Abstract
A high-density fraction of high-molecular-weight DNA was isolated from the human lymphoid cell line Raji. This cell line contains 50 to 60 virus genome equivalents of Epstein-Barr virus DNA per cell, and the high-density DNA fraction was 10-fold enriched in such viral sequences. Sedimentation analysis on neutral glycerol gradients, followed by hybridization experiments with viral complementary RNA, showed that most of the intracellular viral DNA sequences in this material did not cosediment with the cellular DNA, but were recovered as two distinct species with sedimentation coefficients of 100 S and 65 S. These two forms sediment 1.70-1.75 and 1.10-1.12 times as fast as the linear Epstein-Barr virus DNA from virus particles, and thus have the hydrodynamic properties of a covalently closed circular form and a nicked (containing single-strand breaks) circular form of the virus genome. The 100S form also behaved as a covalently closed circular EBV DNA molecule on gradient centrifugation in CsC1/propidium diiodide, It would appear that latent Epstein-Barr virus DNA has the properties of a mammalian episome, and that both nonintegrated and integrated viral DNA sequences can be isolated from carrier cells.
Full text
PDF
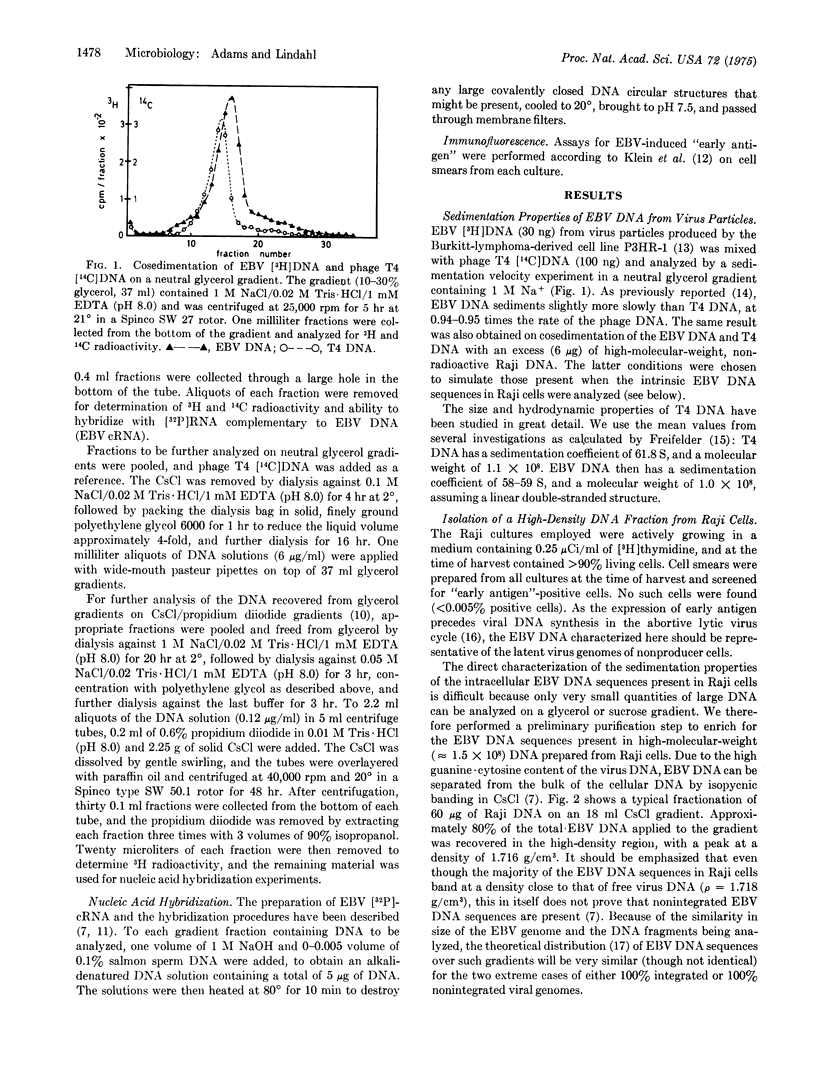

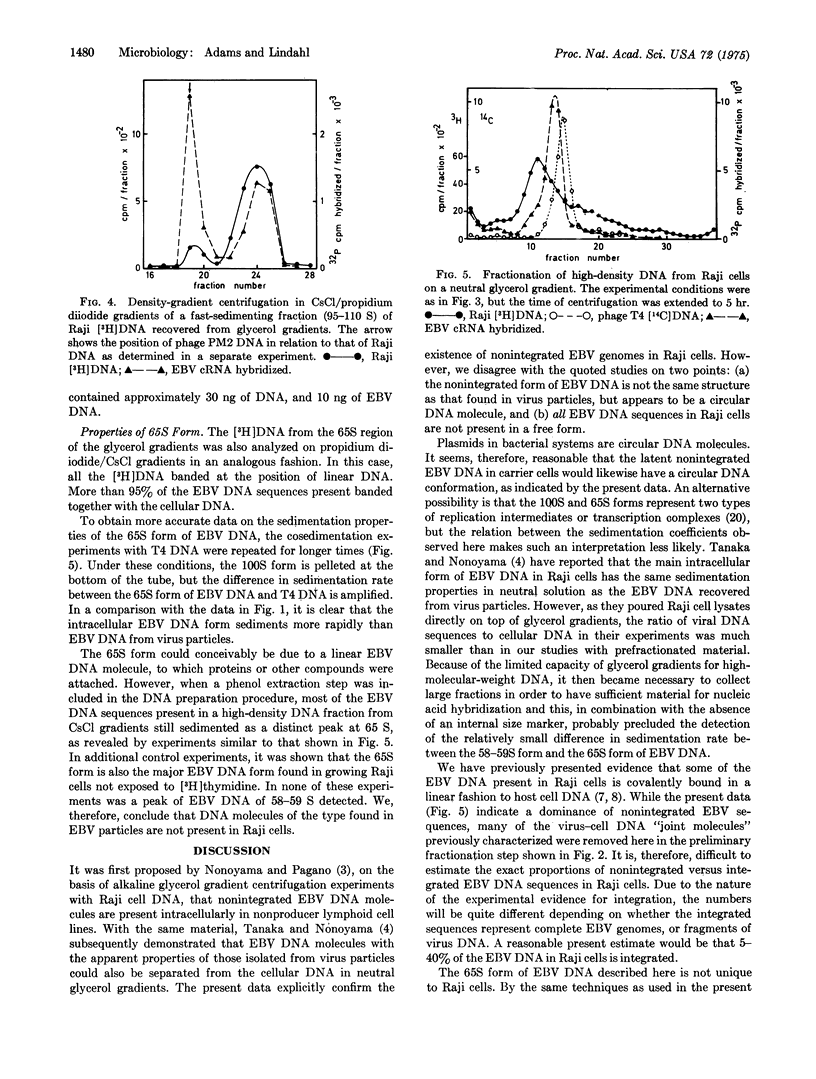

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Rensing U., Philipson L. Intracellular forms of Adenovirus DNA. II. Isolation in dye-buoyant density gradients of a DNA-RNA complex from KB cells infected with Adenovirus type 2. J Virol. 1973 Oct;12(4):793–807. doi: 10.1128/jvi.12.4.793-807.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Gergely L., Klein G., Ernberg I. Appearance of Epstein-Barr virus-associated antigens in infected Raji cells. Virology. 1971 Jul;45(1):10–21. doi: 10.1016/0042-6822(71)90107-3. [DOI] [PubMed] [Google Scholar]
- Helinski D. R., Clewell D. B. Circular DNA. Annu Rev Biochem. 1971;40:899–942. doi: 10.1146/annurev.bi.40.070171.004343. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Jehn U., Lindahl T., Klein C. Fate of virus DNA in the abortive infection of human lymphoid cell lines by Epstein-Barr virus. J Gen Virol. 1972 Sep;16(3):409–412. doi: 10.1099/0022-1317-16-3-409. [DOI] [PubMed] [Google Scholar]
- Klein G., Gergely L., Goldstein G. Two-colour immunofluorescence studies on EBV-determined antigens. Clin Exp Immunol. 1971 Apr;8(4):593–602. [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Klein G., Reedman B. M., Johansson B., Singh S. Relationship between Epstein-Barr virus (EBV) DNA and the EBV-determined nuclear antigen (EBNA) in Burkitt lymphoma biopsies and other lymphoproliferative malignancies. Int J Cancer. 1974 Jun 15;13(6):764–772. doi: 10.1002/ijc.2910130605. [DOI] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. A defective P1 prophage with a chromosomal location. Virology. 1970 Jan;40(1):144–151. doi: 10.1016/0042-6822(70)90386-7. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA J. I., ANRAKU N. MOLECULAR MECHANISMS OF GENETIC RECOMBINATION IN BACTERIOPHAGE. II. JOINING OF PARENTAL DNA MOLECULES OF PHAGE T4. J Mol Biol. 1964 Apr;8:516–540. doi: 10.1016/s0022-2836(64)80009-7. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Nonoyama M. Latent DNA of Epstein-Barr virus: separation from high-molecular-weight cell DNA in a neutral glycerol gradient. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4658–4661. doi: 10.1073/pnas.71.12.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Rosner A. Effect of adenosine and deoxyadenosine on the incorporation and breakdown of thymidine in Escherichia coli K-12. J Bacteriol. 1970 Aug;103(2):417–421. doi: 10.1128/jb.103.2.417-421.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


