Abstract
Strongyloides venezuelensis is a parasitic nematode of rodents frequently used to obtain heterologous antigens for the immunological diagnosis of human strongyloidiasis. The aim of this study was to evaluate membrane fractions from S. venezuelensis for human strongyloidiasis immunodiagnosis. Soluble and membrane fractions were obtained in phosphate saline (SS and SM) and Tris-HCl (TS and TM) from filariform larvae of S. venezuelensis. Ninety-two serum samples (n = 92) were obtained from 20 strongyloidiasis patients (Group I), 32 from patients with other parasitic diseases (Group II), and 40 from healthy individuals (Group III), and were analyzed by enzyme-linked immunosorbent assay (ELISA). Soluble fractions (SS and TS) showed 90.0% sensitivity and 88.9% specificity, whereas the membrane fractions (SM and TM) showed 95.0% sensitivity and 94.4% specificity. The present results suggest the possible use of membrane fractions of S. venezuelensis as an alternative antigen for human strongyloidiasis immunodiagnosis.
Keywords: Strongyloides venezuelensis, Parasitological diagnosis, Immunological diagnosis, Membrane fractions
Abstract
Strongyloides venezuelensis é um nematódeo parasita de roedores, frequentemente usado como antígeno heterólogo para o diagnóstico imunológico da estrongiloidíase humana. O objetivo deste estudo foi avaliar frações de membrana de S. venezuelensis para o imunodiagnóstico da estrongiloidíase humana. Para tanto, frações solúveis e de membrana foram obtidas em solução salina fosfato (SS e MS) e Tris-HCl (ST e MT) de larvas filarioides de S. venezuelensis. Amostras de soro de 92 indivíduos, sendo 20 com estrongiloidíase (Grupo I); 32 com outras parasitoses (Grupo II), e 40 indivíduos saudáveis (Grupo III), foram analisadas pelo teste Imunoenzimático (ELISA). As frações solúveis (SS e ST) apresentaram 90,0% e 88,9%, enquanto que as frações de membrana (MS e MT) demonstraram 95,0% e 94,4%, de sensibilidade e especificidade, respectivamente. Os resultados obtidos permitem indicar as frações de membranas como antígeno alternativo para o diagnóstico da estrongiloidíase humana.
Strongyloidiasis is a human intestinal infection caused by Strongyloides stercoralis, which affects between 30-100 million people in the world2, and is endemic in tropical and subtropical regions15. In immunocompetent hosts it is often an asymptomatic infection. However, immunocompromised hosts may develop a fatal hyperinfection syndrome or disseminated strongyloidiasis9,17.
Strongyloidiasis diagnosis usually depends on the identification of larvae in stool samples9. However, parasitological diagnosis presents low sensitivity, due to an intermittent larval shedding19. Immunological methods, such as enzyme-linked immunosorbent assay (ELISA), are an alternative for the diagnosis of strongyloidiasis3, especially using heterologous antigenic extracts from Strongyloides venezuelensis 4,5,6. The development of more accurate immunological methods, using purified parasitic antigens, can improve the diagnostic sensitivity and specificity4,5,7. The surface of parasitic nematodes has been shown to be antigenic in many infected hosts12, but membrane antigens have been underexplored. The aim of this study was to verify the use of soluble and membrane antigen extracts obtained from filariform larvae of S. venezuelensis for human strongyloidiasis immunodiagnosis.
Serum samples were obtained at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP) from 92 individuals: 20 patients harboring S. stercoralis larvae (Group I); 32 patients with other parasites (Group II) [Schistosoma mansoni (n = 9), hookworm (n = 4), Ascaris lumbricoides (n = 2), Hymenolepis nana (n = 1), Enterobius vermicularis (n = 1), Giardia lamblia (n = 3); Endolimax nana (n = 3), Blastocystis sp. (n = 3) and six poly-infected samples (S. mansoni, A. lumbricoides, E. coli, Blastocystis sp. and E. nana/hookworm and H. nana/hookworm, S. mansoni, E. coli and E. histolytica/E.dispar/G. intestinalis and E. nana/A. lumbricoides and Blastocystis sp./S. mansoni, E. nana and Blastocystis sp.)]; and 40 apparently healthy individuals based on their clinical observation, without evidence of contact with S. stercoralis infection or previous history of strongyloidiasis (Group III). All feces samples were analyzed by the LUTZ method11, a gravity sedimentation technique, the RUGAI method18, based on positive larval termo-hydrotropism, and agar plate culture method16, by the observation of larvae tracks over the agar. Due to the difficulty of obtaining three or more stool samples, a decision was made to analyze a single sample using more sensitive techniques for the detection of S. stercoralis larvae, such as Rugai and culture on an agar plate. It has been shown that the combination of these two methods facilitates detection of 95% of infections caused by S. stercoralis when only one sample is analyzed16. The study received approval from the Research Ethics Committee of Universidade de São Paulo, state of São Paulo, Brazil (protocol 266.046).
For antigenic extraction, S. venezuelensis filariform larvae (L3) were obtained from charcoal cultures of feces of experimentally infected Rattus norvegicus (Wistar), protocol (CPE-IMT 2011/126). L3 PBS (phosphate-buffered saline, 0.01M pH 7.2) or Tris-HCl 25mM pH 7.5, containing protease inhibitors (Sigma-Aldrisch, St. Louis, MO, USA) were added to 400,000 samples and disrupted in an ice bath using a tissue homogenizer for five cycles of five minutes each. The suspensions were centrifuged at 12,400g for 30 minutes at 4 °C and the supernatant was collected (soluble fractions SS and TS, PBS and Tris-HCl, respectively). SS pellets were resuspended in 1% SDS, heated to 100 °C for five minutes, centrifuged at 12,400g for 30 minutes at 4 °C and the supernatant was collected (membrane fraction SM). ST pellets were resuspended in 5M urea, 2M thiourea and 4% CHAPS; disrupted in an ice bath using a tissue homogenizer for five cycles of five minutes; centrifuged at 12,400g for 30 minutes at 4 °C and the supernatant was collected (membrane fraction TM). All fractions were analyzed for protein content according to LOWRY et al. 10, subdivided into aliquots and stored at -20 °C until use.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by LAEMMLI8. Fractions were submitted to electrophoresis using a 12% acrylamide separation gel analyzed by silver nitrate.
ELISA was performed according to COSTA-CRUZ et al. 3 with modifications using the four obtained fractions. Briefly, polystyrene microplates were coated with each of the antigenic fractions (SS, TS, SM and TM) at concentrations of 10 µg/mL in carbonate-bicarbonate buffer (0.06 mmol/L, pH 9.6), incubated overnight at 4 °C. Afterwards, the microplates were blocked with PBS containing 0.05% Tween 20 plus 3% non-fat milk (PBS-TM) for 45 minutes at 37 °C. Serum samples diluted 1:200 in PBS-TM and enzyme-conjugated peroxidase-goat anti-human IgG Fc specific (Sigma-Aldrisch, St. Louis, MO, USA) was then added at 1:30,000 in PBS-TM. The assay was developed by adding the enzyme substrate consisting of hydrogen peroxide and orthophenylenediamine to 0.1 mol/L citrate phosphate buffer pH 5.5 for 15 minutes. The reaction was interrupted with H2SO4 (2N). Optical densities (OD) were determined at 492 nm in an ELISA reader (Thermo Fischer Scientific, Waltham, MA, USA).
Statistical analyses were performed using the GraphPad Prism software, version 5.0 (Graph Pad Software Inc. San Diego, USA). The cut-off value, sensitivity and specificity were established by receiver operating characteristic (ROC) curve analysis using Groups II and III as a negative control. The concordance was carried out by analysis of the Kappa coefficient (κ). Statistical significance was set at p < 0.05.
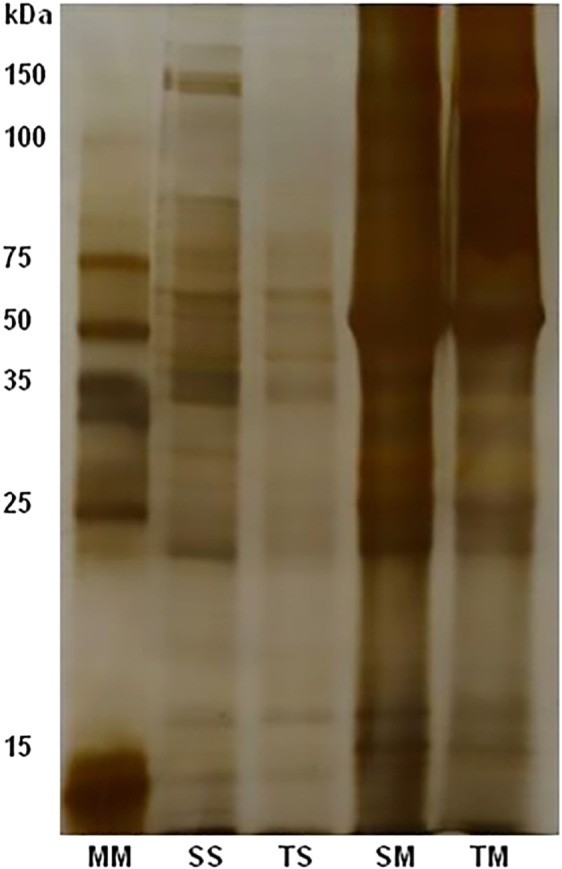
The protein concentrations were 1.3-1.8 mg/mL and 6.2-6.9 mg/mL for soluble and membrane fractions, respectively. Electrophoretical profiles of each antigenic fraction after 12% SDS-PAGE are shown in Figure 1. Extract preparations showed several proteic compounds with molecular weights ranging from < 15 to 150 KDa. In membrane preparation, SM and TM fractions of 50, 70 and 120KDa were evident.
Fig. 1. Electrophoretic profiles of antigenic fractions SS, SM and TS, TM, soluble and membrane fractions from phosphate saline and Tris-HCl, respectively; 12% SDS-PAGE stained by silver nitrate. To each antigenic fraction were used 10 µg/mL.
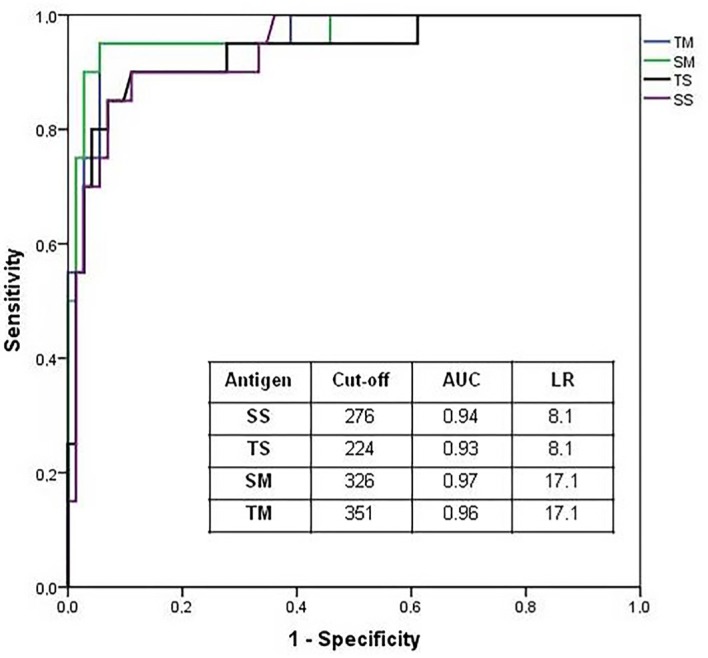
The diagnostic parameters (sensibility and specificity) and diagnostic efficiency of ELISA in the detection of IgG anti S. stercoralis are shown in Table 1. Analysis of the ROC (Fig. 2) showed that SM and TM efficiently distinguished patients from Group I and controls (Groups II and III).
Table 1. Diagnostic parameters of ELISA in detection of IgG anti-S.stercoralis using soluble fractions and membrane antigens.
| Antigens | Se (%) | Sp (%) | κ | DA (CI 95%) (%) |
|---|---|---|---|---|
| SS | 90 | 88.9 | 0.71 | 89.2 (82.9-95.5) |
| TS | 90 | 88.9 | 0.71 | 89.2 (82.9-95.5) |
| SM | 95 | 94.4 | 0.85 | 94.6 (90.0-99.2) |
| TM | 95 | 94.4 | 0.85 | 94.6 (90.0-99.2) |
Se = Sensitivity; Sp = Specificity; κ = Kappa Index; DA = Diagnostic Accuracy; p < 0.05.
Fig. 2. Receiver-operating characteristic curve (ROC-AUC) analysis indicating the optimum point of reactions (cut-off); LR = Likelihood ratio; AUC = Area under curve.
Cross-reactivity in Group II was observed in serum samples from patients infected with S. mansoni (1/9 in SS, SM and TM; 2/9 in ST), hookworms (1/4 in SS and ST) and those polyinfected (1/6 ST).
Considering the difficulties of obtaining more specific antigenic fractions for strongyloidiasis immunodiagnosis, efforts to achieve a reliable diagnostic test are needed. The present study was conducted to verify the use of membrane fractions as a source of antigens for the serological diagnosis of strongyloidiasis. The surface of parasitic nematodes has been shown to be antigenic in many infected hosts11.
Several studies have demonstrated fractionation of Strongyloides antigenic extracts4,5,7. There is a high concern about the antigen preparations used in the tests, particularly in the evaluation of buffers for the extraction of proteins, constituting an essential stage in obtaining antigens. The buffer utilized in the extraction of antigenic fractions is phosphate buffer3,5,6,7. However, the use of Tris-HCl buffer has been reported in an attempt to study the Strongyloides 13,14, but not in direct application to immunodiagnosis.
Sensitivity and specificity using the SS fraction have been described in the literature. Compared to the present work, GONZAGA et al. 6 and FELICIANO et al. 4 showed similar values of sensitivity and specificity using saline extract of the S. venezuelensis. On the other hand, INÊS et al. 7 showed lower values of sensitivity (76.6%) and high specificity (92.9%). The comparison of techniques for the immunodiagnosis of human strongyloidiasis by BISOFFI et al. has recently been reported1. This study showed sensitivity varying from 75.4 to 93.9% and specificity values varying from 94.8 to 100%. In the present study 95% sensitivity and 94.4% specificity were obtained. Then, to obtain membrane antigens, the pellets were treated with detergent, which is a relatively simple and easy procedure, and does not require specialized apparatus, thus reducing the cost of production in the laboratory.
The results showed high sensitivity and specificity of the membrane antigens compared to soluble preparations. In soluble antigen preparation, large amounts of protein present in the pellets are usually discarded. This is not the case when membrane antigens are prepared, so the pellet proteins are not discarded. Besides this, there is the difficulty of obtaining antigen for the diagnosis of human strongyloidiasis. So, it is essential to search for ways to obtain larger amounts of antigen, that can bring results with high sensitivity and specificity in the diagnosis of strongyloidiasis. In conclusion, membrane fractions of S. venezuelensis can be alternative antigens for immunodiagnosis of human strongyloidiasis.
ACKNOWLEDGMENTS
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2013/04236-9)
REFERENCES
- 1.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2013;8:e–2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Concha R, Harrington W, Jr, Rogers AI. Intestinal strongyloidiasis: recognition, management and determinants of outcome. J Clin Gastroenterol. 2005;39:203–11. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 3.Costa-Cruz JM, Madalena J, Silva DA, Sopelete MC, Campos DMB, Taketomi EA. Heterologous antigen extract in the ELISA for the detections of human IgE anti-Strongyloides stercoralis . Rev Inst Med Trop Sao Paulo. 2003;45:265–8. doi: 10.1590/s0036-46652003000500005. [DOI] [PubMed] [Google Scholar]
- 4.Feliciano ND, Gonzaga HT, Gonçalves-Pires MRF, Gonçalves ALR, Rodrigues RM, Ueta MT, et al. Hydrophobic fractions from Strongyloides venezuelensis for use in the human immunodiagnosis of strongyloidiasis. Diagn Microbiol Infect Dis. 2010;67:153–61. doi: 10.1016/j.diagmicrobio.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Gonzaga HT, Ribeiro VS, Cunha-Júnior JP, Ueta MT, Costa-Cruz JM. Usefulness of concanavalin-A non-binding fraction of Strongyloides venezuelensis larvae to detect IgG and IgA in human strongyloidiasis. Diagn Microbiol Infect Dis. 2011;70:78–84. doi: 10.1016/j.diagmicrobio.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Gonzaga HT, Vila-Verde C, Nunes DS, Ribeiro VS, Cunha-Júnior JP, Costa-Cruz JM. Ion-exchange protocol to obtain antigenic fractions with potential for serodiagnosis of strongyloidiasis. Parasitology. 2012;140:69–75. doi: 10.1017/S0031182012001230. [DOI] [PubMed] [Google Scholar]
- 7.Inês E de J, Silva MLS, Souza JN, Teixeira MCA, Soares NM. The role glycosylated epitopes in the serodiagnosis of Strongyloides stercoralis infection. Diagn Microbiol Infect Dis. 2013;76:31–5. doi: 10.1016/j.diagmicrobio.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Liu LX, Weller PF. Strongylodiasis and other intestinal nematode infections. Infect Dis Clin North Am. 1993;37:655–82. [PubMed] [Google Scholar]
- 10.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;93:265–75. [PubMed] [Google Scholar]
- 11.Lutz A. O Schistosomum mansoni e a schistosomatose, segundo observações feitas no Brasil. Mem Inst Oswaldo Cruz. 1919;11:121–55. [Google Scholar]
- 12.Northern C, Grove DI, Warton A, Lovegrove FT. Surface labelling of Strongyloides ratti: stage-specificity and cross-reactivity with S. stercoralis . Clin Exp Immunol. 1989;75:487–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Northern C, Grove DI. Strongyloides stercoralis: antigenic analysis of infective larvae and adult worms. Int J Parasitol. 1990;30:381–7. doi: 10.1016/0020-7519(90)90155-g. [DOI] [PubMed] [Google Scholar]
- 14.Paula FM, Castro-Borges W, Júnior OS, de Souza Gomes M, Ueta MT, Rodrigues V. The ubiquitin-proteasome system in Strongyloididae. Biochemical evidence for developmentally regulated proteolysis in Strongyloides venezuelensis . Parasitol Res. 2009;105:567–76. doi: 10.1007/s00436-009-1430-0. [DOI] [PubMed] [Google Scholar]
- 15.Paula FM, Costa-Cruz JM. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology. 2011;138:1331–40. doi: 10.1017/S003118201100120X. [DOI] [PubMed] [Google Scholar]
- 16.Paula FM, Gottardi M, Corral MA, Chieffi PP, Gryschek RC. Is the agar plate culture a good tool for the diagnosis of Strongyloides stercoralis in candidates for transplantation? Rev Inst Med Trop Sao Paulo. 2013;55:291. [Google Scholar]
- 17.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLOS Negl Trop Dis. 2013;7:e–2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rugai E, Mattos T, Brisola AP. Nova técnica para isolar larvas de nematoides das fezes: modificação do método de Baermann. Rev Inst Adolfo Lutz. 1954;14:5–8. [PubMed] [Google Scholar]
- 19.Uparanukraw P, Phongsri S, Morakote N. Fluctuations of larval excretion in Strongyloides stercoralis infection. Am J Trop Med Hyg. 1999;60:967–73. doi: 10.4269/ajtmh.1999.60.967. [DOI] [PubMed] [Google Scholar]


