Abstract
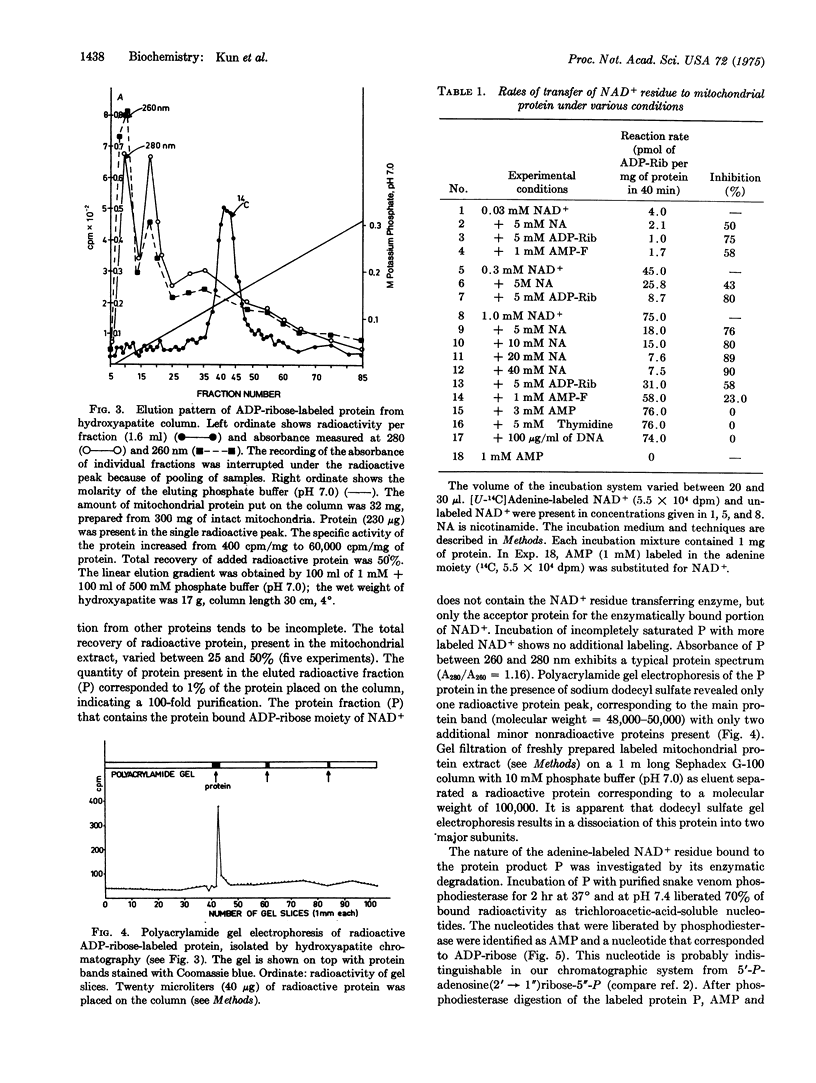
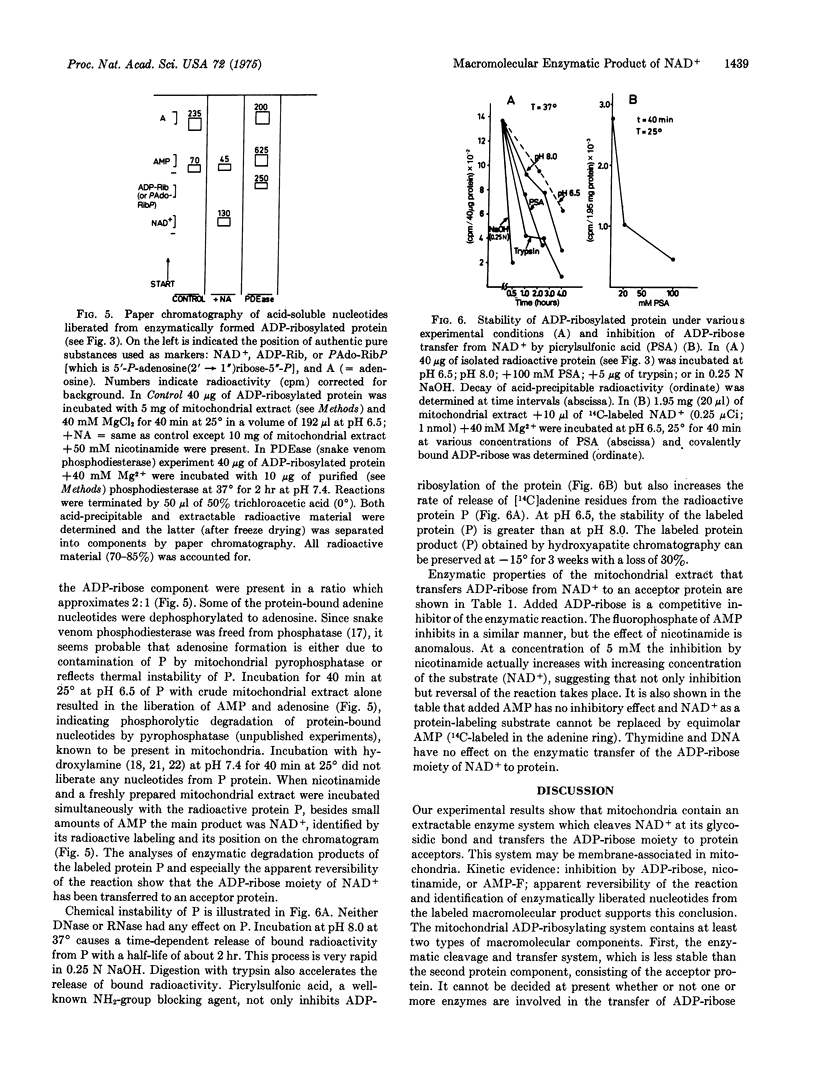
Rat liver mitochondria contain a Mg2+-requiring system that transfers the ADP-ribose moiety of NAD+ to an acceptor protein. The enzyme system was extracted in a soluble form and the ADP-ribosylated protein product was isolated by hydroxyapatite and Sephadex chromatography. The ADP-ribosylated protein product has a molecular weight of 100,000 and can be dissociated into subunits of 50,000 daltons by sodium dodecyl sulfate gel electrophoresis. Incubation of the isotopically labeled ADP-ribosylated protein with nicotinamide and a mitochondrial extract yields labeled NAD+, indicating apparent reversibility of the reaction. Enzymatic degradation of the ADP-ribosylated protein with snake venom phosphodiesterase liberates AMP and ADP-ribose or its isomer. Identification of these products and reversibility of the reaction show that the ADP-ribose moiety of NAD+ is the molecular species that is transferred to the acceptor protein. A fraction of the protein-bound ADP-ribose appears to be present as an an oligomer. The enzymatic protein-ADP-ribosylating reaction is inhibited by nicotinamide, ADP-ribose, the fluorophosphate of AMP, and picrylsulfonic acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamietz P., Bredehorst R., Oldekop M., Hilz H. Nuclear poly (ADPR) and mono (ADPR) residues in tissues with different growth rates. FEBS Lett. 1974 Aug 1;43(3):318–322. doi: 10.1016/0014-5793(74)80670-8. [DOI] [PubMed] [Google Scholar]
- Barath Z., Küntzel H. Cooperation of mitochondrial and nuclear genes specifying the mitochondrial genetic apparatus in Neurospora crassa. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1371–1374. doi: 10.1073/pnas.69.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzio L., Koide S. S. A functional role of polyADPR in DNA synthesis. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1013–1020. doi: 10.1016/0006-291x(70)90894-6. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Uchida T. Diphtheria toxin, protein synthesis, and the cell. Fed Proc. 1973 Apr;32(4):1508–1515. [PubMed] [Google Scholar]
- Goor R. S., Maxwell E. S. A proposed mechanism for ADP ribosylation of aminoacyl transferase II by diphtheria toxin. Cold Spring Harb Symp Quant Biol. 1969;34:609–610. doi: 10.1101/sqb.1969.034.01.070. [DOI] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Adenosine diphosphoribosylation of aminoacyl transferase II by diphtheria toxin. Cold Spring Harb Symp Quant Biol. 1969;34:603–608. doi: 10.1101/sqb.1969.034.01.069. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Kun E. Inhibition of the oxidation of glutamate and isocitrate in liver mitochondria at a specific NADP+-reducing site. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3450–3453. doi: 10.1073/pnas.70.12.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Yoshihara K., Yamamura H., Takeda M., Hayaishi O. Enzymic adenosine diphosphoribosylation of nuclear proteins. Cold Spring Harb Symp Quant Biol. 1969;34:781–786. doi: 10.1101/sqb.1969.034.01.088. [DOI] [PubMed] [Google Scholar]
- Roberts J. H., Stark P., Smulson M. Poly(ADP-ribose): release of template restriction in HeLa cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3212–3216. doi: 10.1073/pnas.71.8.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. A., Henriksen O., Maxwell E. S. Elongation factor 2. Amino acid sequence at the site of adenosine diphosphate ribosylation. J Biol Chem. 1974 Aug 25;249(16):5088–5093. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. R., Shall S. Poly(adenosine diphosphoribose) polymerase in mammalian nuclei. Characterization of the activity in mouse fibroblasts (LS cells). Eur J Biochem. 1973 Sep 21;38(1):146–152. doi: 10.1111/j.1432-1033.1973.tb03044.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yoshihara K., Tanigawa Y., Koide S. S. Inhibition of rat liver Ca2+, Mg2+-dependent endonuclease activity by nicotinamide adenine dinucleotide and poly (adenosine diphosphate ribose) synthetase. Biochem Biophys Res Commun. 1974 Jul 24;59(2):658–665. doi: 10.1016/s0006-291x(74)80030-6. [DOI] [PubMed] [Google Scholar]


