Abstract
Ondansetron is the drug of choice to prevent nausea in women undergoing cesarean surgery and can be used to prevent neonatal abstinence syndrome (NAS). Pharmacokinetics of ondansetron has not been characterized in pregnant women or in newborns. A nonlinear mixed-effects modeling approach was used to analyze plasma samples obtained from 20 non-pregnant and 40 pregnant women following single administration of 4 or 8 mg ondansetron, from umbilical cord blood at delivery, and from neonates after birth. The analysis indicates that: ondansetron disposition is not affected by pregnancy (p>0.05), but influenced by dose (p<0.05), and is characterized by rapid transplacental transfer and longer elimination half-life in neonates compared to their mother. A dosing regimen for prevention of NAS was designed based on the model. The regimen involves IV administration of 4 mg to the mothers shortly before cord clamping, or oral administration of 0.07 mg/kg (or equivalently 0.04 mg/kg IV) to neonates.
Keywords: Ondansetron, Pharmacokinetics, Pregnant, Neonatal Abstinence Syndrome, NONMEM
INTRODUCTION
Ondansetron is a potent and selective 5-hydroxy tryptamine receptor (5-HT3) antagonist that is widely used to prevent and treat nausea and vomiting in surgical patients,1 including women undergoing cesarean surgery.2, 3 A recent study found that ondansetron was effective in preventing narcotic drug withdrawal symptoms in mice and humans,4 suggesting that ondansetron could potentially be used to prevent or treat the narcotic drug withdrawal symptoms that develop in neonates born to mothers who consume narcotics.5 The marked increase in the number of opioid prescriptions and in opioid misuse6 has impacted pregnant mothers and their babies. The constellation of narcotic drug withdrawal signs that develop in infants with prenatal exposure to opioids, which is referred to as neonatal abstinence syndrome (NAS),7 includes: tremors, irritability, hypertonia, seizures, poor feeding, vomiting, diarrhea, dehydration, and fever. At-risk neonates are usually admitted to an intensive care unit for observation, supportive care, and for opioid replacement if needed. The duration of opioid treatment can vary from 1 to 122 days (median 38 days).8, 9 The incidence of NAS has increased 3-fold over the last 10 years, and costs for treatment of this condition have escalated.
We wanted to recommend an ondansetron dosing regimen for expectant mothers and their neonates that could be used to prevent the development of NAS in the off-spring of pregnant women who consume narcotics. For this goal to be achieved, ondansetron pharmacokinetics and trans-placental transmission in mothers undergoing elective cesarean section and their neonates need to be characterized. Ondansetron pharmacokinetics has been previously characterized in healthy volunteers, the elderly,10, 11 and pediatric patients older than 6 months.12, 13 However, its pharmacokinetics have not been evaluated in pregnant women or newborns (0–28 days old), nor has its trans-placental passage been characterized. Ondansetron is primarily eliminated by hepatic phase I metabolism; less than 5% of the intravenously administered drug appears unchanged in the urine.14 The extensive physiological alterations that occur during pregnancy15 and the known differences in neonatal drug metabolism16 may necessitate a dosage adjustment if ondansetron is used to prevent NAS.
RESULTS
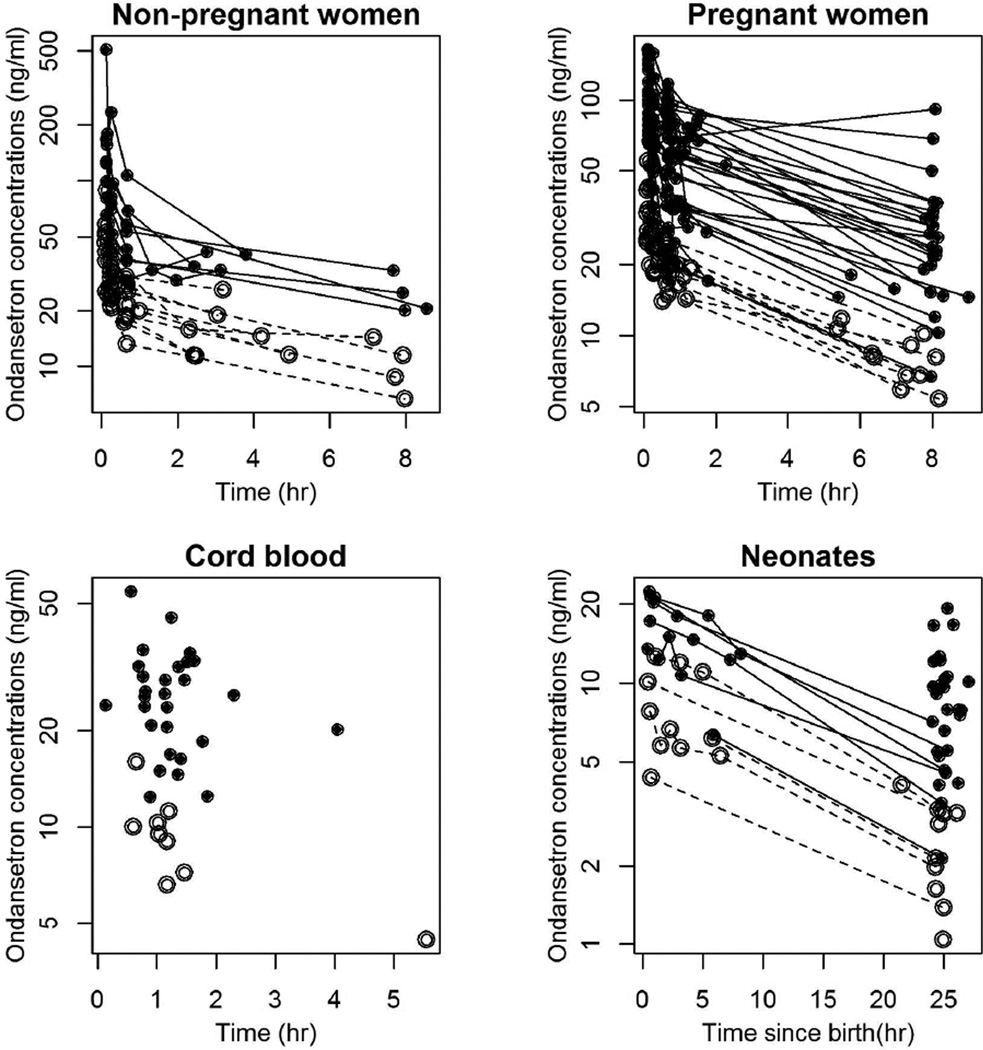
Samples were obtained from 20 nonpregnant and 40 pregnant women and from 39 neonates after birth. Demographic data are listed in Table 1. Of the 371 ondansetron concentrations analyzed, 78 were from nonpregnant women, 191 were from pregnant women, 37 were from umbilical cord blood, and 65 were from neonates. Pharmacokinetic profiles for different groups in our study are shown in Figure 1.
Table 1.
Women and neonatal demographics and gestational data.
| Characteristic | Mean (SD) Or n |
Range | |||
|---|---|---|---|---|---|
| Number of subjects | |||||
| Pregnant | 40 | ||||
| Nonpregnant | 20 | ||||
| Total | 60 | ||||
| Number of subjects on 4 mg or 8 mg dose | |||||
| Pregnant | 10/30 | ||||
| Nonpregnant | 10/10 | ||||
| Total | 20/40 | ||||
| Age (years) | |||||
| Pregnant | 33.0 (5.2) | 23–43 | |||
| Nonpregnant | 37.7 (5.7) | 26–45 | |||
| Total | 34.6 (5.8) | 23–45 | |||
| Weight (kg) | |||||
| Pregnant | 80.7 (12.0) | 60–115 | |||
| Nonpregnant | 71.1 (20.5) | 51–126 | |||
| Total | 77.5 (15.8) | 51–126 | |||
| Height (m) | |||||
| Pregnant | 1.64 (0.06) | 1.5–1.75 | |||
| Nonpregnant | 1.63 (0.06) | 1.4–1.73 | |||
| Total | 1.64 (0.06) | 1.44–1.75 | |||
| Gestation period (weeks) | 39.1 (0.746) | 36.4– 40.4 | |||
| Delay until delivery after dose (min) | 80.8 (55.5) | 8.0–333 | |||
| Number of neonates | 38 | ||||
| Weight of neonate at birth(kg) | 3.49 (0.49) | 2.47–4.58 | |||
| Height of neonate at birth (cm) | 50.5 (2.12) | 46–55 | |||
| Neonatal Gender (n) | |||||
| Male | 18 | ||||
| Female | 20 | ||||
Figure 1.
Ondansetron plasma logarithmic concentration-time profiles for non-pregnant women, pregnant-women, cord blood, and neonates. Open circles and dashed lines represent observations following administration of the 4 mg dose to the women. Closed circles and solid lines represent observations following administration of the 8 mg dose to the women. Lines connect observations obtained in the same individual.
During our construction of the covariate model, we found that: the dosing level was the most important covariate affecting its CL and VSS, and that none of the other covariates (including pregnancy status) had a significant effect on the model.
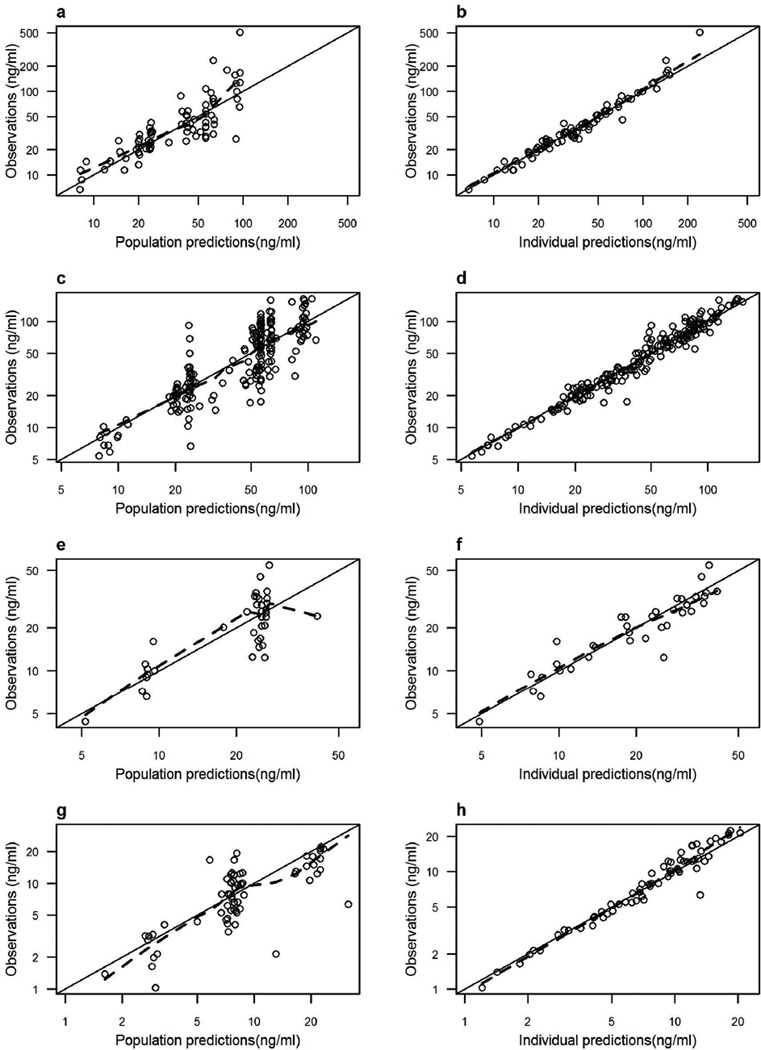
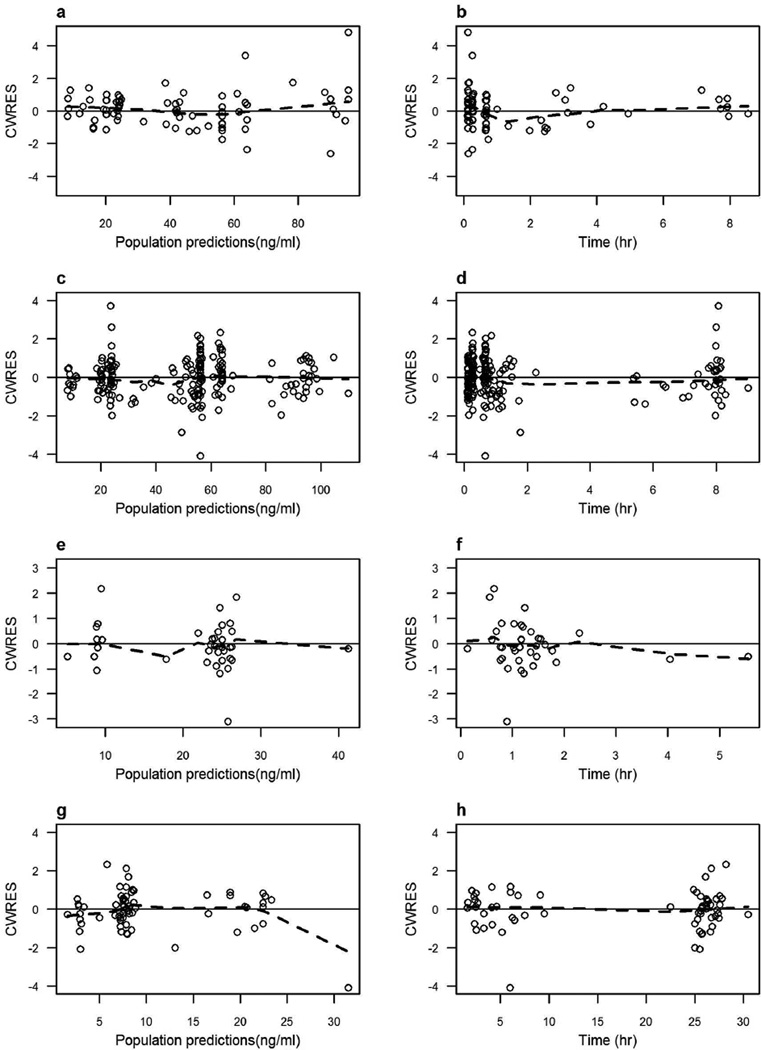
The population PK model parameter estimates calculated for women (pooled data from non-pregnant and pregnant groups), cord blood, and neonates; along with their between-subject variability and 95% confidence interval are summarized in Table 2. Good estimation precision was noticed for the model fixed-effects parameters (%SE < 18%). The parameters showed Gaussian distribution around the point estimates, as indicated by the overlap between the asymptotic and log-likelihood profiling confidence intervals. Analysis of the results shown in Table 2 indicate that: (1) increasing the dose from 4 mg to 8 mg decreased ondansetron clearance and steady-state volume of distribution by 31% and 26%, respectively; and (2) the elimination half-life of ondansetron in newborns is 2.5 times longer than the β-half-life in adults on the 4-mg dose (15 hours versus 5.6 hours). Figure 1 shows that the relationship between observed ondansetron concentrations and the model predictions involves symmetric distribution of population predictions around the identity line, and good agreement with individual predicted ondansetron concentrations. This indicates that the data obtained were accurately predicted at the population and subject levels using the model. No patterns were observed in the plot of conditionally weighted residuals versus population-predicted concentrations, or time (Figure 2), indicating lack of systemic bias. Umbilical cord blood concentrations were linearly correlated with maternal predicted concentrations at delivery (Pearson’s r2 = 0.90), indicating lack of delayed distribution between the maternal and fetal blood and confirming rapid trans-placental transfer.
Table 2.
Ondansetron population pharmacokinetic parameter estimates.
| Parameters | Point estimate (%SE) |
Symmetrical 95% CI |
Nonsymmetrical 95% CI |
Residual variability (%CV) |
Between-subject variability (%CV) |
|---|---|---|---|---|---|
| Women: | 21.6 | ||||
| CL (L/h) | 40.1 | ||||
| CL0 | 21.8 (6.7) | 18.9, 24.6 | 17.2, 27.2 | ||
| θCL | 0.69 (11) | 0.54, 0.84 | 0.52, 0.92 | ||
| V (L) | 27.9 (17) | 18.5, 37.3 | 13.6, 45.0 | 56.1 | |
| Q(L/h) | 329 (9.5) | 268, 390 | 256, 401 | 51.3 | |
| VSS (L) | 32.4 | ||||
| VSS0 | 167 (4.7) | 151, 182 | 140, 198 | ||
| θVss | 0.75 (7.8) | 0.63, 0.86 | 0.61, 0.91 | ||
| Cord: | 23.5 | ||||
| KP | 0.47 (5.1) | 0.42, 0.52 | 0.44, 0.51 | ||
| Neonates: | 21.9 | ||||
| KN0 (1/h) | 0.046 (6.2) | 0.041, 0.052 | 0.040, 0.052 | 29.6 | |
%SE: relative standard error; CV: coefficient of variation; CI: confidence interval; CL clearance in women; CL0 clearance for 4-mg dose; θCL fractional change in CL0 for the 8-mg dose; V distribution volume in women; Q inter-compartmental clearance in women; KP placenta-to-
Figure 2.
Plot of ondansetron observed versus the final pharmacokinetic model population predicted (left panel) and individual predicted (right panel) concentrations in non-pregnant women (a and b), pregnant women (c and d), cord blood (e and f), and neonates (g and h). The solid line is the line of identity. The dashed line is a lowess smother. The observed concentrations, population predictions, and individual predictions were transformed into their logarithms.
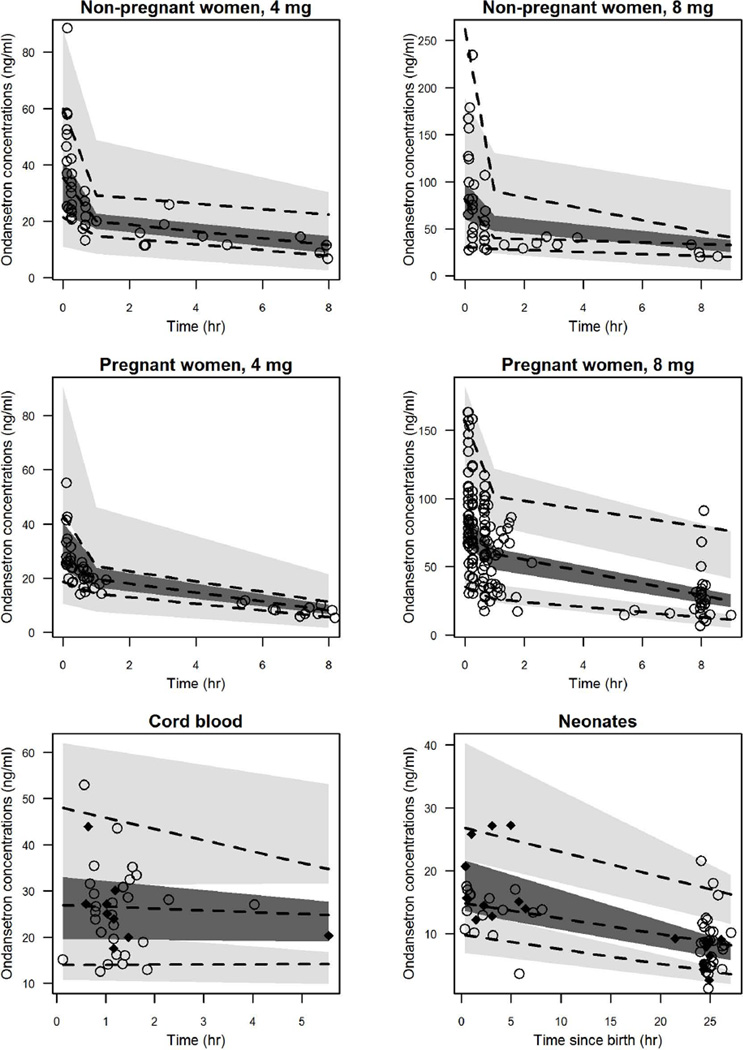
The VPC plots (Figure 4) show that the observed ondansetron concentration percentiles were consistently within the 95% confidence intervals of the population PK model simulated concentration percentiles. The only exception was for the 8-mg dose of ondansetron administered to the control group, where the observed concentration was slightly under-predicted at the initial sampling time. These results indicate that the characteristics of the real world data can be adequately replicated using the model and ensure the correct estimation of the model variability parameters.
Figure 4.
Visual predictive check of the final pharmacokinetic model for ondansetron concentrations non-pregnant women, pregnant women, cord blood, and neonates. Dashed lines represent the 5th, 50th, and 95th percentile of observed concentrations. Shaded areas represent the 95% CI for the 5th, 50th, and 95th percentile of simulated concentrations. Points represent the observed concentrations. Observations in cord blood and neonates were prediction-corrected. In cord blood and neonatal plots, closed squares and open circles represent observations following administration of the 4 mg and 8 mg doses to the mothers, respectively.
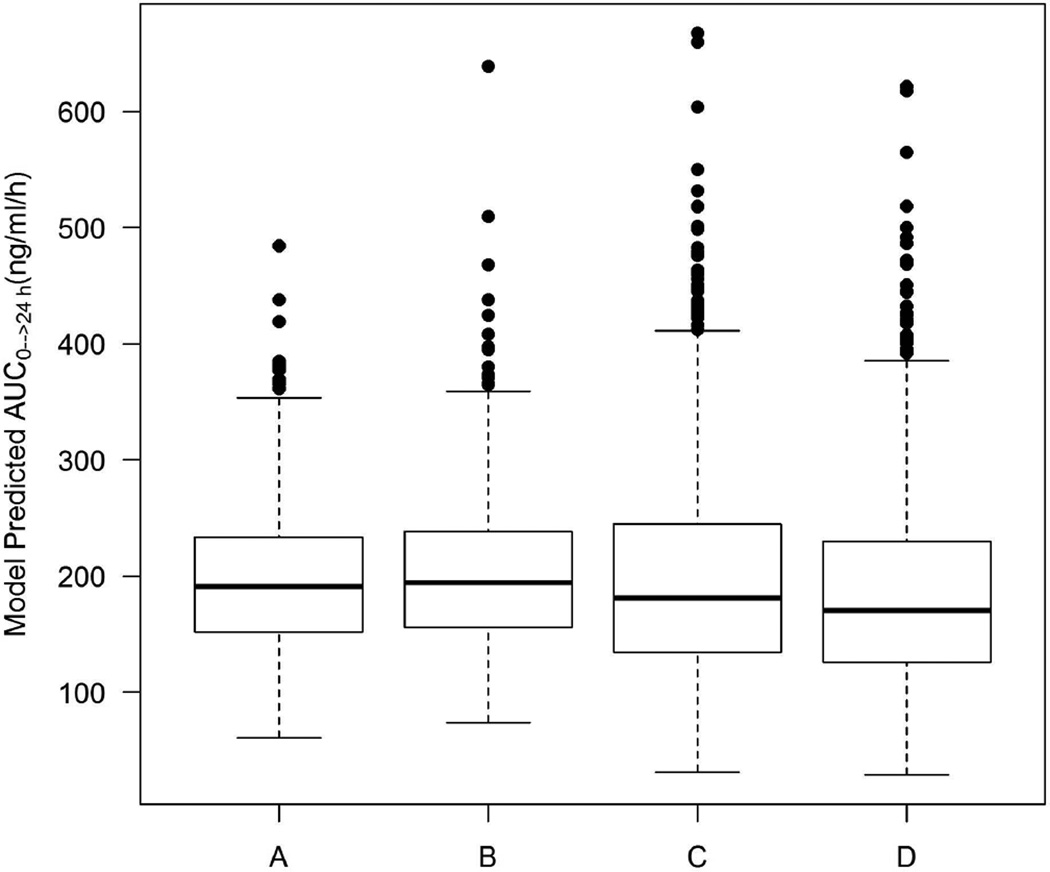
Simulations were then performed using the model to devise an ondansetron dose in neonates that would be effective for preventing the development of NAS. Figure 5 shows that IV administration of 4 mg to the mothers 15 minutes before cord clamping, or oral administration of 0.07 mg/kg (or equivalently 0.04 mg/kg IV) to neonates, produces an exposure level in neonates (AUC0→24 h) that is similar to that in adults treated with an 4 mg oral dose twice a day.
Figure 5.
Boxplot of model predicted AUC0–24 h in adults following the oral administration of 4 mg twice a day (A); in neonates following the IV administration of 4 mg to the mothers 15 minutes before delivery (B); in neonates following the administration of oral 0.07 mg/kg (C); or IV 0.04 mg/kg (D) to neonates.
DISCUSSION
Ondansetron pharmacokinetic parameters have not been characterized in pregnant women or in neonates. In order to develop a treatment regimen that could reduce the public health problem caused by NAS, we need a detailed analysis of ondansetron pharmacokinetics in this population. Our analysis of ondansetron pharmacokinetics in pregnant women and neonates, and its trans-placental passage, enabled us to develop a predictive pharmacokinetic model. Based on the findings of our analysis, ondansetron dosing does not need to be altered in pregnancy, and it readily crosses the placenta. However, a dosage adjustment is required in neonates because of its longer half-life, which is likely to be due to reduced clearance. Although most of the microsomal enzymes that are responsible for ondansetron biotransformation are present at birth, their activities are considerably reduced.16 CYP3A4 activity in full-term neonates is ~20% of adult levels, and does not reach adult levels until 6–12 months after birth.17 CYP2D6 expression in neonates < 7 days is substantially reduced;18 and CYP1A2, which is the last hepatic CYP450 to appear,19 reaches 35% of adult levels after one year.17
Our findings are consistent with the pharmacokinetic properties of ondansetron that were measured in other non-pregnant adult populations. Our finding that ondansetron kinetics follow a bi-exponential disposition,13 and the measured clearance parameters (Table 2) are in good agreement with previously reported result.10 Because 95% of ondansetron’s clearance is mediated by hepatic oxidation,20 the dose-dependent clearance is probably due to saturation of hepatic metabolic enzymes, as previously reported.21 The rapid trans-placental passage of ondansetron is also not surprising, because ondansetron is a highly lipid-soluble drug that readily crosses tissue membranes via passive diffusion.22 However, the dose-dependent volume of distribution at steady-state observed here suggests a parallel carrier-mediated transport pathway. Neither weight nor age was a significant factor affecting the ondansetron pharmacokinetics in our study, and this can be attributed to the narrow range of weights and ages evaluated here.
Ondansetron is metabolized by multiple cytochrome P450 forms, including CYP1A1, CYP1A2, CYP2D6, and the CYP3A subfamily, with no single form of cytochrome P450 dominating the overall metabolism and the role played by CYP2D6 is minor.23 The effects of pregnancy on the activity of cytochrome P450 forms are different: CYP1A2 activity decreases, while CYP2D6 and CYP3A4 activity increases during pregnancy.15 This bidirectional effect could explain why pregnancy did not alter ondansetron clearance. Because ondansetron is only 70%–76% bound to plasma proteins24 and its clearance rate is ~40% of the hepatic blood flow,20 it is not likely that pregnancy-induced alterations in protein binding or liver blood flow influences ondansetron’s hepatic clearance. Ondansetron’s distribution volume is larger than that of total body water,21 which suggests that the drug distributes into fatty tissues. Hence, the increased plasma volume associated with pregnancy15 is not likely to affect ondansetron dilution. This may explain the lack of pregnancy effect on the distribution volume of ondansetron.
Scarce information is available on ondansetron effective dose for treating physical dependence or alleviating withdrawal symptoms when drug use is stopped in adults. Single IV administration of 8 mg in 8 healthy male volunteers significantly reduced naloxone-induced withdrawal symptoms in 7 of them.4 A case report indicated that oral 8 mg administered twice a day eliminated oxycodone-induced withdrawal symptoms in a 44 year-old female.25 A preliminary controlled clinical trial in adults suggested that oral administration of 4 mg of ondansetron twice a day is promising for treating cocaine dependence.26
Ondansetron is not approved for use in pediatric patients younger than one-month old. The dosing regimen described in the package insert for treatment of post-operative nausea and vomiting in one-month old patients is a single IV 0.1 mg/kg. Assuming an adult dose for dependence treatment of 8 mg twice a day,25 the equivalent exposure in neonates, based on our model, will be achieved by combined administration of IV 8 mg to the mothers before labor and external administration of doses ranging between 0.07 and 0.1 mg/kg in newborns. This dose is higher than the labeled dosing for older patients closest in age to the neonates. Shifting to an adult dose of 4 mg twice a day26 resulted in a higher safety margin for neonates.
Age-related differences in gastric pH, gastrointestinal motility, bile salts, pancreatic function, intestinal pH, and intestinal drug-metabolizing enzymes and transporters result in differences in absorption rate and extent between adults and newborns.27 This should be more evident for drugs suffering first-pass metabolism like ondansetron. Accordingly, setting the absorption rate and bioavailability in neonates to be equal to that in adults is a simplification that may influence the validity of predictions following oral administration in neonates. However, there is no straightforward and accurate method for scaling absorption parameters from adults to newborns. This is usually done through complex PBPK models, which are beyond the scope of this work.
Because external ondansetron dose was not administered to neonates, it was not possible to uniquely identify clearance or volume of distribution. However, the assumption of equal distribution volumes in neonates and 1–48 month old infants is valid, since the birth weight of our neonates is in range of the weights studied by Mondick et al.13
Assessing ondansetron pharmacokinetics in women at the time of cesarean delivery and following delivery would have reduced the inter-subject variability and better served one of the purposes of this study which is showing the effect of pregnancy. However, this study had been designed to follow the standard of care protocol developed by Stanford University Hospitals and Clinics for patients undergoing surgical operations.
It is possible that mothers who consume opioids metabolized by cytochrome P450 will exhibit different ondansetron pharmacokinetic profiles compared to opioid free women examined in this study, due to ondansetron/opioid interaction. Because of underdeveloped hepatic enzymatic activity in neonates, it is very unlikely that this will be the case in the off-spring of both populations. However, the dose regimens rationalized by our model need to be evaluated in neonatal populations at risk of NAS development before they get approved in clinical practice.
In conclusion, we have found that pregnancy does not affect the pharmacokinetics of ondansetron. We found that ondansetron rapidly crosses the placental barrier, but it undergoes significantly slower neonatal clearance in the first day of life. The pharmacokinetic information and model developed here enables a dosing regimen for ondansetron to be developed, which can be used to prevent the development of NAS in babies born to mothers who consume narcotics.
METHODS
Patients, ondansetron dosing, and sampling
Following approval of the Institutional Review Board (IRB) at Stanford University, healthy nonpregnant women undergoing routine elective surgery and pregnant women undergoing elective cesarean surgery and their neonates were admitted to this prospective, open label study. Informed consent was obtained at the time of anesthesia evaluation. Informed consent for the neonates was obtained from the mother, and from both parents when possible.
Before intravenous administration of 4 or 8 mg of ondansetron, blood samples were taken from all of the women. Samples were taken once more 7, 15, and 40 minutes, and 8 hours after the drug was administered. A randomized design determined in advance of the study was employed for ondansetron dose assignment. The umbilical cord was sampled at time of delivery. After collection of blood samples, immediate centrifugation was performed and the plasma was separated and frozen for batched assay. Post-natal dried blood spots (DBS) were obtained from the neonates at 30 and 90 minutes, and 2, 6, and 24 hours after birth using a heel prick for capillary blood, in accordance with the Clinical and Laboratory Standards Institute’s (CLSI) newborn screening guidelines.28
Analytical Method
Analysis of ondansetron in human plasma used in this study has been published29 with modification.
Chemicals and Reagents
Ondansetron and the internal standard ondansetron-D,3 were purchased from Toronto Research Chemical, Inc. (Toronto, Ontario, Canada). High-performance, liquid-chromatography- (HPLC) grade water, methanol, acetonitrile, and dimethyl sulfoxide (DMSO) were used for sample extraction and as the mobile phase (Fisher Scientific, Fairlawn, NJ). Standard stock solutions of ondansetron 1 mg/mL were prepared by dissolving the pure compound in DMSO. Stock solutions were stored at −20°C. Working standard solutions of the ondansetron were prepared by diluting three-fold ondansetron 0.4 mg/mL stock solution with DMSO.
Preparation of calibration standards and quality control samples
Human K2EDTA plasma, and DBS calibration, and quality control standards of ondansetron were prepared by spiking the 2.5-µL working standard solution of ondansetron into 50 µL of drug-free human EDTA plasma or blood (1:20) [v/v].
Plasma sample preparation
The plasma samples were prepared by mixing each sample with 300 µL of acetonitrile containing the internal standard ondansetron-D3 at a concentration of 100 ng/mL. The samples were then vortexed for 2.5 min and centrifuged (4°C, 13,000 g, 5 min). After centrifugation, 100 µL of supernatants were transferred into a 96-well plate. The well plates contained 100 µL of HPLC water. The standard curve range was from 0.338 ng/mL to 741 ng/mL.
DBS sample preparation
For each DBS sample, 50 µL of spiked blood was transferred on Whatman 903 filter paper cards. After drying for 1 hour, the DBS were punched (6.4 mm in diameter, containing 20 µL blood) and reconstituted with 100 µL of HPLC-grade water. Then, 500 µL of the protein precipitation solution, methanol 0.2 M ZnSO4 (7:3, v/v) containing the internal standard ondansetron-D3 at concentrations of 50 and 10 ng/mL, was added to the ondansetron samples. The samples were vortexed for 2.5 min and centrifuged (4°C, 8000g, 5min). After centrifugation, the supernatants were transferred into HPLC vials. The calibration curve range for ondansetron was from 0.338 ng/mL to 82.3 ng/mL.
HPLC-MS/MS Analysis
The extracts were analyzed using an LC-MS/MS system. First, 10 µL of the sample supernatant was injected onto an analytical column Phenomenex (Torrance CA) PFP 2.6 u 100×4.6 mm. A gradient was then run from 95% solvent A to 95% solvent B over 2 min. The mobile phase was solvent A: 0.1 % formic acid in acetonitrile and solvent B: 0.1% formic acid in water. The flow was 1 mL/min with a 5.0-min runtime. An Agilent (Santa Clara, CA) 1100 Series HPLC system was interfaced with the AB Sciex (Framingham, MA) API5000 tandem quadrupole mass spectrometer using a positive ESI source. The mass spectrometer was run using positive multiple reaction monitoring (MRM). For ondansetron, the following ion transition was monitored: 294.3 [M+H]+ → 170.0. For the ondansetron D3 internal standard, the following ion transition was monitored: 297.4 [M+H]+ → 173.3. The total run time was 5 min. The HPLC and the mass spectrometer were controlled using Analyst Software (version 1.4.1).
Population Pharmacokinetic (PK) Analysis
Modeling strategy
A PK model was developed to simultaneously analyze ondansetron concentration-time data in the blood from nonpregnant and pregnant women, the umbilical cord, and neonates. A two-compartment linear disposition model was used to describe the data obtained from the women. The model determined parameters were total clearance (CL), inter-compartmental clearance (Q), central (V), and steady-state (VSS) volume of distribution. A linear model best described the relationship between ondansetron concentrations in cord blood (CUC) and in maternal blood (CMAT,1):
| (1) |
where KP is the placenta-to-blood partition coefficient. The model in equation 1 is based on the assumption that the umbilical cord compartment (fetal unit) is in equilibrium with the maternal central compartment. After delivery, the fetal compartment was disconnected from the maternal model and developed to a neonatal one-compartment model, with the distinct first-order elimination pathway KN0:
| (2) |
where CN is ondansetron neonatal concentration and Tdel is delivery time, t’ is time since birth (t’ = t−Tdel), and + is a truncation sign (+ = t−Tdel for t ≥ Tdel and 0 otherwise).
The model’s analytical solutions were specified in the $PRED section of the nonlinear mixed-effects modeling software program NONMEM (version VII; Icon Development Solutions, Ellicott City, MD). The first-order conditional estimation (FOCE) with η-ε interaction was used to estimate the model’s parameters. The convergence criterion was determined to be three significant digits.
Under the assumption that the PK parameters are log-normally distributed, an exponential model was used to represent the inter-individual variability. A diagonal variance-covariance matrix was used. Log-transformation of plasma concentrations was performed before model fitting, and an additive model of residual (intra-individual) variability for PK observations was used. Discrimination between inter- and intra-subject random effects was not possible for cord data since only one observation was available per individual. Therefore, we only used residual variability.
A sequential approach was adopted to search for the best base model. We initially evaluated the one- and two-compartment models as structural models using the plasma concentrations from the women. Then, we obtained post hoc PK parameter estimates for each mother and used them to compute subject-specific predictions of ondansetron concentrations over the entire time range. Based on those maternal predictions, a linear model (assumes that equilibrium is rapidly achieved between the maternal central compartment and fetal unit) and effect-compartment model (the fetal unit acts as a virtual compartment that does not affect the maternal mass balance equations) were evaluated as structural models for the cord blood PK data. Akaike information criterion (AIC), and relative standard error (%SE) of the estimate were used for selection between rival models. AIC was computed as the NONMEM minimum objective function (−2 log likelihood) value (OFV) plus two times the number of model-estimated parameters. %SE was calculated as the percentage of NONMEM $COVARIANCE step; standard error to the parameter estimate.
We investigated the effect of pregnancy, dosing level, women’s weight, neonatal birth weight, women’s age, gestational age, and neonatal gender on maternal total clearance and volume parameters, as well as on neonatal elimination rate constant using stepwise forward addition and likelihood ratio testing at significance level of 5%. Linear, exponential, or power functions were used to model the relationship between a continuous covariate and a PK parameter. The covariate was centered or normalized using the corresponding mean value in the population investigated. Relationship between a categorical covariate and a PK parameter was developed to get a particular parameter estimate for every cluster. The effect of pregnancy was investigated as a categorical covariate, and as a continuous relationship with gestational age (GA):
| (3) |
where PREG is 0 for nonpregnant women and is 1 for pregnant women.
Model evaluation
Standard goodness-of-fit plots including observed versus predicted concentrations, observed and predicted concentrations versus time, and conditional weighted residuals versus population predictions and time were used as primary model diagnostics. Nonsymmetrical 95% confidence interval (CI) from log-likelihood profiling, using Perl-speaks-NONMEM30 was used to assess the precision of the final model parameters. This approach, unlike the symmetrical, standard-error-based method, does not assume normal distribution of the parameters.31
Ability of the final model to predict the distribution of measured ondansetron concentrations was evaluated through visual predictive check (VPC). The approach adopted in this work was the confidence interval VPC as described by Karlsson and Holford.32 One-thousand replicates of a dataset having the same number of subjects, sampling times, delivery times, and dosing levels as the observed dataset were simulated using the final model. To calculate summary statistics and graph the results, we stratified the observed and simulated plasma concentrations from the women by pregnancy status and dosing level. Owing to sparse data, stratification of the observed cord and neonatal concentrations was not applicable, so we used prediction-corrected VPC.33 Unlike standard VPC methods, observations and simulations are normalized based on the median of typical population predictions for specific time bin.
Simulation
A simulation study was performed to recommend a dosing regimen for prevention of NAS development. We hypothesized that an effective dose for preventing the development of NAS in neonates would produce an exposure similar to that obtained when 4 mg of ondansetron is administered orally twice a day in adults.
Our developed model in adults was adjusted by inclusion of a first-order absorption route. The bioavailability was set to 62%, which corresponds to the mean absolute bioavailability for 8 mg of ondansetron administered to adults in three previous studies,10, 34, 35 weighted by the number of subjects. The absorption rate constant, 1.05 L/h, was taken from a study of ondansetron absorption following administration of 8 mg of solution in six healthy adults,35
Three administration modes were considered in neonates: 1) maternal administration of IV dose prior to cord clamping, 2) external administration of an oral dose to neonates, and 3) external administration of an IV bolus dose to neonates. The first administration mode depends on transplacental transfer for delivering the drug load to neonates (as considered in our developed model, Eqs. 1 and 2). The external administration modes are based on the assumption that ondansetron disposition in neonates follows a one-compartment model with a first-order elimination rate constant, KN0. Absorption parameters were set to values equal to that in adults, as explained on the previous paragraph. The volume of distribution for the external administration in neonates was set to 3.5 L/kg (steady-state distribution volume in 1–48 month old infants13).
For each group, plasma time-concentration profiles were simulated in 1000 virtual subjects using the fixed-effects, intra-, and inter-subject variability parameter estimates of the final population model. Different doses and maternal administration times before delivery were examined. Values for AUC0→24 h were calculated for each subject and their distributions were compared between adults and neonates.
Figure 3.
Conditional weighted residuals (CWRES) versus population predicted concentrations (left panel) and time (right panel) for the final ondansetron pharmacokinetic model in non-pregnant women (a and b), pregnant women (c and d), cord blood (e and f), and neonates (g and h). The solid line is the zero line. The dashed line is a lowess smother.
STUDY HIGHLIGHTS.
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
Ondansetron is the drug of choice to prevent nausea and vomiting in surgical patients, including women undergoing cesarean surgery. Ondansetron is effective in preventing narcotic drug withdrawal symptoms in mice and humans, suggesting that it can potentially be used to prevent NAS. Pharmacokinetics of ondansetron has not been characterized in pregnant women or in newborns.
WHAT QUESTION DID THIS STUDY ADDRESS?
We characterized the pharmacokinetics of ondansetron in non-pregnant, and pregnant women, and in neonates, as well as its trans-placental passage. Our ultimate goal was to derive an optimal dose for prevention of NAS.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE?
The study indicates that: (1) ondansetron disposition is not affected by pregnancy; (2) dose is the most important covariate affecting its pharmacokinetics; and (3) ondansetron readily crosses the placenta, but (4) has a significantly longer elimination half-life in neonates compared to their mother.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS?
Ondansetron dose does not need to be altered during pregnancy. We propose a dosing regimen for prevention of NAS.
ACKNOWLEDGEMENT
This study was funded by a grant from the NICHD (Eunice Kennedy Shriver National Institute of Child Health and Human Development) 1 R01 HD070795-01A1. The authors would like to thank Carol Cohane, RN (research nurse) for recruitment and study related work.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
M.H.E., G.P., and D.R.D. wrote the manuscript.
D.R.D., G.P., and B.C. designed research.
P.S., M.W., C.C., J.L.G. and M.H.E. performed research.
M.H.E. analyzed data.
M.W., C.C. and J.L.G. contributed new reagents/analytical tools.
REFERENCES
- 1.Hsu ES. A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics. Am J Ther. 2010;17:476–486. doi: 10.1097/MJT.0b013e3181ea7821. [DOI] [PubMed] [Google Scholar]
- 2.Pan PH, Moore CH. Comparing the efficacy of prophylactic metoclopramide, ondansetron, and placebo in cesarean section patients given epidural anesthesia. J Clin Anesth. 2001;13:430–435. doi: 10.1016/s0952-8180(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 3.George RB, Allen TK, Habib AS. Serotonin receptor antagonists for the prevention and treatment of pruritus, nausea, and vomiting in women undergoing cesarean delivery with intrathecal morphine: a systematic review and meta-analysis. Anesth Analg. 2009;109:174–182. doi: 10.1213/ane.0b013e3181a45a6b. [DOI] [PubMed] [Google Scholar]
- 4.Chu LF, et al. From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics. 2009;19:193–205. doi: 10.1097/FPC.0b013e328322e73d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307:1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 6.Paulozzi L. The epidemiology of unintentional drug poisoning in the United States. 2007 [Google Scholar]
- 7.Maas U, Kattner E, Weingart-Jesse B, Schafer A, Obladen M. Infrequent neonatal opiate withdrawal following maternal methadone detoxification during pregnancy. J Perinat Med. 1990;18:111–118. doi: 10.1515/jpme.1990.18.2.111. [DOI] [PubMed] [Google Scholar]
- 8.Seligman NS, Salva N, Hayes EJ, Dysart KC, Pequignot EC, Baxter JK. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol. 2008;199:396, e1–e7. doi: 10.1016/j.ajog.2008.06.088. [DOI] [PubMed] [Google Scholar]
- 9.Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2010;106:574–580. doi: 10.1111/j.1360-0443.2010.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colthup PV, Felgate CC, Palmer JL, Scully NL. Determination of ondansetron in plasma and its pharmacokinetics in the young and elderly. J Pharm Sci. 1991;80:868–871. doi: 10.1002/jps.2600800913. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard JF, Bryson JC, Kernodle AE, Benedetti TL, Powell JR. Age and gender effects on ondansetron pharmacokinetics: evaluation of healthy aged volunteers. Clin Pharmacol Ther. 1992;51:51–55. doi: 10.1038/clpt.1992.7. [DOI] [PubMed] [Google Scholar]
- 12.Spahr-Schopfer IA, Lerman J, Sikich N, Palmer J, Jorch U. Pharmacokinetics of intravenous ondansetron in healthy children undergoing ear, nose, and throat surgery. Clin Pharmacol Ther. 1995;58:316–321. doi: 10.1016/0009-9236(95)90248-1. [DOI] [PubMed] [Google Scholar]
- 13.Mondick JT, et al. Population pharmacokinetics of intravenous ondansetron in oncology and surgical patients aged 1–48 months. Eur J Clin Pharmacol. 2010;66:77–86. doi: 10.1007/s00228-009-0730-8. [DOI] [PubMed] [Google Scholar]
- 14.Saynor DA, Dixon CM. The metabolism of ondansetron. Eur J Cancer Clin Oncol. 1989;25(Suppl 1):S75–S77. [PubMed] [Google Scholar]
- 15.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 16.Morselli PL, Francomorselli R, Bossi L. Clinical Pharmacokinetics in Newborns and Infants - Age-Related Differences and Therapeutic Implications. Clinical Pharmacokinetics. 1980;5:485–527. doi: 10.2165/00003088-198005060-00001. [DOI] [PubMed] [Google Scholar]
- 17.Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45:683–704. doi: 10.2165/00003088-200645070-00004. [DOI] [PubMed] [Google Scholar]
- 18.Stevens JC, et al. Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos. 2008;36:1587–1593. doi: 10.1124/dmd.108.021873. [DOI] [PubMed] [Google Scholar]
- 19.Sonnier M, Cresteil T. Delayed ontogenesis of CYP1A2 in the human liver. Eur J Biochem. 1998;251:893–898. doi: 10.1046/j.1432-1327.1998.2510893.x. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard JF. Ondansetron metabolism and pharmacokinetics. Semin Oncol. 1992;19:9–15. [PubMed] [Google Scholar]
- 21.Roila F, Del Favero A. Ondansetron clinical pharmacokinetics. Clin Pharmacokinet. 1995;29:95–109. doi: 10.2165/00003088-199529020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Gan LS, Hsyu PH, Pritchard JF, Thakker D. Mechanism of intestinal absorption of ranitidine and ondansetron: transport across Caco-2 cell monolayers. Pharm Res. 1993;10:1722–1725. doi: 10.1023/a:1018965929419. [DOI] [PubMed] [Google Scholar]
- 23.Dixon CM, et al. Multiple forms of cytochrome P450 are involved in the metabolism of ondansetron in humans. Drug Metab Dispos. 1995;23:1225–1230. [PubMed] [Google Scholar]
- 24.Colthup PV, Palmer JL. The determination in plasma and pharmacokinetics of ondansetron. Eur J Cancer Clin Oncol. 1989;25(Suppl 1):S71–S74. [PubMed] [Google Scholar]
- 25.Wakim JH. Alleviating symptoms of withdrawal from an opioid. Pain Ther. 2012;1:4. doi: 10.1007/s40122-012-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson BA, et al. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of cocaine dependence. Drug Alcohol Depend. 2006;84:256–263. doi: 10.1016/j.drugalcdep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Mooij MG, de Koning BA, Huijsman ML, de Wildt SN. Ontogeny of oral drug absorption processes in children. Expert Opin Drug Metab Toxicol. 2012;8:1293–1303. doi: 10.1517/17425255.2012.698261. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute (CLSI) Blood Collection on Filter Paper for Newborn Screening Programs, Approved Standard. Fifth Edition. Wayne, Pa: Clinical and Laboratory Standards Institute; 2007. [Google Scholar]
- 29.Chandrasekar D, Ramakrishna S, Diwan PV. A rapid, sensitive and validated method for the determination of ondansetron in human plasma by reversed-phase high-pressure liquid chromatography. Arzneimittelforschung. 2004;54:655–659. doi: 10.1055/s-0031-1297017. [DOI] [PubMed] [Google Scholar]
- 30.Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit--a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Sheiner LB. Analysis of pharmacokinetic data using parametric models. III. Hypothesis tests and confidence intervals. J Pharmacokinet Biopharm. 1986;14:539–555. doi: 10.1007/BF01059660. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson MO, Holford NH. A tutorial on visual predictive checks (Abstr 1434) 2008 < http://www.page-meeting.org/pdf_assets/8694-Karlsson_Holford_VPC_Tutorial_hires.pdf>. [Google Scholar]
- 33.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baber N, Palmer JL, Frazer NM, Pritchard JF. Clinical pharmacology of ondansetron in postoperative nausea and vomiting. Eur J Anaesthesiol Suppl. 1992;6:11–18. [PubMed] [Google Scholar]
- 35.Hsyu PH, et al. Comparison of the pharmacokinetics of an ondansetron solution (8 mg) when administered intravenously, orally, to the colon, and to the rectum. Pharm Res. 1994;11:156–159. doi: 10.1023/a:1018974501232. [DOI] [PubMed] [Google Scholar]