Abstract
Pneumonia causes around 750 000 child deaths per year in sub-Saharan African (SSA) countries. The lack of accessibility to prompt and effective treatment is an important contributor to this burden. Community case management of pneumonia (CCMp) uses trained community health workers (CHWs) to administer antibiotics to suspected child pneumonia cases in villages. This strategy has been gaining momentum in low- and middle-income countries, and the World Health Organization and United Nations children’s fund have recently encouraged countries to broaden community case management to other diseases. Recommendations in favour CCMp are based on three meta-analyses showing its efficacy to reduce childhood mortality and morbidity attributable to pneumonia although most of the studies in the meta-analyses were conducted in Asian countries. This is problematic as community case management strategies have been implemented in very different ways in Asian and SSA countries, partly due to differences in malaria prevalence. Therefore, we conducted a narrative synthesis to systematically review the evidence on CCMp in SSA. Results show that there is a lack of evidence concerning its efficacy and effectiveness in SSA, irrespective of whether case management is integrated with other diseases or not. CHWs encounter difficulties in counting the respiratory rate. Their adherence to the guidelines is poorer when they are required to manage several illnesses or children with severe signs. CCMp thus encompasses issues of over-treatment and missed treatment, with potentially negative consequences such as increased lethality in severe cases and antibiotics resistance. The current lack of evidence concerning its efficacy, effectiveness and the factors leading to successful implementation, coupled with CHWs' poor adherence, demand a thorough examination of the legitimacy of implementing CCMp in SSA countries.
Keywords: Case management, community health, systematic reviews, acute respiratory infections
Introduction
Pneumonia is the leading cause of death in children under 5 years old (WHO 2008b). In sub-Saharan Africa (SSA) alone, ∼750 000 child deaths were caused by pneumonia in 2008 (Black et al. 2010). The lack of prompt and accessible treatment for vulnerable populations is a major and well-known contributor to this burden (Kallander et al. 2008). In 1982, the World Health Organization (WHO) launched the acute respiratory infections programme with the objective of establishing clinical guidelines to help healthcare personnel diagnose and treat pneumonia (Riley 1982). A decade later, these guidelines would be broadened to other diseases (namely malaria, diarrhoea and malnutrition) in the integrated management of childhood diseases (IMCI) strategy (Gove 1997).
In the meantime, studies suggested that primary healthcare personnel could also manage pneumonia with the help of a simplified algorithm (Shann et al. 1984). This led the WHO and United Nations children’s fund (UNICEF) to launch in 1986 the strategy known as the community case management of pneumonia (CCMp), which consists in the selection, training and supervision of community health workers (CHWs) who administer antibiotics in simple pneumonia cases (WHO 1986). Studies conducted in Asia have shown that under good implementation conditions (defined as those with regular supervision, support from the health sector and adequate initial training), CHWs can significantly reduce childhood mortality and morbidity attributable to pneumonia in countries with insufficient human resources for health care (Winch et al. 2005). A 2008 survey showed that 12 of the 16 Asian countries who had been recommended for this strategy had at that time implemented the CCMp (Marsh et al. 2008)—some of them at a large-scale level (Dawson et al. 2008).
Until recently, CCMp had received less attention in SSA, both from researchers and programme planners (Winch et al. 2005; WHO and UNICEF 2006; Marsh et al. 2008; Yeboah-Antwi et al. 2010). In SSA countries where malaria is endemic, the WHO had recommended from 1992 until recently that primary healthcare personnel administer presumptive antimalarial treatments for non-complicated fevers in children (WHO Regional Office for Africa 1992; WHO 2004). The malaria prevalence and mortality in SSA countries (WHO 2010b; Meyrowitsch et al. 2011) combined with the lack of resources for laboratory diagnosis (Ukwaja et al. 2011) justified this presumptive strategy. But the systematic association of fever with malaria limited the diagnosis of pneumonia and the adoption of community-based interventions against it. Consequently, many pneumonia cases remained untreated, worsening the burden of the disease (Kallander et al. 2008). In 2008, a WHO report entitled Community-directed interventions for major health problems in Africa still did not mention pneumonia (WHO 2008a)—the combination of malaria and pneumonia management at the community level had been envisaged in 2004 (WHO and UNICEF 2004), but was not officially endorsed until 2012 when the WHO and UNICEF issued a statement promoting integrated community case management (iCCM) (WHO and UNICEF 2012).
In 2010, the fear of resistance to artemisinin-combination therapy (WHO 2010a) and the spread of rapid diagnostic tests (RDTs) (D'Acremont et al. 2009) led the WHO to recommend that antimalarial treatment be administered only after parasitological confirmation, even in febrile children (WHO 2010a). As a result, the issue of non-malarial acute undifferentiated fevers—febrile illnesses with no indication of an organ-specific disease after diagnosis of malaria has been excluded (Joshi et al. 2008)—has been emerging and is a potential avenue to further promote efforts against pneumonia in SSA (Rutebemberwa et al. 2009; Baiden et al. 2011b; Chanda et al. 2011). A UNICEF-piloted survey in the ‘countdown to 2015 countries’ found that out of the 40 SSA countries with available data, 28 had implemented CCMp programmes in 2010—compared to only 11 in 2005 (UNICEF 2012).
While there are increasingly more countries implementing CCMp, the consequences for this strategy in SSA countries where malaria is endemic are still being debated among scientists, healthcare professionals and community health programme planners. Based on recent studies indicating that community case management of malaria (CCMm) is effective in several SSA countries (Ghana, Uganda, Nigeria, Ethiopia, Malawi and Burkina Faso) and acceptable to local communities (Chinbuah et al. 2006; Ajayi et al. 2008a,b; Akweongo et al. 2011), that CHWs can perform malaria RDTs (Harvey et al. 2008; Chanda et al. 2011; Ishengoma et al. 2011; Mubi et al. 2011), and on meta-analyses showing that CCMp is effective in reducing child mortality (Sazawal and Black 1992; Sazawal et al. 2003; Theodoratou et al. 2010), the potential impacts of a combined malaria and pneumonia CCM are under investigation (Hildenwall et al. 2007; Kallander et al. 2008; Marsh et al. 2008). Some go further and see an opportunity to implement iCCM, which replicates the IMCI strategy at the community level (Perez et al. 2009; Ukwaja et al. 2011; Chopra et al. 2012; WHO and UNICEF 2012). In this context they highlight CHWs’ effectiveness to treat or prevent the deadliest diseases, and explore their contribution to reducing childhood mortality and reaching the ‘2015 Millennium Development Goals’ (Haines et al. 2007; Lewin et al. 2010). There are some reservations concerning iCCM because of the numerous barriers and difficulties to sustainably implement such interventions at a large scale (Liu et al. 2011). The negative prospect associated with creating a parallel, second-rate, health system—with dangerous consequences in severe cases—is also anticipated (Aubouy 2011). Finally, the introduction of malaria RDTs could trigger a non-rational use of antibiotics if they become routinely administered to febrile cases with negative results (Baiden et al. 2011b). This inappropriate use may increase resistance to antibiotics (English and Gaur 2010). If CHWs are to use diagnostic tests at the community level, these should arguably better distinguish between parasitological, viral and bacterial infections.
To our knowledge, there has been no systematic examination of CCMp interventions implemented in SSA countries. Three meta-analyses have examined CCMp impacts (Sazawal and Black 1992; Sazawal et al. 2003; Theodoratou et al. 2010), but only one of the 15 included studies took place in SSA (Mtango and Neuvians 1986)—its results are discussed below. This is problematic since community health programmes have been implemented in different ways in Asian and SSA countries, partly due to distinct epidemiological, socioeconomic and political contexts. Indeed, the African context is characterized by a higher prevalence of malaria, by lower overall literacy rates and by fewer public services, with more limited access (Winch et al. 2005; Marsh et al. 2008).
Consequently, the aim of this article is to produce a review of the evidence on CCMp programmes in SSA. Adhering to the instructions for producing a narrative synthesis (Popay et al. 2006), we delimited our review question to: ‘What is the current state of knowledge concerning the effects and the implementation of a CCMp intervention in SSA?’
Methodology
We chose to produce a narrative synthesis, which can be defined as ‘approach to the systematic review and synthesis of findings from multiple studies that relies primarily on the use of words and texts to summarize and explain the finding of the synthesis’ (Popay et al. 2006). In contrast to ‘narrative reviews’ and ‘evidence syntheses’, narrative syntheses entail a systematic and pre-defined search strategy, but they are more focused on producing a textual synthesis than other types of systematic reviews such as quantitative meta-analyses. However, narrative synthesis can be used to review quantitative as well as qualitative data justifying its use in the current study. In the present case, a quantitative meta-analysis was not an option because there were very few experimental or quasi-experimental studies and their study outcome varied.
We followed the steps suggested in the Popay et al. (2006) framework to improve the quality of our narrative synthesis. Once our review question was defined, we conducted a systematic review of studies evaluating or describing CCMp interventions in SSA countries. We considered all types of evaluations (impact, effectiveness, efficacy, implementation, feasibility and acceptability) and study outcomes. Broader interventions—implementing an integrated management of several diseases—were included in our review as long as pneumonia management was specifically addressed.
Search strategy
Twelve databases were searched for free-text words (Medline, Embase, CAB Abstracts, Popline, Web of Knowledge, African Journals on line, Business Source Premier, Cumulative Index to Nursing and Allied Health Literature, Banque de données en santé publique, Educational Resources Information Center, Evidence-Based Medicine Reviews and Current Contents). The exact expression ‘CCMp’ was systematically searched for, as well as a combination of terms using two Boolean logic operators (AND, OR) and a truncation sign (*) as an open-ended term (Box 1). The language used in the search was English, but articles written in French were also included. When available, we used filters to limit the search from January 1986—year of the introduction of the CCMp strategy—to present (September 30, 2012). We also looked for cross-references in three literature reviews and a WHO database on acute respiratory infections (https://apps.who.int/chd/-publications/ari/aripub.htm). Duplicates were identified and removed by listing all citations in an Excel spreadsheet.
KEY MESSAGES.
There is a lack of evidence concerning the impacts of community case managements of pneumonia on childhood mortality and morbidity in African countries, irrespective of whether the management is integrated to other diseases or not.
Community health workers' (CHWs) performance in managing pneumonia suffers from challenges associated with counting the respiratory rate, which entails severe issues of over-treatment and miss-to-treat.
African policy planners should use great caution in interpreting the efficacy of community case management of pneumonia extrapolated from the scientific literature because most of the studies have been conducted in Asian countries.
Innovative approaches to increase CHWs' adherence to the guidelines must be tested before integrating community case management to other diseases, namely malaria.
Eligibility criteria
Papers were screened in a two-stage process (Box 2). First, we screened titles and abstracts using three exclusion criteria. Papers that undoubtedly met at least one exclusion criterion were removed. Two inaccessible papers were also discarded. Second, we read retained papers in their entirety to determine if they respected all three inclusion criteria. Screening the papers for inclusion criteria required establishing three definitions.
Box 2 Inclusion and exclusion criteria.
Exclusion criteria (stage 1)
The document was not published in a peer-reviewed journal.
The title or abstract of the document mention that the focus is a disease other than acute lower respiratory infections or pneumonia.
The title or abstract of the document mention that the study was conducted in a country outside Africa.
Inclusion criteria (stage 2)
The article presents original empirical data.
The article concerns community health workers who respect the three basic criteria of the WHO definition.
The article evaluates a community CCMp intervention.
Box 1 Search terms used for systematic review on CCMp evaluations in Africa.
Exact expression [all fields]
community case management of pneumonia.
Boolean expression [all fields]
(community health worker* OR lay health worker* OR village health worker* OR community health volunteer* OR lay health volunteer* OR village health volunteer*) AND
(pneumonia OR acute lower respiratory infection*) AND
(antibiotic* OR amoxicillin OR cotrimoxazole).
First, we used the WHO definition of CHWs as actors (1) working in the community in which they live, (2) with shorter training than professional workers and (3) partially integrated or supported by the formal health sector (WHO 1989). We did not take into consideration two common CHW’s characteristics (selection by and answerable to their community) as most studies do not give information about those. Second, we defined a CCMp intervention as the management by CHW (with antibiotics administration) of children with acute lower respiratory infections, including pneumonia. Evaluations of interventions implementing an integrated management of several diseases were included if acute lower respiratory infections or pneumonia was one of them. Third, we defined a child as an individual ranging from 1 to 59 months of age. We therefore purposely excluded neonatal infections, for which different programmes of CHW management exist.
In this second stage of the screening process, all studies meeting the four inclusion criteria were included regardless of their design or methodology; we assessed quality in a subsequent stage (see ‘Analysis’ section). We retrieved original articles from the relevant meta-analyses and checked their conformity to the exclusion and inclusion criteria. We also hand-searched the reference list of eligible papers for additional references. Then, we listed all references and looked for the citation using the Thomson Reuters Web of Knowledge® (formerly ISI) database.
Analysis
Following the Popay et al. (2006) framework, we focus on three elements. First, we conducted a preliminary analysis of the content. This allowed us to identify three recurrent themes of evaluation: impact on mortality/morbidity attributable to pneumonia, CHWs’ adherence to the guidelines and implementation issues including contextual factors. We relied upon this 3-fold categorization to separate study outcomes (impact/effects, adherence of CHWs to the guidelines, implementation) and organize the results. We chose to separate CHWs’ adherence from the implementation theme due to the importance given to the former in the material. Second, we extracted and presented as much information as possible on the design study and evaluation procedures allowing for the comparison of results across different studies. Design descriptions follow evaluation conventions presented in Shadish et al. (2001). Links between the study authors and the implementers of the intervention were either mentioned in the article or induced by reading the affiliations, acknowledgments and methodology sections. Third, we considered the quality of the studies by applying the ‘Mixed methods appraisal tool (MMAT)’ (Pluye 2013), because some studies mixed quantitative and qualitative analysis of data. Despite the absence of an uncontested tool to assess simultaneously the quality of quantitative, qualitative and mixed methods studies, the MMAT showed intra-class correlations ranging from 0.7 to 0.9 (Pluye et al. 2009; Pace et al. 2012). In addition, our desire to influence policy led us to reflect deeply on the current debate about the relevance of combining the community management of pneumonia with other diseases.
Results
Description of the material
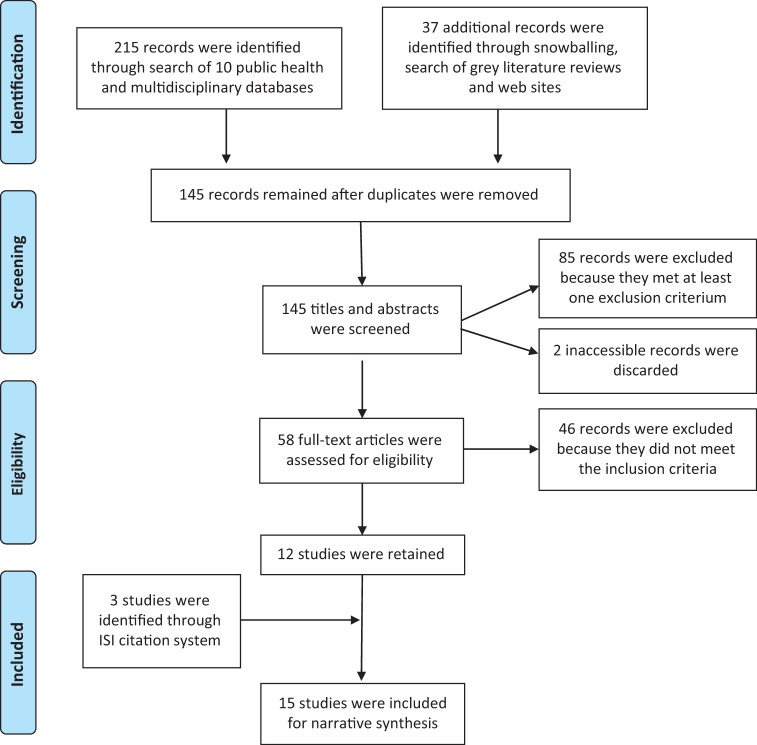
Results of our research strategy are detailed in Figure 1. We retained 15 articles that we coded from 1 to 15 (for the coding assignment, see ‘References’ section), presenting the results originating from nine distinct research sites (Table 1). The selected studies varied widely in terms of their design, objective and hypotheses, scope and duration and the type of programme considered (Table 2). Only one study evaluated a programme implemented under real-life conditions [2]. In most of the studies (8 of 15), the evaluation was not entirely independent—the implementer (co-)authored the study [1, 6, 7, 10–14].
Figure 1.
Flow chart of the research strategy.
Table 1.
Description of the programmes under study
| # | First author | Year of publication | Country | Type of programme | CHW’s training | Implementer of the programme/ intervention | Programme scope | # CHWs under study |
|---|---|---|---|---|---|---|---|---|
| 7 | Mtango | 1986 | Tanzania | CCMp | ? | A co-operation agency and WHO | 1 district | ? |
| 2 | Greenwood | 1990 | Gambia | Integrated intervention | 42 days | National authorities | National | 41 |
| 6 | Kelly | 2001* | Kenya | Integrated intervention | 21 days | An NGO | 1 district | 322 |
| 13 | Sylla | 2004** | Senegal | CCMp | 3 days | UNICEF | 4 districts | 107 |
| 4 | Kallander | 2006 | Uganda | CCMp | 2 days | Research team | 1 district | 96 |
| 10 | Rowe | 2006* | Kenya | Integrated intervention | 15 days | An NGO | 1 district | 103 |
| 11 | Rowe | 2007* | Kenya | Integrated intervention | 15 days | An NGO | 1 district | 125 |
| 14 | Sylla | 2007** | Senegal | CCMp | 3 days | UNICEF | 4 districts | 107 |
| 12 | Rowe | 2007b* | Kenya | Integrated intervention | 15 days | An NGO | 1 district | 114 |
| 1 | Degefie | 2009 | Ethiopia | Integrated intervention | 7 days | An NGO | 1 district | 38 |
| 15 | Yeboah-Antwi | 2010*** | Zambia | CCMp + m | 5 days | Research team | 1 hospital catchment area | 37 |
| 8 | Mukanga | 2011**** | Uganda | CCMp + m | 8 days | Research team | 1 sub-county | 14 |
| 3 | Hamer | 2012*** | Zambia | CCMp + m | 5 days | Research team | 1 hospital catchment area | 18 |
| 9 | Mukanga | 2012**** | Uganda | CCMp + m | 8 days | Research team | 1 sub-county | 7 |
| 5 | Kalyango | 2012 | Uganda | CCMp + m | 6 days | Research team | 1 health and demographic surveillance site | 125 |
Asterisks indicate studies coming from an identical research site.
#, number attributed to the paper; NGO, non-governmental organization.
Table 2.
Description of the evaluation approach
| # | First author | Beginning of the study compared to the intervention introduction | Study designa | Follow-up duration | Study outcome | Links between the implementer and the authors | MMAT scoreb |
|---|---|---|---|---|---|---|---|
| 7 | Mtango | Identical | CR X O O | 2 years | Impact on mortality | One of the authors is the implementer | 0% |
| CR O X O | |||||||
| 2 | Greenwood | 1 year before | O X O | 4 years | Impact on mortality | None | 33% |
| O O | |||||||
| 6 | Kelly | 10, 31 and 47 months later | X O1 O2 O3 | NA | CHWs' performance | One of the authors is the implementer | 100% |
| The implementer funded the study | |||||||
| 13 | Sylla | Identical | X O | NA | CHWs' performance | Two of the authors are the implementer | 50% |
| 4 | Kallander | Identical | X O | NA | CHWs' performance | Same (interventional research) | 75% |
| 10 | Rowe | 5 years later | X Oi=1 Oi=90 | 3 months | CHWs' performance | One of the authors is the implementer | 100% |
| 5 years later | X O | NA | CHWs' performance | The implementer funded the study | |||
| 11 | Rowe | 4 years later | X O | NA | CHWs' performance | One of the authors is the implementer | 100% |
| The implementer funded the study | |||||||
| 14 | Sylla | Identical | X Oi=1 Oi=365 | 1 year | CHWs' performance | Three of the authors are the implementer | 0% |
| 12 | Rowe | 2 years later | X Oi=1 Oi=1825 | 5 years | CHWs' performance | One of the authors is the implementer | 100% |
| 1 | Degefie | 1 year later | X O | NA | Impact on care-seeking practices | Five of the authors are the implementer | 75% |
| CHWs' performance | |||||||
| Implementation determinants | |||||||
| 15 | Yeboah-Antwi | Identical | CR X O | 1 year | Impact on morbidity | Same (interventional research) | 75% |
| CR O | |||||||
| 8 | Mukanga | 3 days later | X O | NA | CHWs' performance | Same (interventional research) | 100% |
| 3 | Hamer | ? | X Oi=1 Oi=365 | 1 year | Impact on knowledge | Same (interventional research) | 75% |
| CHWs' performance | |||||||
| 9 | Mukanga | 1 year later | X O | NA | Implementation determinants | Same (interventional research) | 100% |
| 15 | Kalyango | Identical | CR X O | 2 years | CHWs' performance | Same (interventional research) | 33% |
| CR O |
#, number attributed to the paper; NA, not applicable; CR, cluster-randomized.
aDesign descriptions follow evaluation conventions presented in Shadish et al. (2001). O represents an outcome measure, and X indicates the introduction of the intervention. For experimental or quasi-experimental designs, control groups are represented on the line below.
bMMAT scores were calculated following methods explained by Pluye (2013). Details are available in the Supplementary File 2.
One study combined two designs to evaluate the same outcome [10]. The majority of studies (11 of 15) examined primarily the CHWs’ performance, and all but one of these was observational. Of the four experimental or quasi-experimental designs, two measured the impact on mortality (they took place more than 20 years ago), one evaluated the effects on treatment failures and health-seeking practices and one compared CHWs’ performance in CCMm vs CCMm + p interventions. Longitudinal studies lasted from 3 months to 4 years. Essential characteristics of CCMp interventions showed considerable heterogeneity across studies—e.g. the length of the CHWs’ training ranged from 2 to 42 days. A summary of main results is available in the Supplementary File 1.
The small number of studies, the fact that all but two were conducted in East Africa and their methodological heterogeneity call for caution concerning the comparability and generalizability of the results to other contexts. Most of the studies provided very little information on CHWs’ characteristics and the context of implementation. While the performance of CHWs was the most frequent study outcome, measurement methods were not always similar—the evaluation was either made by clinicians in unusual work conditions for CHWs or by the examination of inconsistencies in CHWs’ registries. In addition, the management algorithms and the type of antibiotics administered varied.
Results of the review of study quality are presented in Table 2. Based on the mixed methods evaluation tool, five studies had a score equal or inferior to 50% [2, 5, 7, 13, 14], which means that they met two or fewer of the four criteria used to appraise quality (for details, see the Supplementary File 2) (Pluye 2013). However, these scores should be considered with caution, given the aforementioned diversity of the studies. Moreover, the tool used is still under development and may not consider the same elements of methodological quality as other tools developed for quantitative studies. Results are therefore presented to allow for the reader’s own critical assessment. Due to the absence of an undisputed definition of quality, the small number of studies included and the congruence of their results, we chose not to exclude any of the 15 studies on the grounds of their quality appraisal or time of publication. The reader should keep in mind that most of the studies were observational.
CHWs’ adherence to guidelines
To evaluate CHWs’ adherence to guidelines, three studies relied upon their registries [3, 12, 14], six directly observed CHWs in a health facility [1, 4, 6, 8, 11, 13] and two did both [5, 10]. One specifically compared the two methods of assessment, revealing that CHWs evaluated under direct observation changed their management practices and provided treatment more consistent with the guidelines compared to their classifications in routine conditions [10]. While some only presented average overall adherence scores [11, 12], the majority of studies separated the process into four chronological steps: assessment, classification, treatment and referral.
Assessment
If a child shows difficulty in breathing or coughs, the IMCI guidelines classify a child with one sign (high respiratory rate) as having moderate pneumonia and a child with two signs (chest in-drawing and stridor) as having severe pneumonia (Benguigui and Stein 2006). Studies assessing the ability of the CHWs in identifying these signs showed that CHWs had difficulty assessing fast breathing in children, even when performed at the end of training in a clinical setting—between 29% and 51% of respiratory counts were more than 5 breaths per minute different from the gold standard, i.e. the assessment by a trained physician [4, 5]. CHWs correctly assessed 41–81% of children with fast breathing [6, 8]. The concordance rate between the gold standard and CHWs in discriminating between normal and fast breathing in children ranged between 79% and 84% [4, 8]. Assessment of severe pneumonia was somewhat difficult for CHWs—the average sensitivity of chest in-drawing detected by CHWs varied between 19% and 60% [6]. No study evaluated the CHWs’ ability to assess stridor; apparently the majority of interventions did not include that symptom during the CHWs’ training.
Classification
When they had to differentiate between simple and severe pneumonia, CHWs correctly classified 32–55% of children having non-severe pneumonia (as defined by the gold standard), and 18–53% of children having severe pneumonia [6]. This sensitivity score increased to 75–84% if there was no differentiation of severity in pneumonia diagnosis [4, 8]. The concordance rate between CHWs’ classification and the gold standard varied between 75% and 78% [4, 5]. Arguably, misclassification was mainly due to assessment errors, since more than 95% of children having pneumonia (as assessed by the CHW) were correctly classified [1, 3, 14]. However, this was not the case for children having severe pneumonia (as assessed by CHWs), for whom the sensitivity score of CHWs’ classification was 63% [14].
Treatment
About 80% or more uncomplicated pneumonia cases (as classified by CHWs) were coherently prescribed antibiotics [3, 5, 8, 14]. CHWs’ treatment scores were lower when compared to the gold standard, i.e. the treatment recommended by a trained physician using the guidelines, especially if they had to make distinctions between severe and moderate pneumonia. In these cases, 50–65% of children with moderate pneumonia (as defined by the gold standard) were given an appropriate treatment by the CHWs [6]. Dosage errors are an additional concern—in one study, 85% of antibiotics prescriptions were correctly dosed [14], while another mentioned that dosage errors were frequent [6].
Referral
In the Sylla et al. study [14], CHWs referred to the nearest facility 88% of the children they had classified as having severe pneumonia or a danger sign. In another study, three successive evaluations showed that 11–60% of children classified by the gold standard (a trained physician using the guidelines) with severe pneumonia were appropriately referred by CHWs [6].
Adherence determinants
It is not clear from this literature review what determines CHWs’ adherence to the CCMp or IMCI guidelines. One study found a significant association between CHWs’ education level and the quality of care [11], in contrast to two others that did not [4, 13]—however, these two recruited only literate CHWs. In integrated interventions, adherence was lower when children had several concurrent classifications, when they were <2 years old or when a danger sign was present [11]. In addition, the use of treatment cards, the number of CHWs’ benefits and the recording of complaints during the assessment phase were positively correlated to adherence [11]. Surprisingly, supervision and refresher training sessions were not always beneficial. A longitudinal analysis showed that they can have a direct negative effect on adherence scores, and even a negative long-term impact [12]. An interaction was observed between refresher training sessions and the severity of a child’s illness—the refresher session increased the adherence score of CHWs for patients with a severe classification but decreased it in cases of patients with a non-severe classification.
Antibiotic misuse
Antibiotic misuse, including over-treatment and failure-to-treat, is a major concern from a public health perspective. There are different situations in which the inappropriate use of antibiotics by CHWs occurs. The first occurs when the treatment is in disagreement with the CHW’s own classification—between 2% and 23% of CHW-classified non-pneumonia cases were administered antibiotics [5, 8, 14] while up to 18% of children classified as having pneumonia received no antibiotics at all [5]. In the Kalyango et al. study [5], 9% of all antibiotics treatments were administered to children with no pneumonia symptoms. A second possibility is that a CHW provides antibiotic treatment consistent with his diagnosis, but the diagnosis is incorrect according to that made by a trained physician using the IMCI guidelines, i.e. ‘the gold standard’. Mukanga et al. [8] report that 16% of all antibiotic treatments were given to children without pneumonia (as established by the gold standard). In contrast, 16% of children diagnosed with pneumonia by a physician received no antibiotics because the CHWs did not classify them as such [8]. The third type of antibiotic misuse concerns situations where the diagnosis according to the gold standard mistakenly administers a treatment; indeed, the case management guidelines have limited specificity and sensitivity (Weber et al. 1997). Taking these cumulative risks into consideration, Kallander et al. [4] estimate that the proportion of over-treatment of pneumonia could reach 40% depending on the local pneumonia prevalence as could the risk of failure-to-treat. Thus, as the true prevalence of pneumonia increases, the risk of failure-to-treat grows and the risk of over-treatment diminishes.
CCMp implementation and uptake of CHW services
A house survey examined implementation obstacles to an integrated intervention and revealed difficulties with drug and cash management [1]. Despite these obstacles and the presence of modest user fees, community care-seeking practices for pneumonia symptoms increased from 30% to 84% during the intervention [1]. Another survey showed that CHWs were the first option of treatment for 40% of sick children and that 98% of mothers were satisfied with the services they provided; in this context, using respiration rate timers and RDTs apparently reinforced CHWs’ credibility [9]. However, caregivers in the households also mentioned several key reasons for not consulting a CHW: frequent shortages of drugs, dislike of CHW services and their lack of availability. These factors were also cited in other studies, in addition to the sickness perceived as too severe [2, 5, 15]. Furthermore, proximity of the households to the CHW is a determinant in favour of their use, while the presence of a primary health centre in close proximity to the households is a deterrent against their use [9, 15].
Impacts on mortality and morbidity attributable to pneumonia
Two studies evaluated the impact on mortality [2, 7]. The first one measured mortality at the outset and 1 year following intervention by CCMp. It revealed that the overall childhood mortality rate dropped from 40 per thousand to 35 per thousand and that the mortality attributable to pneumonia fell from 14 per thousand to 12 per thousand [7], though there was no mention if this was statistically significant. The second study evaluated the impact of an integrated intervention 3 years following its introduction. It found no significant reductions in overall childhood mortality rates between the intervention area and a control area with no community intervention [2]. The child mortality rate attributable to acute respiratory infections decreased from 10.7 per thousand to 6.8 per thousand, but this change was not statistically significant. Repeated clinical measures showed no significant variation in the prevalence of acute lower respiratory infection and fever.
In a cluster-randomized control trial (C-RCT) conducted in Zambia, Yeboah-Antwi et al. [15] compared the impact on morbidity in two arms: (1) an intervention group where CHWs (equipped with malaria RDTs) treated childhood malaria and pneumonia vs; (2) a control group where CHWs (without RDTs) presumptively treated malaria and referred children with pneumonia symptoms to a health centre. Children classified with pneumonia experienced significantly less treatment failures in the intervention group (11%) than in the control group (20%)—treatment failures were defined as the persistence of pneumonia symptoms 5–7 days after initial visit to the CHW. The probability of receiving early and appropriate treatment for non-severe pneumonia cases was also significantly higher in the intervention group (68%) than in the control group (13%).
The combined management of pneumonia with others diseases
The five most recent articles (presenting results from three study sites) examined the combined management of malaria and pneumonia. These studies showed that CHWs’ performance was consistently better if the child presented only with malaria rather than the combination of malaria and pneumonia or pneumonia alone. One experimental study showed that CHWs managing both diseases (with RDTs) significantly reduced treatment failure at Day 5 for children with non-severe pneumonia compared to CHWs managing malaria only (without RDTs) and did so without generating any differences in treatment or outcome for children with malaria [15]. Another experimental study comparing CCMm and CCMm + p showed no significant difference between the two groups of CHWs concerning their knowledge and performance to manage malaria [5]. The combined use of diagnostic tools (thermometer, malaria RDT and respiratory rate timer) was appropriate and acceptable in the Mukanga et al. studies [8, 9], but they observed more frequent difficulties in counting the respiratory rate than in measuring the temperature or using the RDT.
The integrated management of several diseases (mainly malaria, pneumonia, diarrhoea and malnutrition) has rarely been evaluated. A natural experiment showed that an integrated intervention had no positive impact on childhood mortality or morbidity (general or disease-specific) [2]. Rather, 1-year after its introduction, the authors observed an increase of incidence of marasmus (children with weight-for-age <60%) in both arms of the study, and this increase was significantly higher in the intervention area compared to the control area. In two other evaluations of integrated interventions, the complexity of IMCI guidelines was deemed the cause for the poor performance of CHWs and low adherence levels [6, 12].
Discussion
The aim of this article is to synthesize the evidence on the CCMp at a turning point in the fight against infectious diseases for children in SSA. Indeed, while RDTs for malaria are increasing in availability and utilization, and presumptive antimalarial treatment is no longer recommended for febrile children, the issue of non-malarial acute undifferentiated fevers is emerging (Joshi et al. 2008). The management of pneumonia by CHWs—key actors in the ‘renewed primary healthcare’ approach currently promoted by the WHO—is a potential answer to this challenge that has attracted a lot of attention, as shown by the increasing number of SSA countries implementing it (de Sousa et al. 2012; UNICEF 2012).
Our review shows that there have been very few studies in SSA evaluating CCMp interventions or interventions where CHWs simultaneously manage several diseases (including pneumonia). A disproportionate number of them focus on the performance of CHWs in following the guidelines and all but one of the studies considered in this review took place under controlled conditions comparable to pilot project evaluations. In addition, countries where these studies were conducted were limited in their number and regional location. The consequent paucity of evidence is striking, especially when compared with the evidence coming from studies piloted in Asian countries. However, the publication of a Supplement by the American Journal of Tropical Medicine and Hygiene in November 2012 on the iCCM interventions in Africa indicates that research is growing on the topic.
The evidence of impact on mortality is particularly poor and over 20 years out-of-date. In fact, we found only one evaluation of a CCMp intervention that observed a significant change in child mortality [7]. This is the only study conducted in SSA out of the 15 included in the last two meta-analyses on CCMp impacts on mortality (Sazawal et al. 2003; Theodoratou et al. 2010). Since differences have been pointed out between Asian and African community case management strategies (see ‘Introduction’ section), policy planners from SSA countries should use great caution in interpreting the efficacy of CCMp extrapolated from this scientific literature. There may be ‘stronger evidence for this model [CCMp] than for any of the other[s] [models of community case management]’ (Winch et al. 2005), but generalizing the model to the context of malaria-endemic African countries is questionable.
We observed a similar lack of evidence regarding the efficacy of combined or integrated case management models to reduce mortality or morbidity in countries where malaria is prevalent. In terms of the combined management of malaria and pneumonia, the Yeboah-Antwi study [15] suggests that CCMm + p might have a positive effect on child morbidity related to pneumonia. However, its efficacy on mortality remains to be proven—a C-RCT conducted in SSA but published after our review found no significant differences between CCMm and CCMm + p interventions in terms of childhood mortality reduction (Chinbuah et al. 2012). Concerning the iCCM strategy, our review reveals that there is no evidence of a positive impact on the pneumonia-related burden in SSA. This corroborates a previous review documenting the lack of evidence concerning the efficacy of this strategy in reducing child mortality or morbidity (Winch et al. 2005). Henceforth, there are little indications that using CHWs as ‘mini-doctors’ (Walt and Gilson 1990) to prescribe or administer treatments for multiple conditions is an appropriate answer to the challenge of extending child survival interventions at the community level (Bryce et al. 2005). In spite of the limited evidence, the combined and integrated models are already being implemented and promoted in several African countries (Marsh et al. 2008; Chanda et al. 2011).
Depending on the methods of evaluation, the phase of the diagnostic process, and the complexity of the guidelines, adherence to the pneumonia classification and treatment guidelines by the CHWs varied considerably across studies. CHWs generally obtained high adherence scores (around 80% or above) to classify, treat and refer children in accordance with their initial assessment in the diagnostic algorithm. A multi-country C-RCT conducted in Africa after the review showed similar performance of CHWs in administering antibiotic treatments to children they had classified as having pneumonia (Mukanga et al. 2012). However, our review reveals that when compared with the gold standard, these scores diminished, notably because CHWs experienced numerous difficulties during the first phase (assessment). Accurately counting the respiratory rate remains a major problem for them, despite the use of respiratory timers that were expressly developed by the WHO and UNICEF to address this challenge (Rasmussen et al. 2000).
Our analysis revealed two key findings in terms of low adherence by CHWs. The first factor relates to the integrated management of several diseases. We observed that adherence scores were lower in integrated interventions than in CCMp interventions. In addition, in integrated interventions, these scores decreased when a child had several concurrent illnesses diagnosed by the CHW. Moreover, CHWs managing both malaria and pneumonia cases tended to miss treating a large proportion of children with pneumonia only—echoing the difficulties in distinguishing pneumonia from malaria on clinical grounds that have been reported for decades, even in hospitals (Bassat et al. 2011). While the debate concerning the number of roles or functions a CHW can effectively perform is still open (Haines et al. 2007), these results suggest that CHWs might encounter difficulties in managing several diseases, particularly if they are concomitant. Given the high prevalence of co-morbidities in children in these contexts and their lethality (Fenn et al. 2005; Gwer et al. 2007), this is of great concern.
The second key finding is that CHWs’ performance when following both the CCMp and IMCI guidelines was weaker for severe pneumonia than for non-severe pneumonia. Because of their potentially fatal outcomes the algorithms usually demand that severe forms of diseases are referred to the nearest health facility. Therefore, failures-to-refer by CHWs are of grave concern and research should establish that there is no additional risk of mortality associated with those community interventions for children with severe pneumonias. A recent randomized controlled trial conducted in Pakistan has just demonstrated the safety of CHWs treating severe cases of pneumonia (instead of referring them to the health centre) (Soofi et al. 2012). This might be an opportunity to test a simplified protocol of pneumonia management in the SSA context, with the prospect of improving adherence to the guidelines.
To our knowledge, no evaluation of a CCMp intervention has ever established that CHWs’ low performance could be detrimental to the intervention’s efficacy to reduce child mortality. This relation may not be straightforward, as suggested by studies that found no statistical association between clinicians’ low performance to follow IMCI guidelines and clinical outcomes (Rowe et al. 2001; Baiden et al. 2011a). But a noteworthy consequence of the low adherence by CHWs is the misuse of antibiotics. A study comparing CHWs’ treatments to the gold standard [8] found that 16% of antibiotics treatments prescribed by CHWs were unnecessary—by comparison, a study conducted in Nepal reported only 3% of antibiotics misuse (Dawson et al. 2008). Admittedly, the practice of overprescribing antibiotics in pneumonia management is not limited to CHWs; it is also a concern with IMCI-trained health personnel in primary care facilities or hospitals (Schellenberg et al. 2004; Osterholt et al. 2009). However, the fact that the guidelines are simplified to suit CHWs is likely to further reduce their specificity and sensitivity [4], increasing the number of unnecessary treatments. The resulting unnecessary costs and increased risk of antibiotics resistance call for caution concerning CCMp (Simoes 2012), especially as the proportion of cases due to bacterial pneumonia varies significantly from region to region and is not always precisely known (Cashat-Cruz et al. 2005; Rudan et al. 2008). Furthermore, no research has demonstrated that equipping CHWs with RDTs for malaria does not increase the proportion of antibiotics prescribed unnecessarily. In its present form, the CCMm + p with RDTs might reflect an unfortunate paradox—the modification of antimalarial treatment recommendations in an effort to slow down the development of artemisinin resistance could increase the emergence of antibiotic resistance by introducing the algorithm-based treatment of pneumonia by CHWs.
We are not arguing that CHWs managing pneumonia cases should be categorically proscribed or that they should not use malaria RDTs to help their diagnosis. The potential positive outcomes for children’s health should be evaluated—for example, the increased use of antibiotics after the introduction of malaria RDTs in health facilities was associated with a reduction of treatment failures (Msellem et al. 2009). Despite poor adherence to the guidelines, the algorithm-based administration of antibiotics by CHWs might be appropriate depending on the local epidemiological context or the level of child vulnerability—for instance, the lethality of bacterial pneumonia is much higher in cases of concurrent HIV infection or malnutrition (Graham et al. 2008). On the other hand, as discussed earlier, the more complex the algorithm, the worse the CHWs’ adherence to guidelines. Research should address these issues and trade-offs to ensure that the administration of antibiotics, either presumptive or algorithm-based in the CCMp strategy, is not based on weak evidence—as was apparently the policy promoting systematic antibiotic treatments to children with simple malnutrition (Alcoba et al. 2013).
Three additional important issues require further elucidation. First, very little is known about CCMp implementation, while serious problems have been previously identified in similar CHWs interventions. These include drug supply and preservation, motivation and retention of CHWs, power dynamics inside the community, integration with the health system and competition with local drug distributors. Intervention research on CCMp effectiveness and implementation in real-life conditions is needed. Second, an assessment of the unintended impact of CCMp on other healthcare(-seeking) practices is needed. In particular, it is plausible that the administration of antibiotic treatments in villages alters prescribing practices in primary care centres, and undermines decade-long endeavours to promote a rational use of antibiotics (le Grand et al. 1999). Finally, impacts of CCMp on health inequities have not been examined yet, even though it is known that population interventions can increase the burden carried by vulnerable populations (Ridde 2007). Evaluations of CCM of malaria showed mixed results concerning the equitable distribution of benefits among households of different socioeconomic status (Nsungwa-Sabiiti et al. 2007; Siekmans et al. 2013).
The current lack of evidence concerning the efficacy, effectiveness and factors of successful implementation coupled with poor CHWs’ adherence results, demand a thorough examination of the legitimacy of implementing CCMp in SSA countries, whether integrated with other diseases or not. Before further implementation, innovative approaches to increase CHWs’ adherence must be tested. Diagnostic tests distinguishing between viral, bacterial and parasitological infections are also needed (Baiden et al. 2011b). Meanwhile, other options than CHWs prescribing antibiotic treatment—or mothers, as has just been tested (Sangho et al. 2012)—should be considered. Their use as actors of social change inside their community to increase impacts of interventions to prevent pneumonia could be promising (Holloway et al. 2009).
Limits
The search process, data extraction and assessment of study quality were primarily done by only one of the authors. We opted for this strategy because the ideal procedure (two authors reviewing the data independently and comparing their results) was not feasible, and we preferred that at least one author reviews material in its entirety. In addition, reports from non-governmental organizations and international organizations were not considered if not published in peer-reviewed journals. The absence of a methodical gathering of non-published material prevented us from proceeding to a systematic review, which is an essential element of narrative syntheses.
One limitation comes from the fact that this systematic review was conducted a few months before a Supplement was issued in the American Journal of Tropical Medicine and Hygiene. The Supplement covers several aspects of iCCM in SSA countries and presents some empirical studies that would have passed through the selection criteria. We reflect as much as possible on the implications of these studies in our discussion.
Conclusion
This review showed that there is a lack of evidence concerning CCMp impacts on childhood mortality and morbidity in SSA. Similarly, the efficacy of combined or integrated interventions to reduce the burden attributable to pneumonia remains to be evaluated. CHWs’ performance in managing pneumonia suffers from challenges associated with counting the respiratory rate. In integrated interventions, performance scores in managing pneumonia were always lower than for the other diseases. In addition, very few studies evaluated the capacity of CHWs to distinguish severe from moderate pneumonia cases, and the available results suggest there are major problems in doing so. Consequently, CCMp encompasses issues of over-treatment and missed treatment. A more comprehensive and contextual understanding of CHWs’ performance is needed, which requires evaluations of CCMp implementation in natural conditions. While the integrated case management of several diseases is currently recommended for CHWs, this review shows important issues and lack of evidence concerning the management of pneumonia, and calls for more context-specific research.
Supplementary Data
Supplementary data are available at HEAPOL online.
Acknowledgements
The authors would like to thank the Student thinking group on user fees abolition in West-African countries and Dr Federica Fregonese for their comments made on a previous version of the manuscript.
Glossary
Abbreviations
- C-RCT
cluster-randomized control trial
- CCMm
community case management of malaria
- CCMp
community case management of pneumonia
- CHW
community health worker
- iCCM
integrated community case management
- IMCI
integrated management of childhood illnesses
- MMAT
mixed methods appraisal tool
- RDT
rapid diagnostic test for malaria
- SSA
sub-Saharan Africa
- UNICEF
United Nations children's fund
- WHO
World Health Organization
Funding
This research received no specific grant from any funding agency. T. Druetz is a Strategic Training Fellow in Global Health Research of the Canadian Institutes of Health Research and Québec Population Health Research Network; he is also funded by the Québec Health Research Fund (FRQS). V. Ridde is a New Investigator of the Canadian Institutes of Health Research.
Conflict of interest
None declared.
References
- Ajayi IO, Browne EN, Bateganya F, et al. Effectiveness of artemisinin-based combination therapy used in the context of home management of malaria: a report from three study sites in sub-Saharan Africa. Malaria Journal. 2008a;7:190. doi: 10.1186/1475-2875-7-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi IO, Browne EN, Garshong B, et al. Feasibility and acceptability of artemisinin-based combination therapy for the home management of malaria in four African sites. Malaria Journal. 2008b;7:6. doi: 10.1186/1475-2875-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akweongo P, Agyei-Baffour P, Sudhakar M, et al. Feasibility and acceptability of ACT for the community case management of malaria in urban settings in five African sites. Malaria Journal. 2011;10:240. doi: 10.1186/1475-2875-10-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoba G, Kerac M, Breysse S, et al. Do children with uncomplicated severe acute malnutrition need antibiotics? A systematic review and meta-analysis. PLoS One. 2013;8:e53184. doi: 10.1371/journal.pone.0053184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubouy A. Promotion of malaria home-based treatment in Africa: the dangers of creating a second health system. International Health. 2011;3:219–20. doi: 10.1016/j.inhe.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Baiden F, Owusu-Agyei S, Bawah J, et al. An evaluation of the clinical assessments of under-five febrile children presenting to primary health facilities in rural Ghana. PLoS One. 2011a;6:1–8. doi: 10.1371/journal.pone.0028944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiden F, Webster J, Owusu-Agyei S, Chandramohan D. Would rational use of antibiotics be compromised in the era of test-based management of malaria? Tropical Medicine & International Health. 2011b;16:142–4. doi: 10.1111/j.1365-3156.2010.02692.x. [DOI] [PubMed] [Google Scholar]
- Bassat Q, Machevo S, O'Callaghan-Gordo C, et al. Distinguishing malaria from severe pneumonia among hospitalized children who fulfilled integrated management of childhood illness criteria for both diseases: a hospital-based study in Mozambique. American Journal of Tropical Medicine and Hygiene. 2011;85:626–34. doi: 10.4269/ajtmh.2011.11-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benguigui Y, Stein F. Integrated management of childhood illness: an emphasis on the management of infectious diseases. Seminars in Pediatric Infectious Diseases. 2006;17:80–98. doi: 10.1053/j.spid.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- Bryce J, Victora CG, Habicht JP, et al. Programmatic pathways to child survival: results of a multi-country evaluation of Integrated Management of Childhood Illness. Health Policy and Planning. 2005;20(Suppl. 1):i5–17. doi: 10.1093/heapol/czi055. [DOI] [PubMed] [Google Scholar]
- Cashat-Cruz M, Morales-Aguirre JJ, Mendoza-Azpiri M. Respiratory tract infections in children in developing countries. Seminars in Pediatric Infectious Diseases. 2005;16:84–92. doi: 10.1053/j.spid.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Chanda P, Hamainza B, Moonga HB, Chalwe V, Pagnoni F. Community case management of malaria using ACT and RDT in two districts in Zambia: achieving high adherence to test results using community health workers. Malaria Journal. 2011;10:158. doi: 10.1186/1475-2875-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinbuah AM, Gyapong JO, Pagnoni F, Wellington EK, Gyapong M. Feasibility and acceptability of the use of artemether-lumefantrine in the home management of uncomplicated malaria in children 6-59 months old in Ghana. Tropical Medicine & International Health. 2006;11:1003–16. doi: 10.1111/j.1365-3156.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Chinbuah MA, Kager PA, Abbey M, et al. Impact of community management of fever (using antimalarials with or without antibiotics) on childhood mortality: a cluster-randomized controlled trial in Ghana. American Journal of Tropical Medicine and Hygiene. 2012;87:11–20. doi: 10.4269/ajtmh.2012.12-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Binkin NJ, Mason E, Wolfheim C. Integrated management of childhood illness: what have we learned and how can it be improved? Archives of Disease in Childhood. 2012;97:350–4. doi: 10.1136/archdischild-2011-301191. [DOI] [PubMed] [Google Scholar]
- D'Acremont V, Lengeler C, Mshinda H, et al. Time to move from presumptive malaria treatment to laboratory-confirmed diagnosis and treatment in African children with fever. PLoS Medicine. 2009;6:e252. doi: 10.1371/journal.pmed.0050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P, Pradhan Y, Houston R, et al. From research to national expansion: 20 years' experience of community-based management of childhood pneumonia in Nepal. Bulletin of the World Health Organization. 2008;86:339–43. doi: 10.2471/BLT.07.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa A, Tiedje KE, Recht J, Bjelic I, Hamer DH. Community case management of childhood illnesses: policy and implementation in Countdown to 2015 countries. Bulletin of the World Health Organization. 2012;90:183–90. doi: 10.2471/BLT.11.093989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English BK, Gaur AH. The use and abuse of antibiotics and the development of antibiotic resistance. In: Finn A, Curtis N, Pollard AJ, editors. Hot Topics in Infection and Immunity in Children. New York: Springer; 2010. pp. 73–82. [Google Scholar]
- Fenn B, Morris SS, Black RE. Comorbidity in childhood in northern Ghana: magnitude, associated factors, and impact on mortality. International Journal of Epidemiology. 2005;34:368–75. doi: 10.1093/ije/dyh335. [DOI] [PubMed] [Google Scholar]
- Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bulletin of the World Health Organization. 1997;75(Suppl. 1):7–24. [PMC free article] [PubMed] [Google Scholar]
- Graham SM, English M, Hazir T, Enarson P, Duke T. Challenges to improving case management of childhood pneumonia at health facilities in resource-limited settings. Bulletin of the World Health Organization. 2008;86:349–55. doi: 10.2471/BLT.07.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwer S, Newton CR, Berkley JA. Over-diagnosis and co-morbidity of severe malaria in African children: a guide for clinicians. American Journal of Tropical Medicine and Hygiene. 2007;77:6–13. [PMC free article] [PubMed] [Google Scholar]
- Haines A, Sanders D, Lehmann U, et al. Achieving child survival goals: potential contribution of community health workers. Lancet. 2007;369:2121–31. doi: 10.1016/S0140-6736(07)60325-0. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Jennings L, Chinyama M, et al. Improving community health worker use of malaria rapid diagnostic tests in Zambia: package instructions, job aid and job aid-plus-training. Malaria Journal. 2008;7:160. doi: 10.1186/1475-2875-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildenwall H, Rutebemberwa E, Nsabagasani X, et al. Local illness concepts—implications for management of childhood pneumonia in eastern Uganda. Acta Tropica. 2007;101:217–24. doi: 10.1016/j.actatropica.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Holloway KA, Karkee SB, Tamang A, et al. Community intervention to promote rational treatment of acute respiratory infection in rural Nepal. Tropical Medicine & International Health. 2009;14:101–10. doi: 10.1111/j.1365-3156.2008.02191.x. [DOI] [PubMed] [Google Scholar]
- Ishengoma DS, Francis F, Mmbando BP, et al. Accuracy of malaria rapid diagnostic tests in community studies and their impact on treatment of malaria in an area with declining malaria burden in north-eastern Tanzania. Malaria Journal. 2011;10:176. doi: 10.1186/1475-2875-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R, Colford JM, Jr, Reingold AL, Kalantri S. Nonmalarial acute undifferentiated fever in a rural hospital in central India: diagnostic uncertainty and overtreatment with antimalarial agents. American Journal of Tropical Medicine and Hygiene. 2008;78:393–9. [PubMed] [Google Scholar]
- Kallander K, Hildenwall H, Waiswa P, et al. Delayed care seeking for fatal pneumonia in children aged under five years in Uganda: a case-series study. Bulletin of the World Health Organization. 2008;86:332–8. doi: 10.2471/BLT.07.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Grand A, Hogerzeil HV, Haaijer-Ruskamp FM. Intervention research in rational use of drugs: a review. Health Policy and Planning. 1999;14:89–102. doi: 10.1093/heapol/14.2.89. [DOI] [PubMed] [Google Scholar]
- Lewin S, Munabi-Babigumira S, Glenton C, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database of Systematic Reviews. 2010:CD004015. doi: 10.1002/14651858.CD004015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Sullivan S, Khan M, Sachs S, Singh P. Community health workers in global health: scale and scalability. Mount Sinai Journal of Medicine. 2011;78:419–35. doi: 10.1002/msj.20260. [DOI] [PubMed] [Google Scholar]
- Marsh DR, Gilroy KE, Van de Weerdt R, Wansi E, Qazi S. Community case management of pneumonia: at a tipping point? Bulletin of the World Health Organization. 2008;86:381–9. doi: 10.2471/BLT.07.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrowitsch DW, Pedersen EM, Alifrangis M, et al. Is the current decline in malaria burden in sub-Saharan Africa due to a decrease in vector population? Malaria Journal. 2011;10:188. doi: 10.1186/1475-2875-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msellem MI, Martensson A, Rotllant G, et al. Influence of rapid malaria diagnostic tests on treatment and health outcome in fever patients, Zanzibar: a crossover validation study. PLoS Medicine. 2009;6:e1000070. doi: 10.1371/journal.pmed.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtango FD, Neuvians D. Acute respiratory infections in children under five years. Control project in Bagamoyo District, Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986;80:851–8. doi: 10.1016/0035-9203(86)90241-5. [DOI] [PubMed] [Google Scholar]
- Mubi M, Janson A, Warsame M, et al. Malaria rapid testing by community health workers is effective and safe for targeting malaria treatment: randomised cross-over trial in Tanzania. PLoS One. 2011;6:e19753. doi: 10.1371/journal.pone.0019753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukanga D, Tiono AB, Anyorigiya T, et al. Integrated community case management of fever in children under five using rapid diagnostic tests and respiratory rate counting: a multi-country cluster randomized trial. American Journal of Tropical Medicine and Hygiene. 2012;87:21–9. doi: 10.4269/ajtmh.2012.11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsungwa-Sabiiti J, Peterson S, Pariyo G, et al. Home-based management of fever and malaria treatment practices in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:1199–207. doi: 10.1016/j.trstmh.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Osterholt DM, Onikpo F, Lama M, Deming MS, Rowe AK. Improving pneumonia case-management in Benin: a randomized trial of a multi-faceted intervention to support health worker adherence to Integrated Management of Childhood Illness guidelines. Human Resources for Health. 2009;7:77. doi: 10.1186/1478-4491-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace R, Pluye P, Bartlett G, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. International Journal of Nursing Studies. 2012;49:47–53. doi: 10.1016/j.ijnurstu.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Perez F, Ba H, Dastagire SG, Altmann M. The role of community health workers in improving child health programmes in Mali. BMC International Health and Human Rights. 2009;9:28. doi: 10.1186/1472-698X-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluye P. Critical appraisal tools for assessing the methodological quality of qualitative, quantitative and mixed methods studies included in systematic mixed studies reviews. Journal of Evaluation in Clinical Practice. 2013;19:722. doi: 10.1111/jep.12017. [DOI] [PubMed] [Google Scholar]
- Pluye P, Gagnon MP, Griffiths F, Johnson-Lafleur J. A scoring system for appraising mixed methods research, and concomitantly appraising qualitative, quantitative and mixed methods primary studies in mixed studies reviews. International Journal of Nursing Studies. 2009;46:529–46. doi: 10.1016/j.ijnurstu.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews; a Product from the ESRC Methods Programme. 2006. Unpublished report, University of Lancaster, UK. [Google Scholar]
- Rasmussen Z, Pio A, Enarson P. Case management of childhood pneumonia in developing countries: recent relevant research and current initiatives. International Journal of Tuberculosis and Lung Disease. 2000;4:807–26. [PubMed] [Google Scholar]
- Ridde V. Reducing social inequalities in health: public health, community health or health promotion? Promotion and Education. 2007;14:63–7, 111–4. doi: 10.1177/10253823070140021401. [DOI] [PubMed] [Google Scholar]
- Riley I. Guidelines for research on acute respiratory infections: memorandum from a WHO meeting. Bulletin of the World Health Organization. 1982;60:521–33. [PMC free article] [PubMed] [Google Scholar]
- Rowe AK, Onipko F, Lama M, Cokou F, Deming MS. Management of childhood illness at health facilities in benin: problems and their causes. American Journal of Public Health. 2001;91:1625–35. doi: 10.2105/ajph.91.10.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutebemberwa E, Nsabagasani X, Pariyo G, et al. Use of drugs, perceived drug efficacy and preferred providers for febrile children: implications for home management of fever. Malaria Journal. 2009;8:131. doi: 10.1186/1475-2875-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangho H, Keita AS, Keita HD, et al. [Training mothers: a strategy to improve the treatment of acute respiratory infections among children in Mali] Sante Publique. 2012;24(Spec No):23–31. [PubMed] [Google Scholar]
- Sazawal S, Black RE. Meta-analysis of intervention trials on case-management of pneumonia in community settings. Lancet. 1992;340:528–33. doi: 10.1016/0140-6736(92)91720-s. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Pneumonia Case Management Trials G. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infectious Disease. 2003;3:547–56. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- Schellenberg A, Bryce J, De Savigny D, et al. The effect of integrated management of childhood illness on observed quality of care of under-fives in rural Tanzania. Health Policy and Planning. 2004;19:1–10. doi: 10.1093/heapol/czh001. [DOI] [PubMed] [Google Scholar]
- Shadish W, Cook T, Campbell D. Experimental and Quasi-experimental Designs for Generalized Causal Interferences. Boston: Houghton Mifflin; 2001. [Google Scholar]
- Shann F, Hart K, Thomas D. Acute lower respiratory tract infections in children: possible criteria for selection of patients for antibiotic therapy and hospital admission. Bulletin of the World Health Organization. 1984;62:749–53. [PMC free article] [PubMed] [Google Scholar]
- Siekmans K, Sohani S, Kisia J, et al. Community case management of malaria: a pro-poor intervention in rural Kenya. International Health. 2013;5:196–204. doi: 10.1093/inthealth/iht017. [DOI] [PubMed] [Google Scholar]
- Simoes EA. Community case management of severe pneumonia. Lancet. 2012;379:1703. doi: 10.1016/S0140-6736(12)60717-X. author reply 1703–4. [DOI] [PubMed] [Google Scholar]
- Soofi S, Ahmed S, Fox MP, et al. Effectiveness of community case management of severe pneumonia with oral amoxicillin in children aged 2-59 months in Matiari district, rural Pakistan: a cluster-randomised controlled trial. The Lancet. 2012;379:729–37. doi: 10.1016/S0140-6736(11)61714-5. [DOI] [PubMed] [Google Scholar]
- Theodoratou E, Al-Jilaihawi S, Woodward F, et al. The effect of case management on childhood pneumonia mortality in developing countries. International Journal of Epidemiology. 2010;39(Suppl. 1):i155–71. doi: 10.1093/ije/dyq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukwaja KN, Aina OB, Talabi AA. Clinical overlap between malaria and pneumonia: can malaria rapid diagnostic test play a role? Journal of Infection in Developing Countries. 2011;5:199–203. doi: 10.3855/jidc.945. [DOI] [PubMed] [Google Scholar]
- UNICEF. Community Case Management of Diarrhea, Malaria and Pneumonia, Tracking Science to Policy and Practice in Sub-Saharan. New York: United Nations Children's Fund; 2012. [Google Scholar]
- Walt G, Gilson L. Community Health Workers in National Programmes: Just Another Pair of Hands? Milton Keynes & Philadelphia: Open University Press; 1990. [Google Scholar]
- Weber MW, Mulholland EK, Jaffar S, et al. Evaluation of an algorithm for the integrated management of childhood illness in an area with seasonal malaria in the Gambia. Bulletin of the World Health Organization. 1997;75(Suppl. 1):25–32. [PMC free article] [PubMed] [Google Scholar]
- WHO. Basic Principles for Control of Acute Respiratory Infections in Children in Developing Countries; A Joint WHO/UNICEF Statement. Geneva: World Health Organization; 1986. [PubMed] [Google Scholar]
- WHO. Strengthening the Performance of Community Health Workers in Primary Health Care. Report of a WHO Study Group. Technical Report Series 780 [microform] Geneva: World Health Organization; 1989. [PubMed] [Google Scholar]
- WHO. Scaling up Home-based Management of Malaria: from Research to Implementation. Geneva: World Health Organization; 2004. [Google Scholar]
- WHO. Community-directed Interventions for Major Health Problems in Africa; A Multi-country Study; Final Report. Geneva: World Health Organization; 2008a. [Google Scholar]
- WHO. The Global Burden of Disease, 2004 Update. Geneva: World Health Organization; 2008b. [Google Scholar]
- WHO. Guidelines for the Treatment of Malaria. 2nd. Geneva: World Health Organization; 2010a. [PubMed] [Google Scholar]
- WHO. World Malaria Report 2010. Geneva: World Health Organization; 2010b. [Google Scholar]
- WHO Regional Office for Africa. Guidelines for the Diagnosis and Treatment of Malaria in Africa: Based on the Report of an Informal Consultation of Experts on Malaria in the African Region. Brazzaville: Regional Office for Africa of the World Health Organization; 1992. [Google Scholar]
- WHO, UNICEF. Management of Pneumonia in Community Settings. New York: World Health Organization & UNICEF; 2004. [Google Scholar]
- WHO, UNICEF. Management of Sick Children by Community Health Workers; Intervention Models and Programme Examples. Geneva: World Health Organization & UNICEF; 2006. [Google Scholar]
- WHO, UNICEF. Integrated Community Case Management (iCCM) Geneva: World Health Organization & UNICEF; 2012. [Google Scholar]
- Winch PJ, Gilroy KE, Wolfheim C, et al. Intervention models for the management of children with signs of pneumonia or malaria by community health workers. Health Policy and Planning. 2005;20:199–212. doi: 10.1093/heapol/czi027. [DOI] [PubMed] [Google Scholar]
- Yeboah-Antwi K, Pilingana P, Macleod WB, et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Medicine. 2010;7:e1000340. doi: 10.1371/journal.pmed.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coding table of the 15 studies included for narrative synthesis
- 1.Degefie T, Marsh D, Gebremariam A, et al. Community case management improves use of treatment for childhood diarrhea, malaria and pneumonia in a remote district of Ethiopia. Ethiopian Journal of Health Development. 2009;23:120–6. [Google Scholar]
- 2.Greenwood BM, Bradley AK, Byass P, et al. Evaluation of a primary health care programme in The Gambia. II. Its impact on mortality and morbidity in young children. Journal of Tropical Medicine and Hygiene. 1990;93:87–97. [PubMed] [Google Scholar]
- 3.Hamer DH, Brooks ET, Semrau K, et al. Quality and safety of integrated community case management of malaria using rapid diagnostic tests and pneumonia by community health workers. Pathogens and Global Health. 2012;106:32–9. doi: 10.1179/1364859411Y.0000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallander K, Tomson G, Nsabagasani X, et al. Can community health workers and caretakers recognise pneumonia in children? Experiences from western Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100:956–63. doi: 10.1016/j.trstmh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Kalyango JN, Rutebemberwa E, Alfven T, et al. Performance of community health workers under integrated community case management of childhood illnesses in eastern Uganda. Malaria Journal. 2012;11:282. doi: 10.1186/1475-2875-11-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly JM, Osamba B, Garg RM, et al. Community health worker performance in the management of multiple childhood illnesses: Siaya District, Kenya, 1997-2001. American Journal of Public Health. 2001;91:1617–24. doi: 10.2105/ajph.91.10.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mtango FD, Neuvians D. Acute respiratory infections in children under five years. Control project in Bagamoyo District, Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986;80:851–8. doi: 10.1016/0035-9203(86)90241-5. [DOI] [PubMed] [Google Scholar]
- 8.Mukanga D, Babirye R, Peterson S, et al. Can lay community health workers be trained to use diagnostics to distinguish and treat malaria and pneumonia in children? Lessons from rural Uganda. Tropical Medicine and International Health. 2011;16:1234–42. doi: 10.1111/j.1365-3156.2011.02831.x. [DOI] [PubMed] [Google Scholar]
- 9.Mukanga D, Tibenderana JK, Peterson S, et al. Access, acceptability and utilization of community health workers using diagnostics for case management of fever in Ugandan children: a cross-sectional study. Malaria Journal. 2012a;11:121. doi: 10.1186/1475-2875-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe SY, Olewe MA, Kleinbaum DG, et al. The influence of observation and setting on community health workers' practices. International Journal for Quality in Health Care. 2006;18:299–305. doi: 10.1093/intqhc/mzl009. [DOI] [PubMed] [Google Scholar]
- 11.Rowe SY, Kelly JM, Olewe MA, et al. Effect of multiple interventions on community health workers' adherence to clinical guidelines in Siaya district, Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007a;101:188–202. doi: 10.1016/j.trstmh.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Rowe SY, Olewe MA, Kleinbaum DG, et al. Longitudinal analysis of community health workers' adherence to treatment guidelines, Siaya, Kenya, 1997-2002. Tropical Medicine and International Health. 2007b;12:651–63. doi: 10.1111/j.1365-3156.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 13.Sylla A, Sarr CS, Gueye EH, et al. [Assessment of management training for low-level community health workers providing care for children with acute respiratory infections in four districts of Senegal] Revue d'épidémiologie et de Santé Publique. 2004;52:243–7. doi: 10.1016/s0398-7620(04)99049-9. [DOI] [PubMed] [Google Scholar]
- 14.Sylla A, Gueye EH, N'Diaye O, et al. [Low level educated community health workers training: a strategy to improve children access to acute respiratory treatment in Senegal] Archives de Pediatrie. 2007;14:244–8. doi: 10.1016/j.arcped.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Yeboah-Antwi K, Pilingana P, Macleod WB, et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Medicine. 2010;7:e1000340. doi: 10.1371/journal.pmed.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.