Abstract
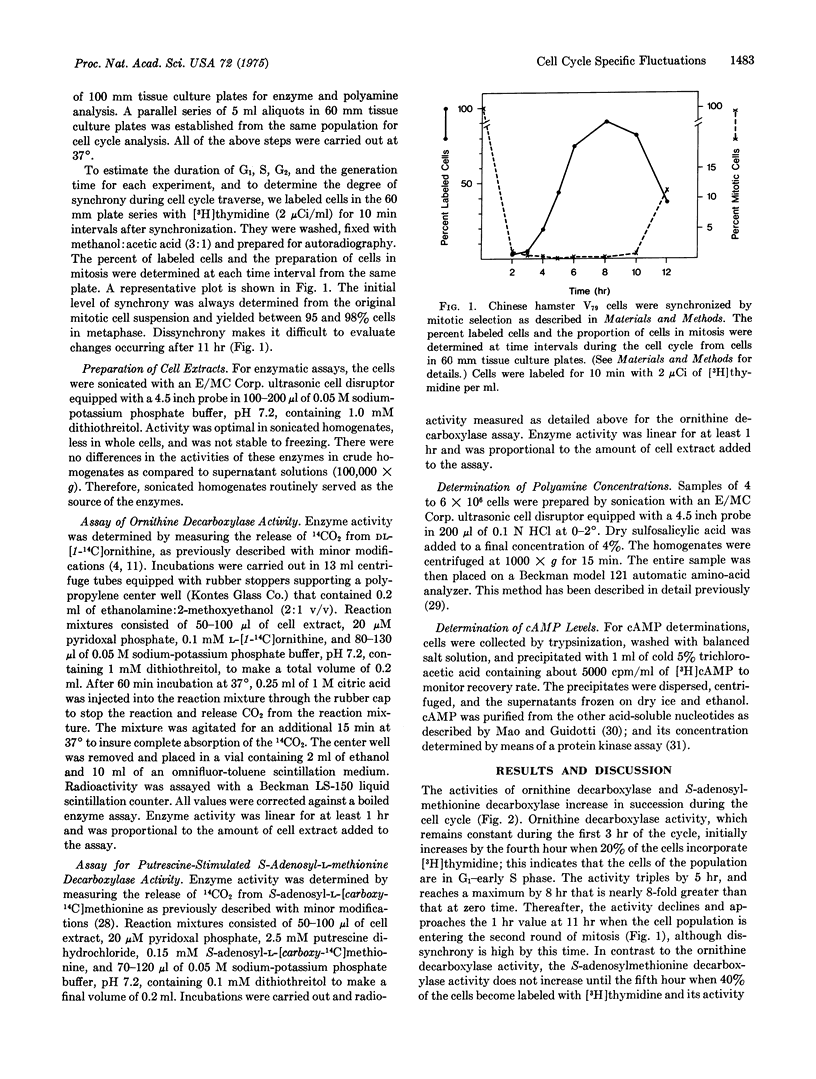
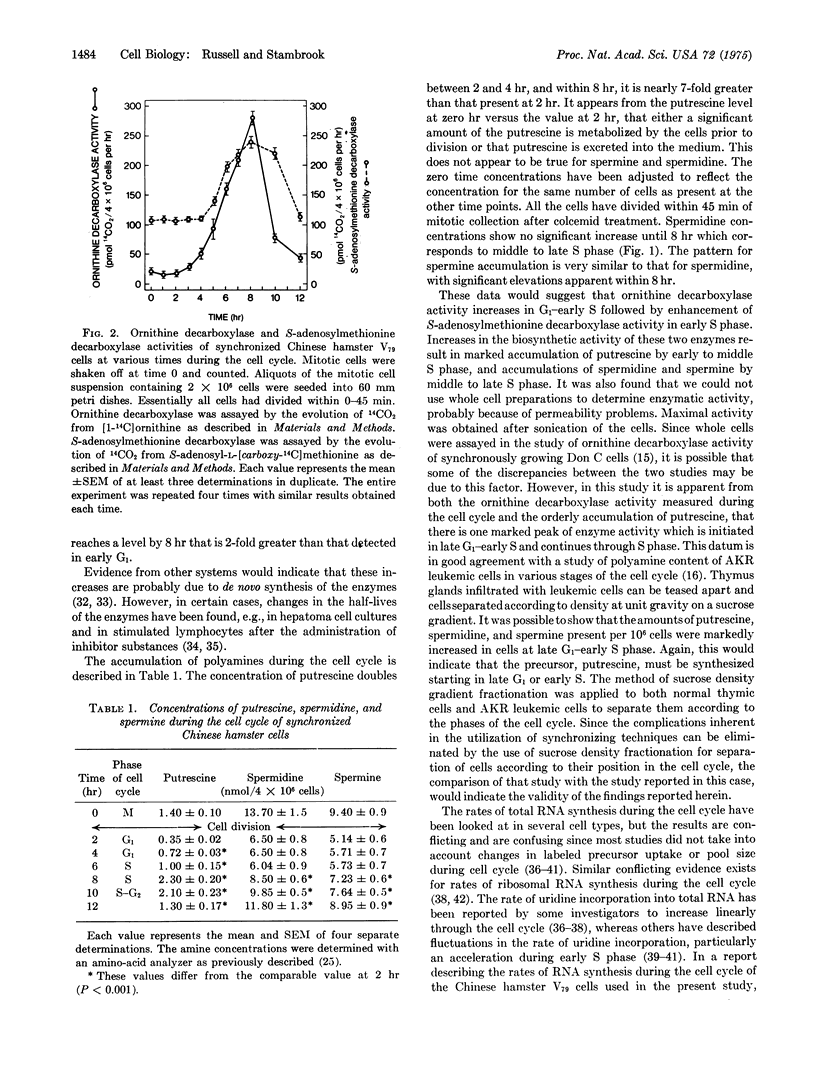
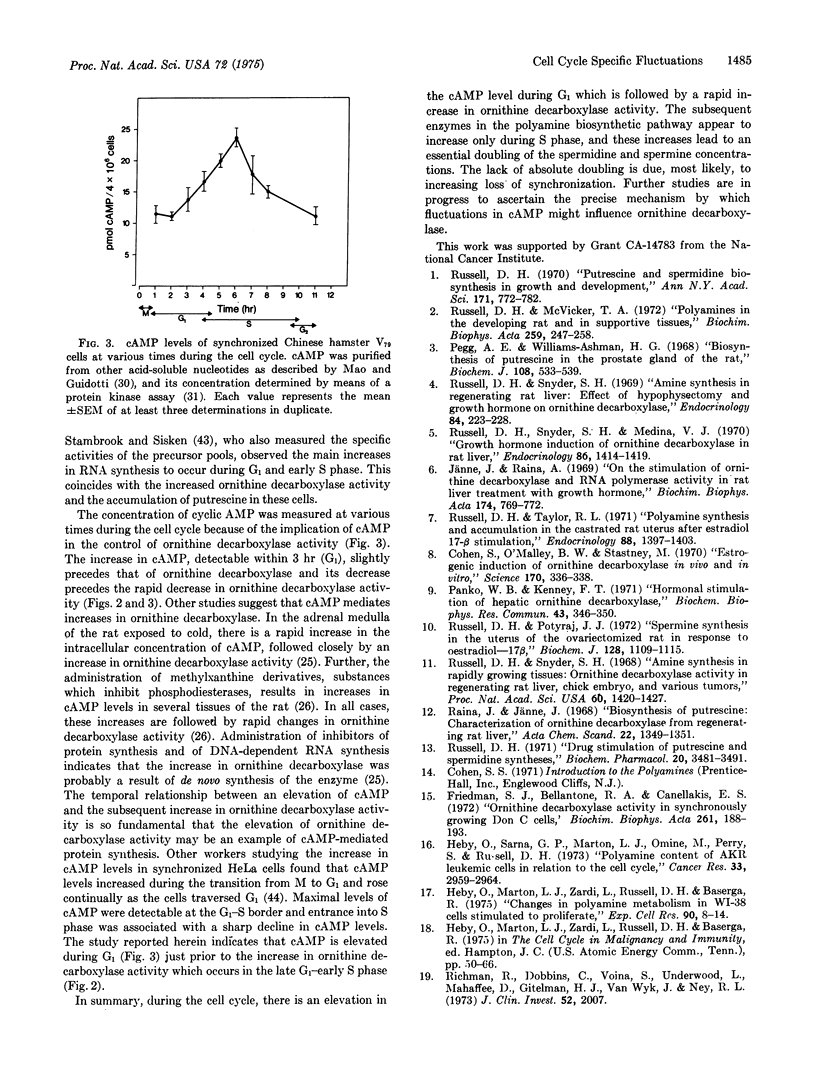
Chinese hamster V79 cells were synchronized by mitotic selection, which resulted in approximately 95% synchrony. The adenosine 3':5'-cyclic monophosphate level was elevated within 3 hr (G1 phase) and reached a level 2-fold higher than in early G1 within 6 hr (early S phase). An increase in ornithine decarboxylase activity (6-ornithine carboxy-lyase, EC 4.1.1.17), the initial enzyme in the polyamine biosynthetic pathway, was detected within 4 hr and was maximal at 8 hr. Since about 20% of the cells were labeled with [3-H]thymidine at 4 hr, ornithine decarboxylase exhibits cell-cycle specific activity starting in late G1 and continuing through middle S phase. The activity of S-adenosylmethionine decarboxylase (S-adenosyl-L-methionine carboxylase, EC 4.1.1.50) increased within 5 hr, i.e., early S phase. It is suggested on the basis of these data and other studies discussed herein that the increase in ornithine decarboxylase activity, which parallels closely the elevation in cyclic AMP, is an example of adenosine 3':5'-cyclic monophosphate-mediated protein synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byus C. V., Russell D. H. Ornithine decarboxylase activity: control by cyclic nucleotides. Science. 1975 Feb 21;187(4177):650–652. doi: 10.1126/science.163486. [DOI] [PubMed] [Google Scholar]
- Cohen S., O'Malley B. W., Stastny M. Estrogenic induction of ornithine decarboxylase in vivo and in vitro. Science. 1970 Oct 16;170(3955):336–338. doi: 10.1126/science.170.3955.336. [DOI] [PubMed] [Google Scholar]
- Enger M. D., Tobey R. A. RNA synthesis in Chinese hamster cells. II. Increase in rate of RNA synthesis during G1. J Cell Biol. 1969 Jul;42(1):308–315. doi: 10.1083/jcb.42.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. S-adenosyl-L-methionine decarboxylase during lymphocyte transformation: decreased degradation in the presence of a specific inhibitor. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1020–1025. doi: 10.1016/0006-291x(73)91039-5. [DOI] [PubMed] [Google Scholar]
- Friedman S. J., Bellantone R. A., Canellakis E. S. Ornithine decarboxylase activity in synchronously growing Don C cells. Biochim Biophys Acta. 1972 Jan 28;261(1):188–193. doi: 10.1016/0304-4165(72)90329-7. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Zardi L., Russell D. H., Baserga R. Changes in polyamine metabolism in WI38 cells stimulated to proliferate. Exp Cell Res. 1975 Jan;90(1):8–14. doi: 10.1016/0014-4827(75)90350-x. [DOI] [PubMed] [Google Scholar]
- Heby O., Sarna G. P., Marton L. J., Omine M., Perry S., Russell D. H. Polyamine content of AKR leukemic cells in relation to the cell cycle. Cancer Res. 1973 Nov;33(11):2959–2964. [PubMed] [Google Scholar]
- Hogan B., Shields R., Curtis D. Effect of cyclic nucleotides on the induction of ornithine decarboxylase in BHK cells by serum and insulin. Cell. 1974 Aug;2(4):229–233. doi: 10.1016/0092-8674(74)90015-4. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Raina A. Stimulation of ornithine decarboxylase and nuclear RNA polymerase activity in rat liver by glucagon and dibutyryl cyclic amp. Acta Endocrinol (Copenh) 1973 Aug;73(4):794–800. doi: 10.1530/acta.0.0730794. [DOI] [PubMed] [Google Scholar]
- Jänne J., Raina A. On the stimulation of ornithine decarboxylase and RNA polymerase activity in rat liver after treatment with growth hormone. Biochim Biophys Acta. 1969 Feb 18;174(2):769–772. doi: 10.1016/0005-2787(69)90310-4. [DOI] [PubMed] [Google Scholar]
- Jänne J., Raina A. Stimulation of spermidine synthesis in the regenerating rat liver: relation to increased ornithine decarboxylase activity. Acta Chem Scand. 1968;22(4):1349–1351. doi: 10.3891/acta.chem.scand.22-1349. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Perez A. G. Ribonucleic acid synthesis in synchronously dividing populations of HeLa cells. Nature. 1965 Aug 28;207(5000):974–975. doi: 10.1038/207974a0. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. An assay method for cyclic AMP and cyclic GMP based upon their abilities to activate cyclic AMP-dependent and cyclic GMP-dependent protein kinases. Adv Cyclic Nucleotide Res. 1972;2:41–50. [PubMed] [Google Scholar]
- Panko W. B., Kenney F. T. Hormonal stimulation of hepatic ornithine decarboxylase. Biochem Biophys Res Commun. 1971 Apr 16;43(2):346–350. doi: 10.1016/0006-291x(71)90759-5. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Biosynthesis of putrescine in the prostate gland of the rat. Biochem J. 1968 Jul;108(4):533–539. doi: 10.1042/bj1080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pfeiffer S. E. RNA synthesis in synchronously growing populations of HeLa S3 cells. II. Rate of synthesis of individual RNA fractions. J Cell Physiol. 1968 Feb;71(1):95–104. doi: 10.1002/jcp.1040710111. [DOI] [PubMed] [Google Scholar]
- Preslock J. P., Hampton J. K., Jr Ornithine decarboxylase stimulation by 3',5'-cyclic AMP in oviduct of Coturnix quail. Am J Physiol. 1973 Oct;225(4):903–907. doi: 10.1152/ajplegacy.1973.225.4.903. [DOI] [PubMed] [Google Scholar]
- Richman R., Dobbins C., Voina S., Underwood L., Mahaffee D., Gitelman, Van Wyk J., Ney R. L. Regulation of adrenal ornithine decarboxylase by adrenocorticotropic hormone and cyclic AMP. J Clin Invest. 1973 Aug;52(8):2007–2015. doi: 10.1172/JCI107385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel D. H., McVicker T. A. Polyamines in the developing rat and in supportive tissues. Biochim Biophys Acta. 1972 Jan 31;259(2):247–258. doi: 10.1016/0005-2787(72)90065-2. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Drug stimulation of putrescine and spermidine syntheses. Relationships to enhancement of ribonucleic acid synthesis. Biochem Pharmacol. 1971 Dec;20(12):3481–3491. doi: 10.1016/0006-2952(71)90453-9. [DOI] [PubMed] [Google Scholar]
- Russell D. H., McVicker T. A. Polyamine metabolism in mouse liver after partial hepatectomy. Biochim Biophys Acta. 1971 Jul 20;244(1):85–93. doi: 10.1016/0304-4165(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Potyraj J. J. Spermine synthesis in the uterus of the ovariectomized rat in response to oestradiol-17 . Biochem J. 1972 Aug;128(5):1109–1115. doi: 10.1042/bj1281109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: effect of hypophysectomy and growth hormone on ornithine decarboxylase. Endocrinology. 1969 Feb;84(2):223–228. doi: 10.1210/endo-84-2-223. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H., Medina V. J. Growth hormone induction of ornithine decarboxylase in rat liver. Endocrinology. 1970 Jun;86(6):1414–1419. doi: 10.1210/endo-86-6-1414. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Taylor R. L. Polyamine synthesis and accumulation in the castrated rat uterus after estradiol-17-beta stimulation. Endocrinology. 1971 Jun;88(6):1397–1403. doi: 10.1210/endo-88-6-1397. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambrook P. J., Sisken J. E. Induced changes in the rates of uridine- 3 H uptake and incorporation during the G 1 and S periods of synchronized Chinese hamster cells. J Cell Biol. 1972 Mar;52(3):514–525. doi: 10.1083/jcb.52.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambrook P. J., Sisken J. E. The relationship between rates of ( 3 H)uridine and ( 3 H)adenine incorporation into RNA and the measured rates of RNA synthesis during the cell cycle. Biochim Biophys Acta. 1972 Sep 29;281(1):45–54. doi: 10.1016/0005-2787(72)90186-4. [DOI] [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res. 1963 Apr;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]


