Abstract
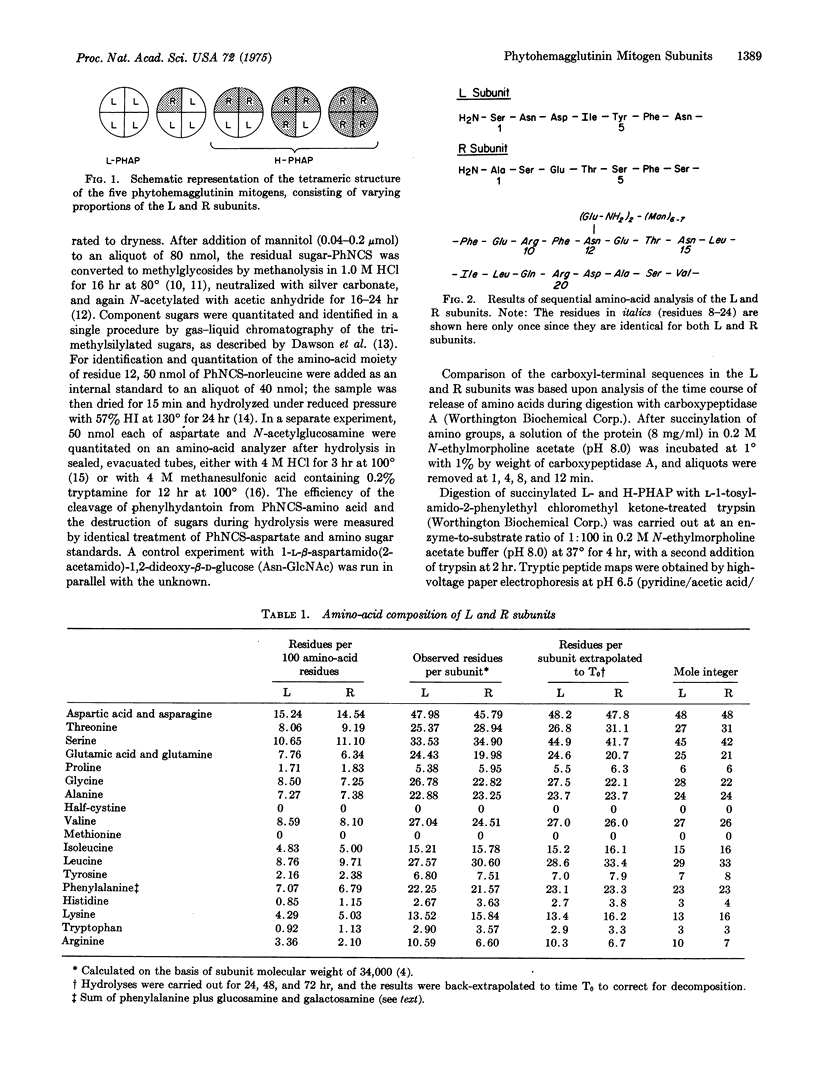
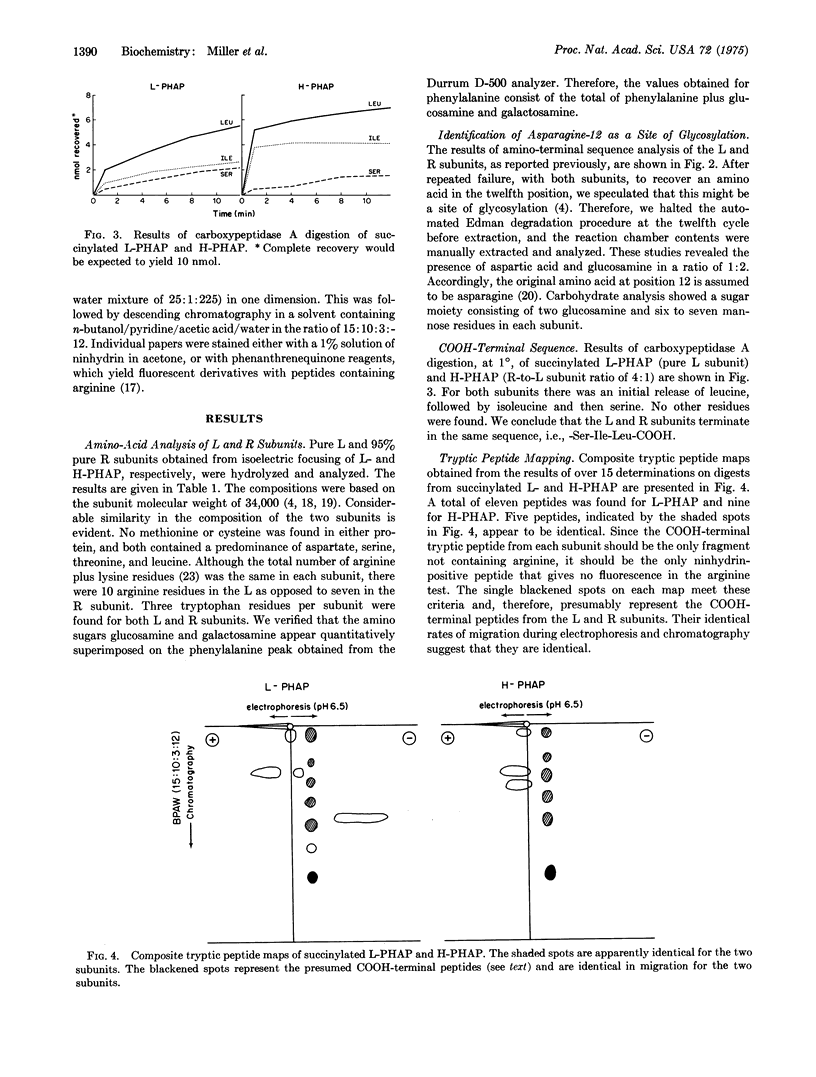
The phytohemagglutinin mitogenic proteins derived from Phaseolus vulgaris comprise a class of five glycoproteins that are isomeric tetramers composed of varying proportions of two different subunits (L and R). Within the native tetramer, the L subunit is a potent leukoagglutinin and mitogen that lacks hemagglutinating properties, whereas the R subunit is a potent hemagglutinin with little or no mitogenic activity. The subunits have been isolated in homogeneous form by isoelectric focusing in 8 M urea. Previous work has shown that they have equal molecular weights and differ in amino-acid sequence from residues 1-7, but are identical in positions 8-24 [(1973) J. Exp. Med. 138, 939-951]. We now report amino-acid composition studies which reveal striking similarities between the subunits. Both lack methionine and cysteine. The twelfth residue in each subunit is a glycosylated asparagine, with the identical carbohydrate composition in each. The last three residues of the subunits, as determined by carboxypeptidase A digestion, are identical. Tryptic peptide mapping of the succinylated phytohemagglutinin subunits reveals a high degree of similarity. We conclude that the substantial difference in biological properties among the tetrameric phytohemagglutinin mitogens is a result of relatively restricted differences in the primary structure of their constituent subunits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. W., Svenson R. H., Yachnin S. Purification of mitogenic proteins derived from Phaseolus vulgaris: isolation of potent and weak phytohemagglutinins possessing mitogenic activity. Proc Natl Acad Sci U S A. 1969 Jun;63(2):334–341. doi: 10.1073/pnas.63.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Dawson G., Hough L. The simultaneous estimation of 6-deoxy-L-galactose (L-fucose), D-mannose, D-galactose, 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) and N-acetylneuraminic acid (sialic acid) in glycopeptides and glycoproteins. Biochim Biophys Acta. 1967 Nov 28;148(2):342–349. doi: 10.1016/0304-4165(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Dawson G., Matalon R., Dorfman A. Glycosphingolipids in cultured human skin fibroblasts. I. Characterization and metabolism in normal fibroblasts. J Biol Chem. 1972 Sep 25;247(18):5944–5950. [PubMed] [Google Scholar]
- FLETCHER A. P., MARKS G. S., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 5. Procedures for the isolation of glycopeptides from hen's-egg albumin and their oxidation by periodate. Biochem J. 1963 May;87:265–273. doi: 10.1042/bj0870265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H., Sharon N., Katchalski E. Identification of the carbohydrate-protein linking group in soybean hemagglutinin. Biochim Biophys Acta. 1969 Nov 18;192(2):364–366. doi: 10.1016/0304-4165(69)90380-8. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Noyes C., Heinrikson R., Kingdon H. S., Yachnin S. Phytohemagglutinin mitogenic proteins. Structural evidence for a family of isomitogenic proteins. J Exp Med. 1973 Oct 1;138(4):939–951. doi: 10.1084/jem.138.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGAS D. A., OSGOOD E. E. Purification and properties of the phytohemagglutinin of Phaseolus vulgaris. J Biol Chem. 1955 Feb;212(2):607–615. [PubMed] [Google Scholar]
- Räsänen V., Weber T. H., Gräsbeck R. Crystalline kidney-bean leucoagglutinin. Eur J Biochem. 1973 Sep 21;38(1):193–200. doi: 10.1111/j.1432-1033.1973.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Sox H. C., Jr, Hood L. Attachment of carbohydrate to the variable region of myeloma immunoglobulin light chains. Proc Natl Acad Sci U S A. 1970 Jul;66(3):975–982. doi: 10.1073/pnas.66.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]
- Weber T. H., Aro H., Nordman C. T. Characterization of lymphocyte-stimulating blood cell-agglutinating glycoproteins from red kidney beans (Phaseolus vulgaris). Biochim Biophys Acta. 1972 Mar 15;263(1):94–105. doi: 10.1016/0005-2795(72)90163-8. [DOI] [PubMed] [Google Scholar]
- Yachnin S., Allen L. W., Baron J. M., Svenson R. H. The potentiation of phytohemagglutinin-induced lymphocyte transformation by cell-cell interaction; a matrix hypothesis. Cell Immunol. 1972 Apr;3(4):569–589. doi: 10.1016/0008-8749(72)90120-7. [DOI] [PubMed] [Google Scholar]
- Yachnin S., Svenson R. H. The immunological and physicochemical properties of mitogenic proteins derived from Phaseolus vulgaris. Immunology. 1972 May;22(5):871–883. [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Takahashi N., Murachi T. The composition and structure of carbohydrate moiety of stem bromelain. Biochemistry. 1970 Jan 6;9(1):25–32. doi: 10.1021/bi00803a004. [DOI] [PubMed] [Google Scholar]


