Abstract
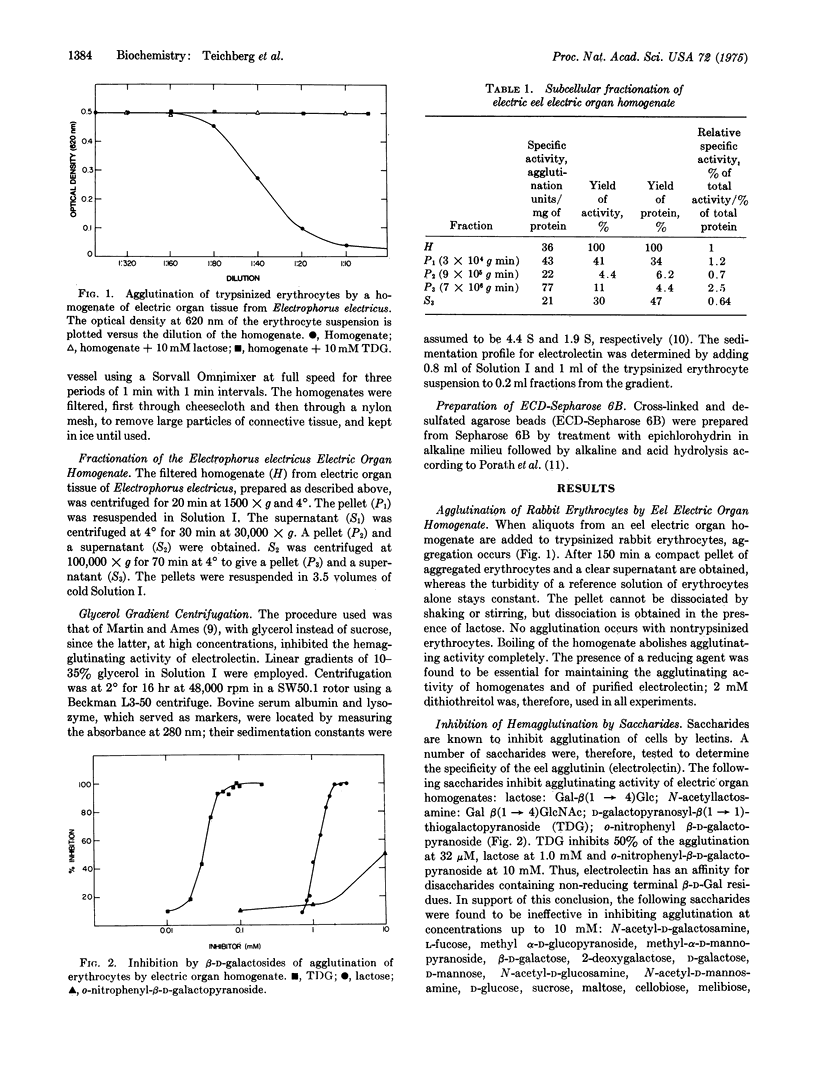
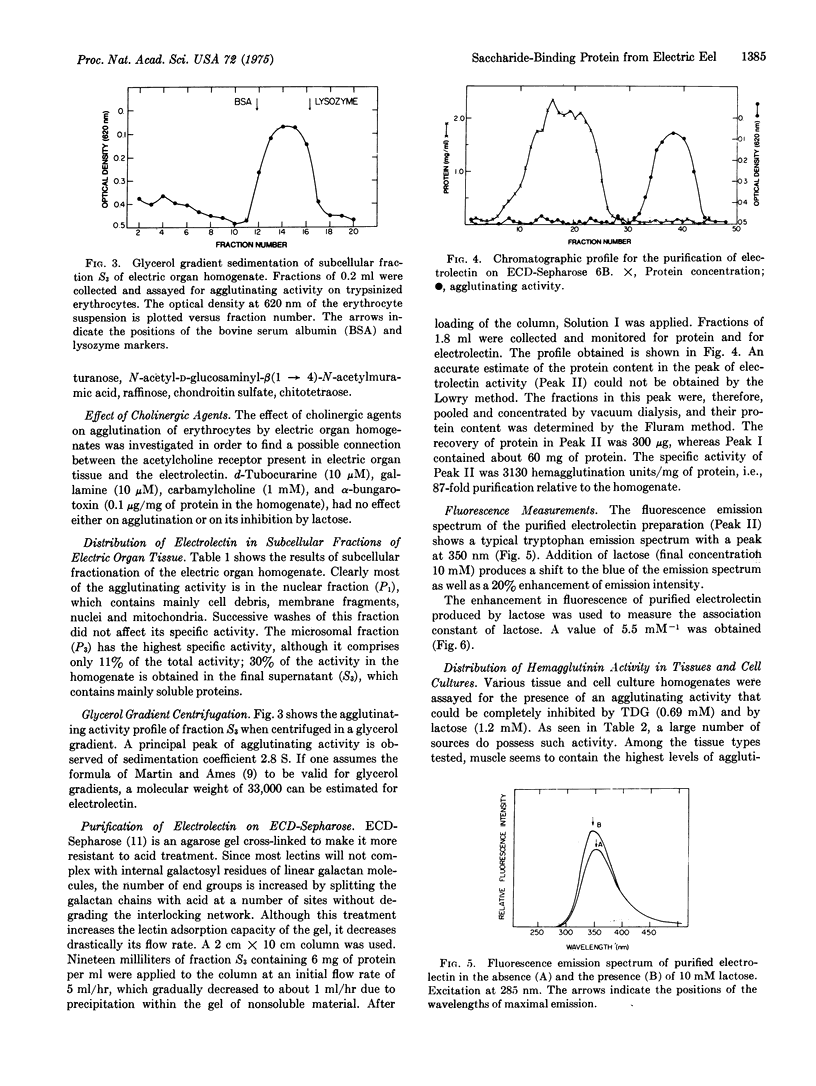
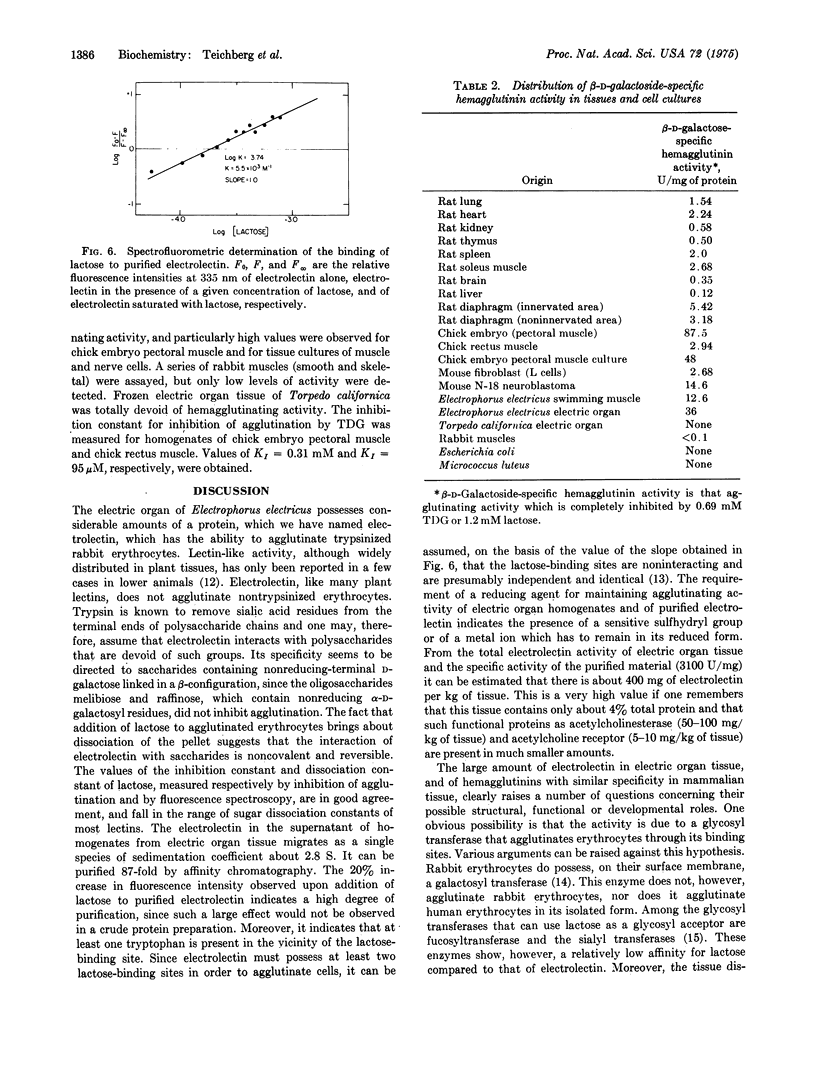
Extracts of electric organ tissue of Electrophorus electricus contain a saccharide-binding protein, named electrolectin, which agglutinates trypsin-treated rabbit erythrocytes and is specifically inhibited by disaccharides containing nonreducing terminal beta-D-galactosyl residues. Electrolectin seems at least partially membrane-bound but is also found in soluble fractions of homoge-nates from which it can be purfied by affinity chromatography on cross-linked and desulfated agarose (ECD-Sepharose) as a protein of molecular weight 33,000. About 400 mg of electrolectin are present per kg of tissue. It has an affinity for lactose of 1.0 mM-1 and 5.5mM-1 as estimated, respectively, by hapten inhibition and fluorescence spectroscopy. Studies on the distribution of beta-D-galactoside-binding activity in animal tissues reveal particularly high levels in sheletal muscle tissue and in cultures of embryonic skeletal muscle and neuroblastoma cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chipman D. M., Grisaro V., Sharon N. The binding of oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid to lysozyme. The specificity of binding subsites. J Biol Chem. 1967 Oct 10;242(19):4388–4394. [PubMed] [Google Scholar]
- Clark D. G., Macmurchie D. D., Elliott E., Wolcott R. G., Landel A. M., Raftery M. A. Elapid neurotoxins. Purification, characterization, and immunochemical studies of -bungarotoxin. Biochemistry. 1972 Apr 25;11(9):1663–1668. doi: 10.1021/bi00759a020. [DOI] [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Pecht I., Maron E., Arnon R., Sela M. Specific excitation energy transfer from antibodies to dansyl-labeled antigen. Studies with the "loop" peptide of hen egg-white lysozyme. Eur J Biochem. 1971 Apr;19(3):368–371. doi: 10.1111/j.1432-1033.1971.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M., La Mont J. T., Isselbacher K. J. Galactosyltransferase and concanavalin A agglutination of cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):904–908. doi: 10.1073/pnas.71.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Simpson D. L., Rosen S. D., Barondes S. H. Discoidin, a developmentally regulated carbohydrate-binding protein from Dictyostelium discoideum. Purification and characterization. Biochemistry. 1974 Aug 13;13(17):3487–3493. doi: 10.1021/bi00714a011. [DOI] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. Mammalian hepatic lectin. Science. 1974 Oct 25;186(4161):365–366. doi: 10.1126/science.186.4161.365. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]


