J. B., Rivera L. R., Cho H.-J., Bravo D. M. and Callaghan B. (2013). The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 1010, 729-740 10.1038/nrgastro.2013.180 [DOI] [PubMed] [Google Scholar]
- Furriols M. and Bray S. (2001). A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr. Biol. 11, 60-64 10.1016/S0960-9822(00)00044-0 [DOI] [PubMed] [Google Scholar]
- Gäde G. (2004). Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annu. Rev. Entomol. 49, 93-113 10.1146/annurev.ento.49.061802.123354 [DOI] [PubMed] [Google Scholar]
- Habib A. M., Richards P., Cairns L. S., Rogers G. J., Bannon C. A. M., Parker H. E., Morley T. C. E., Yeo G. S. H., Reimann F. and Gribble F. M. (2012). Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153, 3054-3065 10.1210/en.2011-2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenelin M., Kramatschek B. and Campos-Ortega J. A. (1990). The pattern of transcription of the neurogenic gene Delta of Drosophila melanogaster. Development 110, 905-914. [DOI] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J. and Young M. W. (1983). The Notch locus of Drosophila melanogaster. Cell 34, 421-433 10.1016/0092-8674(83)90376-8 [DOI] [PubMed] [Google Scholar]
- Lee T. and Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451-461 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Lee W.-C. and Micchelli C. A. (2013). Development and characterization of a chemically defined food for Drosophila. PLoS ONE 8, e67308 10.1371/journal.pone.0067308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianes A. and Spradling A. C. (2013). Physiological and stem cell compartmentalization within the Drosophila midgut. Elife 2, e00886 10.7554/eLife.00886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G., Beucher A., Lobstein V., Michel P., Robine S., Kedinger M. and Gradwohl G. (2010). Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J. Clin. Invest. 120, 1708-1721 10.1172/JCI40794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli C. A. (2014). Whole-mount immunostaining of the adult Drosophila gastrointestinal tract. Methods 68, 273-279 10.1016/j.ymeth.2014.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli C. A. and Perrimon N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475-479 10.1038/nature04371 [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., Sudmeier L., Perrimon N., Tang S. and Beehler-Evans R. (2011). Identification of adult midgut precursors in Drosophila. Gene Expr. Patterns 11, 12-21 10.1016/j.gep.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Montagne C. and Gonzalez-Gaitan M. (2014). Sara endosomes and the asymmetric division of intestinal stem cells Development 141, 2014-2023 10.1242/dev.104240 [DOI] [PubMed] [Google Scholar]
- Nässel D. R. and Wegener C. (2011). A comparative review of short and long neuropeptide F signaling in invertebrates: any similarities to vertebrate neuropeptide Y signaling? Peptides 32, 1335-1355 10.1016/j.peptides.2011.03.013 [DOI] [PubMed] [Google Scholar]
- Ohlstein B. and Spradling A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470-474 10.1038/nature04333 [DOI] [PubMed] [Google Scholar]
- Ohlstein B. and Spradling A. (2007). Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988-992 10.1126/science.1136606 [DOI] [PubMed] [Google Scholar]
- Perdigoto C. N., Schweisguth F. and Bardin A. J. (2011). Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 138, 4585-4595 10.1242/dev.065292 [DOI] [PubMed] [Google Scholar]
- Piper M. D. W., Blanc E., Leitão-Gonçalves R., Yang M., He X., Linford N. J., Hoddinott M. P., Hopfen C., Soultoukis G. A., Niemeyer C. et al. (2014). A holidic medium for Drosophila melanogaster. Nat. Methods 11, 100-105 10.1038/nmeth.2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiher W., Shirras C., Kahnt J., Baumeister S., Isaac R. E. and Wegener C. (2011). Peptidomics and peptide hormone processing in the Drosophila midgut. J. Proteome Res. 10, 1881-1892 10.1021/pr101116g [DOI] [PubMed] [Google Scholar]
- Reimann F., Tolhurst G. and Gribble F. M. (2012). G-protein-coupled receptors in intestinal chemosensation. Cell Metabol. 15, 421-431 10.1016/j.cmet.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Schonhoff S. E., Giel-Moloney M. and Leiter A. B. (2004). Minireview: development and differentiation of gut endocrine cells. Endocrinology 145, 2639-2644 10.1210/en.2004-0051 [DOI] [PubMed] [Google Scholar]
- Scopelliti A., Cordero J. B., Diao F., Strathdee K., White B. H., Sanson O. J. and Vidal M. (2014). Local control of intestinal stem cell homeostasis by enteroendocrine cells in the adult Drosophila midgut. Curr. Biol. 24, 1199-1211 10.1016/j.cub.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei Y., Lu X., Liou A., Zhao X. and Wank S. A. (2011). A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G345-G356 10.1152/ajpgi.00278.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M. and Micchelli C. A. (2011). Quiescent gastric stem cells maintain the adult Drosophila stomach. Proc. Natl. Acad. Sci. USA 108, 17696-17701 10.1073/pnas.1109794108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G. and Basler K. (1993). Organizing activity of wingless protein in Drosophila. Cell 72, 527-540 10.1016/0092-8674(93)90072-X [DOI] [PubMed] [Google Scholar]
- Struhl G., Fitzgerald K. and Greenwald I. (1993). Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74, 331-345 10.1016/0092-8674(93)90424-O [DOI] [PubMed] [Google Scholar]
- Takashima S., Adams K. L., Ortiz P. A., Ying C. T., Moridzadeh R., Younossi-Hartenstein A. and Hartenstein V. (2011). Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev. Biol. 353, 161-172 10.1016/j.ydbio.2011.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U., Speicher S. A. and Knust E. (1991). The Drosophila gene Serrate encodes an EGF-like transmembrane protein with a complex expression pattern in embryos and wing discs. Development 111, 749-761. [DOI] [PubMed] [Google Scholar]
- Van Loy T., Vandersmissen H. P., Poels J., Van Hiel M. B., Verlinden H. and Broeck J. V. (2010). Tachykinin-related peptides and their receptors in invertebrates: a current view. Peptides 31, 520-524 10.1016/j.peptides.2009.09.023 [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. (2009). Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 336, 309-323 10.1007/s00441-009-0769-y [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. and Ida T. (2014). More Drosophila enteroendocrine peptides: Orcokinin B and the CCHamides 1 and 2. Cell Tissue Res. 357, 607-621 10.1007/s00441-014-1880-2 [DOI] [PubMed] [Google Scholar]
- Veenstra J. A., Agricola H.-J. and Sellami A. (2008). Regulatory peptides in fruit fly midgut. Cell Tissue Res. 334, 499-516 10.1007/s00441-008-0708-3 [DOI] [PubMed] [Google Scholar]
- Vodovar N., Vinals M., Liehl P., Basset A., Degrouard J., Spellman P., Boccard F. and Lemaitre B. (2005). Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. USA 102, 11414-11419 10.1073/pnas.0502240102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Wen T., Lee G., Park J. H., Cai H. N. and Shen P. (2003). Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron 39, 147-161 10.1016/S0896-6273(03)00396-9 [DOI] [PubMed] [Google Scholar]
- Zeng X., Chauhan C. and Hou S. X. (2010). Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. Genesis 48, 607-611 10.1002/dvg.20661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke N., Korzelius J., van Straaten M., Bender K., Schuhknecht G. F. P., Dutta D., Xiany J. and Edgar B. (2014). Fly-FUCCI: a versatile tool for studying cell proliferation in complex tissues. Cell Rep. 7, 588-598 10.1016/j.celrep.2014.03.020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.