Abstract
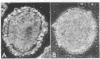
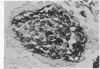
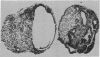
The differentiation in vitro of clonal pluripotent teratocarcinoma cells is reported. The first stage of this process is the formation of simple embryoid bodies which are identical to those found in animals bearing intraperitoneal teratocarcinomas. They consist of an inner core of embryonal carcinoma cells surrounded by a layer of endodermal cells which produce Reichert's membrane. The endodermal cells become apparent shortly after the embryonal carcinoma cells have formed aggregates which are loosely attached to the substratum. One clonal teratocarcinoma line was found to produce complex cystic embryoid bodies in vitro. Following formation of the endodermal cells, extensive differentiation to a wide variety of cell types occurs. There are similarities between the process of embryoid body formation and the early events of differentiation of the mouse embryo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Dubois P., Bennett D., Condamine H., Babinet C., Jacob F. Surface antigens common to mouse cleavage embryos and primitive teratocarcinoma cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2988–2992. doi: 10.1073/pnas.70.10.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanov I., Solter D., Belicza M., Skreb N. Teratomas obtained through extrauterine growth of seven-day mouse embryos. J Natl Cancer Inst. 1971 Mar;46(3):471–passim. [PubMed] [Google Scholar]
- Damjanov I., Solter D., Skreb N. Enzyme histochemistry of experimental embryo-derived teratocarcinomas. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1971;76(4):249–256. doi: 10.1007/BF00304029. [DOI] [PubMed] [Google Scholar]
- Evans M. J. The isolation and properties of a clonal tissue culture strain of pluripotent mouse teratoma cells. J Embryol Exp Morphol. 1972 Aug;28(1):163–176. [PubMed] [Google Scholar]
- Gearhart J. D., Mintz B. Contact-mediated myogenesis and increased acetylcholinesterase activity in primary cultures of mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1734–1738. doi: 10.1073/pnas.71.5.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert M. C., Graham C. F. Cell determination and biochemical differentiation of the early mammalian embryo. Curr Top Dev Biol. 1974;8:151–178. doi: 10.1016/s0070-2153(08)60608-0. [DOI] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jami J., Ritz E. Multipotentiality of single cells of transplantable teratocarcinomas derived from mouse embryo grafts. J Natl Cancer Inst. 1974 May;52(5):1547–1552. doi: 10.1093/jnci/52.5.1547. [DOI] [PubMed] [Google Scholar]
- KLEINSMITH L. J., PIERCE G. B., Jr MULTIPOTENTIALITY OF SINGLE EMBRYONAL CARCINOMA CELLS. Cancer Res. 1964 Oct;24:1544–1551. [PubMed] [Google Scholar]
- Kahan B. W., Ephrussi B. Developmental potentialities of clonal in vitro cultures of mouse testicular teratoma. J Natl Cancer Inst. 1970 May;44(5):1015–1036. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Torosian M., Sarokhan A. J., Teresky A. K. Biochemical criteria for the in vitro differentiation of embryoid bodies produced by a transplantable teratoma of mice. The production of acetylcholine esterase and creatine phosphokinase by teratoma cells. J Cell Physiol. 1974 Oct;84(2):311–317. doi: 10.1002/jcp.1040840217. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Evans M. J. The morphology and growth of a pluripotent teratocarcinoma cell line and its derivatives in tissue culture. Cell. 1974 Jul;2(3):163–172. doi: 10.1016/0092-8674(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Rubin H. Effects of cell adhesion of the substratum on the growth of chick embryo fibroblasts. Exp Cell Res. 1974 Apr;85(2):319–333. doi: 10.1016/0014-4827(74)90133-5. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D. The organization center of the amphibian embryo: its origin, spatial organization, and morphogenetic action. Adv Morphog. 1973;10:1–39. doi: 10.1016/b978-0-12-028610-2.50005-8. [DOI] [PubMed] [Google Scholar]
- PIERCE G. B., DIXON F. J., Jr Testicular teratomas. I. Demonstration of teratogenesis by metamorphosis of multipotential cells. Cancer. 1959 May-Jun;12(3):573–583. doi: 10.1002/1097-0142(195905/06)12:3<573::aid-cncr2820120316>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- PIERCE G. B., Jr, DIXON F. J., Jr, VERNEY E. L. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab Invest. 1960 Nov-Dec;9:583–602. [PubMed] [Google Scholar]
- PIERCE G. B., Jr, MIDGLEY A. R., Jr, RAM J. S., FELDMAN J. D. Pariental yolk sac carcinoma: clue to the histogenesis of Riechert's membrane of the mouse embryo. Am J Pathol. 1962 Nov;41:549–566. [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M. D., Wishnow R. M., Sato G. H. In vitro growth and differetiation of clonal populations of multipotential mouse clls derived from a transplantable testicular teratocarcinoma. J Natl Cancer Inst. 1970 May;44(5):1001–1014. [PubMed] [Google Scholar]
- STEVENS L. C. Embryology of testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1959 Dec;23:1249–1295. [PubMed] [Google Scholar]
- STEVENS L. C. Studies on transplantable testicular teratomas of strain 129 mice. J Natl Cancer Inst. 1958 Jun;20(6):1257–1275. doi: 10.1093/jnci/20.6.1257. [DOI] [PubMed] [Google Scholar]
- Solter D., Biczysko W., Pienkowski M., Koprowski H. Ultrastructure of mouse egg cylinders developed in vitro. Anat Rec. 1974 Oct;180(2):263–279. doi: 10.1002/ar.1091800202. [DOI] [PubMed] [Google Scholar]
- Solter D., Damjanov I., Skreb N. Distribution of hydrolytic enzymes in early rat and mouse embryos--a reappraisal. Z Anat Entwicklungsgesch. 1973 Mar 20;139(2):119–126. doi: 10.1007/BF00523633. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Gierthy J. F., Cholon J. J. An autoradiographic screening test for mycoplasmal contamination of mammalian cell cultures. In Vitro. 1973 May-Jun;8(6):466–472. doi: 10.1007/BF02615948. [DOI] [PubMed] [Google Scholar]
- Tarkowski A. K., Wróblewska J. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morphol. 1967 Aug;18(1):155–180. [PubMed] [Google Scholar]
- Teresky A. K., Marsden M., Kuff E. L., Levine A. J. Morphological criteria for the in vitro differentiation of embryoid bodies produced by a transplantable teratoma of mice. J Cell Physiol. 1974 Oct;84(2):319–332. doi: 10.1002/jcp.1040840218. [DOI] [PubMed] [Google Scholar]
- Ware L. M., Axelrad A. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: evidence that congenic mouse strains SIM and SIM.R differ at the Fv-1 locus. Virology. 1972 Nov;50(2):339–348. doi: 10.1016/0042-6822(72)90385-6. [DOI] [PubMed] [Google Scholar]