Abstract
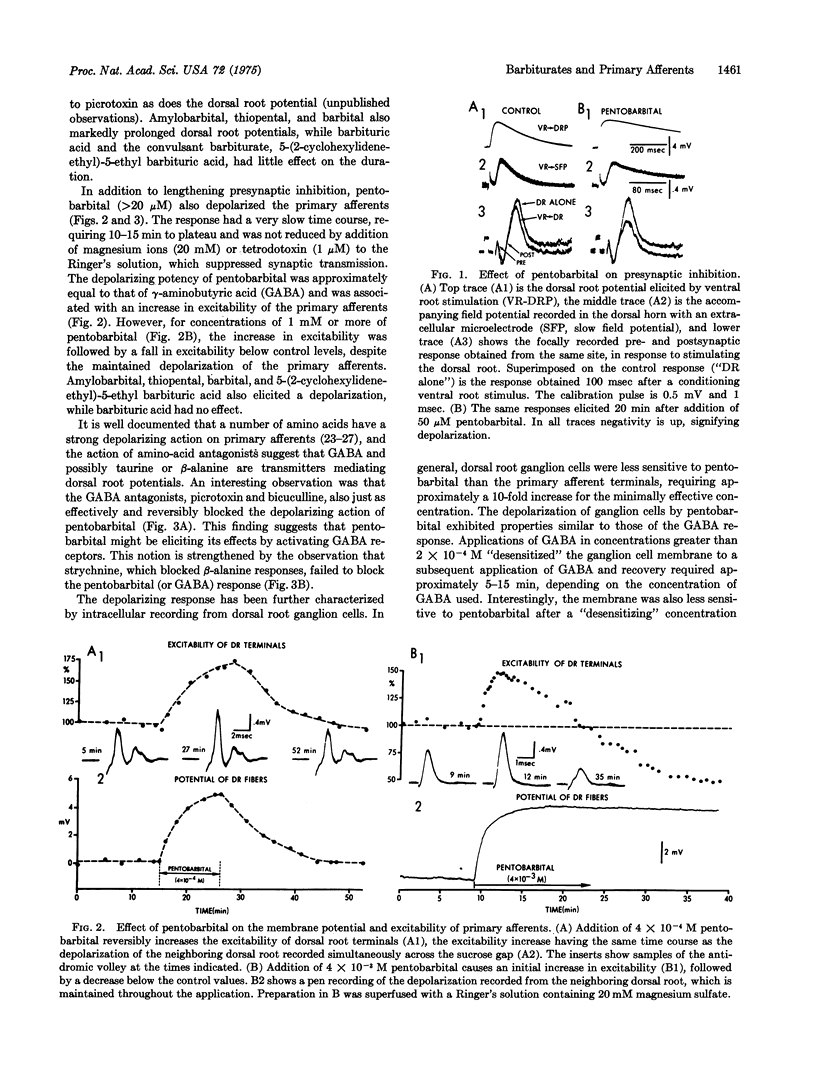
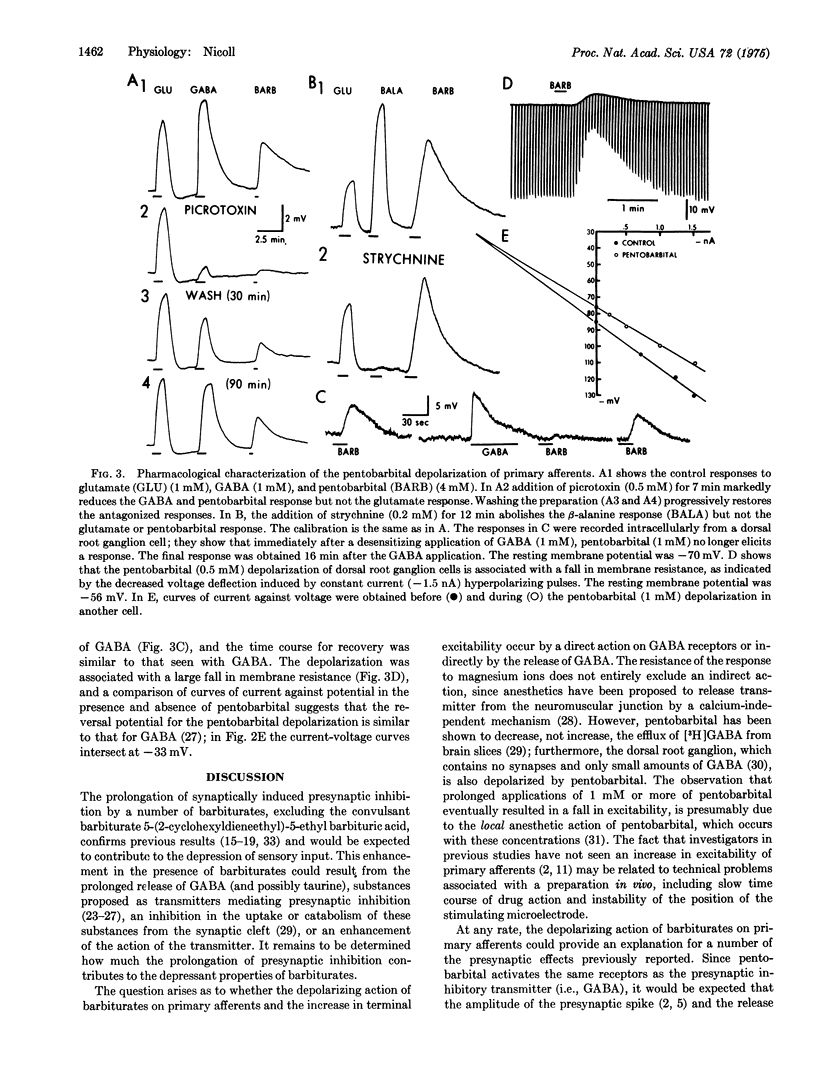
The action of pentobarbital on primary afferents of the isolated frog spinal cord was analyzed with sucrose gap and intracellular recordings techniques. Pentobarbital in concentrations generally considered to be in the anesthetic range greatly prolonged presynaptic inhibition and also depolarized primary afferents. The depolarization was accompanied by an increase in excitability and resulted from activation of gamma-aminobutyric acid receptors, possibly by a direct action on these receptors, since the depolarization was reversibly blocked by gamma-aminobutyric acid, but not by glycine, antagonists, and magnesium ions. Furthermore, dorsal root ganglion cells exhibited a reduced sensitivity to both gamma-aminobutyric acid and pentobarbital after a "desensitizing" dose of gamma aminobutyric acid. The prolongation of presynaptic inhibition and the activation of gamma-aminobutyric acid receptors on primary afferents by pentobarbital should act to reduce the amount of transmitter released from the first synapse in sensory pathways.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banna N. R., Jabbur S. J. Pharmacological studies on inhibition in the cuneate nucleus of the cat. Int J Neuropharmacol. 1969 May;8(3):299–307. doi: 10.1016/0028-3908(69)90051-3. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Gainer H. Pentobarbital: selective depression of excitatory postsynaptic potentials. Science. 1973 Nov 16;182(4113):720–722. doi: 10.1126/science.182.4113.720. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. Gamma-aminobutyric acid: role in primary afferent depolarization. Science. 1972 Jun 2;176(4038):1043–1045. doi: 10.1126/science.176.4038.1043. [DOI] [PubMed] [Google Scholar]
- Bloedel J. R., Roberts W. J. Functional relationship among neurons of the cerebellar cortex in the absence of anesthesia. J Neurophysiol. 1969 Jan;32(1):75–84. doi: 10.1152/jn.1969.32.1.75. [DOI] [PubMed] [Google Scholar]
- Bremer F. Inhibitions intrathalamiques récurrentielles et physiologie du sommeil. Electroencephalogr Clin Neurophysiol. 1970 Jan;28(1):1–16. doi: 10.1016/0013-4694(70)90002-7. [DOI] [PubMed] [Google Scholar]
- Cutler R. W., Markowitz D., Dudzinski D. S. The effect of barbiturates on (3H)GABA transport in rat cerebral cortex slices. Brain Res. 1974 Dec 6;81(2):189–197. doi: 10.1016/0006-8993(74)90935-4. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Gamma-aminobutyric acid antagonism and presynaptic inhibition in the frog spinal cord. Science. 1972 Jan 21;175(4019):331–333. doi: 10.1126/science.175.4019.331. [DOI] [PubMed] [Google Scholar]
- Downes H., Franz D. N. Effects of a convulsant barbiturate on dorsal root ganglion cells and dorsal root discharges. J Pharmacol Exp Ther. 1971 Dec;179(3):660–670. [PubMed] [Google Scholar]
- Downes H., Williams J. K. Effects of a convulsant barbiturate on the spinal monosynaptic pathway. J Pharmacol Exp Ther. 1969 Aug;168(2):283–289. [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Faber D. S., Táboríková H. The action of a parallel fiber volley on the antidromic invasion of Purkyne cells of cat cerebellum. Brain Res. 1971 Jan 22;25(2):335–356. doi: 10.1016/0006-8993(71)90442-2. [DOI] [PubMed] [Google Scholar]
- Galindo A. Effects of procaine, pentobarbital and halothane on synaptic transmission in the central nervous system. J Pharmacol Exp Ther. 1969 Oct;169(2):185–195. [PubMed] [Google Scholar]
- Glusman S., Rudomín P. Presynaptic modulation of synaptic effectiveness of afferent and ventrolateral tract fibers in the frog spinal cord. Exp Neurol. 1974 Dec;45(3):474–490. doi: 10.1016/0014-4886(74)90153-8. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Karczmar A. G., Kitamura R. Acetylcholine depolarization of the dorsal root nerve terminals in the amphibian spinal cord. Int J Neuropharmacol. 1969 Jul;8(4):329–336. doi: 10.1016/0028-3908(69)90018-5. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- Larson M. D., Major M. A. The effect of hexobarbital on the duration of the recurrent IPSP in cat motoneurons. Brain Res. 1970 Jul 14;21(2):309–311. doi: 10.1016/0006-8993(70)90377-x. [DOI] [PubMed] [Google Scholar]
- Miyahara J. T., Esplin D. W., Zablocka B. Differential effects of depressant drugs on presynaptic inhibition. J Pharmacol Exp Ther. 1966 Oct;154(1):119–127. [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. Effect of strychnine on dorsal root potentials and amino acid responses in frog spinal cord. Nat New Biol. 1973 Dec 19;246(155):224–225. doi: 10.1038/newbio246224a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Richards C. D. On the mechanism of barbiturate anaesthesia. J Physiol. 1972 Dec;227(3):749–767. doi: 10.1113/jphysiol.1972.sp010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richens A. Microelectrode studies in the frog isolated spinal cord during depression by general anaesthetic agents. Br J Pharmacol. 1969 Jun;36(2):312–328. doi: 10.1111/j.1476-5381.1969.tb09508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. J., Keen P., Mitchell J. F. The distribution and axonal transport of free amino acids and related compounds in the dorsal sensory neuron of the rat, as determined by the dansyl reaction. J Neurochem. 1973 Jul;21(1):199–209. doi: 10.1111/j.1471-4159.1973.tb04239.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- Somjen G. Effects of anesthetics on spinal cord of mammals. Anesthesiology. 1967 Jan-Feb;28(1):135–143. doi: 10.1097/00000542-196701000-00015. [DOI] [PubMed] [Google Scholar]
- Staiman A., Seeman P. The impulse-blocking concentrations of anesthetics, alcohols, anticonvulsants, barbiturates, and narcotics on phrenic and sciatic nerves. Can J Physiol Pharmacol. 1974 Jun;52(3):535–550. doi: 10.1139/y74-071. [DOI] [PubMed] [Google Scholar]
- Tebecis A. K., Phillis J. W. The use of convulsants in studying possible functions of amino acids in the toad spinal cord. Comp Biochem Physiol. 1969 Mar;28(3):1303–1315. doi: 10.1016/0010-406x(69)90568-4. [DOI] [PubMed] [Google Scholar]
- Thomson T. D., Turkanis S. A. Barbiturate-induced transmitter release at a frog neuromuscular junction. Br J Pharmacol. 1973 May;48(1):48–58. doi: 10.1111/j.1476-5381.1973.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veseliunene M. A., Gutman A. M., Lesene V. A. Vliianie nembutala na tormoznuiu volnu antidromno vyzvannogo potentsiala motornoi kory koshki. Farmakol Toksikol. 1971 Sep-Oct;34(5):520–522. [PubMed] [Google Scholar]
- Wall P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):i3–21. [PMC free article] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]


