Abstract
Traps are widely employed for sampling and monitoring mosquito populations for surveillance, ecological and fauna studies. Considering the importance of assessing other technologies for sampling mosquitoes, we addressed the effectiveness of Mosquito Magnet® Independence (MMI) in comparison with those of the CDC trap with CO2 and Lurex3® (CDC-A) and the CDC light trap (CDC-LT). Field collections were performed in a rural area within the Atlantic Forest biome, southeastern state of São Paulo, Brazil. The MMI sampled 53.84% of the total number of mosquitoes, the CDC-A (26.43%) and CDC-LT (19.73%). Results of the Pearson chi-squared test (χ2) showed a positive association between CDC-LT and species of Culicini and Uranotaeniini tribes. Additionally, our results suggested a positive association between CDC-A and representatives of the Culicini and Aedini tribes, whereas the MMI was positively associated with the Mansoniini and Sabethini as well as with Anophelinae species. The MMI sampled a greater proportion (78.27%) of individuals of Anopheles than either the CDC-LT (0.82%) or the CDC-A traps (20.91%). Results of the present study showed that MMI performed better than CDC-LT or CDC-A in sampling mosquitoes in large numbers, medically important species and assessing diversity parameters in rural southeastern Atlantic Forest.
Keywords: effectiveness, Mosquito Magnet® Independence, diversity, surveillance
Traps are important tools for sampling adult mosquito populations for ecological studies (Forattini et al. 1991, Jones et al. 2004) and for the surveillance of disease vectors (Bisevac et al. 2009). There are several kinds of trap that can be used with or without chemical attractants. Different models of traps possess particular characteristics that may influence the abundance of mosquito species that are potentially attracted by the light and/or chemicals employed with the trap and also in the diversity index estimated with it in a specific environment (Dusfour et al. 2010). According to Gomes et al. (1985) and Forattini (2002), the CDC light trap (CDC-LT) is widely used in entomological studies. The CDC trap was originally designed by Sudia and Chamberlain in 1962. It was constructed to use a point light source. However, over the years CDC trap has been utilised in association with carbon dioxide (CO2) to simulate the presence of a vertebrate (Forattini 2002). The employment of chemicals as attractants (chemical kairomones) in mosquito traps has increased the probability of sampling a larger number of mosquitoes, suggesting that they can be as effective as vertebrate animals in the collection of mosquitoes (Brown et al. 2008). Furthermore, the employment of traps has facilitated comparison of the data obtained in distinct regions since the trap eliminates the bias caused by human ability to capture live mosquitoes.
The efficiency of CDC traps associated with attractants and of CDC-LT has been proved by the results of several studies (Forattini et al. 1991, Hutchings et al. 2005, 2013, Montes 2005, Laporta & Sallum 2011). Furthermore, these traps are considered effective tools for sampling mosquitoes (Cardoso et al. 2011, d, de Sá & Sallum 2013). The performance of different models of Mosquito Magnet® (MM) (Woodstream Corporation, USA) for sampling mosquitoes has been compared with that of other collection methods, including that of human attraction. As a result, distinct models of MM have shown good performance for sampling mosquito populations under different environmental and climate conditions (Pucci et al. 2003, Brown et al. 2008, Xue et al. 2008, Kitau et al. 2010, Morrow et al. 2010 , Hiwat et al. 2011a, Jawara et al. 2011, Missawa et al. 2011, d, de Sá & Sallum 2013).
The performance of the model Mosquito Magnet® Independence (MMI) has been addressed in two previous studies conducted in areas on the Brazilian Atlantic Forest coast. In the first, de Sá and Sallum (2013) assessed the effectiveness of MMI in comparison to those of the CDC with CO2 and Lurex® (CDC-A) and with CDC-LT in three rural areas. A second study was conducted in an area of preserved forest (Chaves et al. 2014), employing the same sampling methods and traps utilised by de Sá and Sallum (2013). The results of both studies have confirmed that the effectiveness of the MMI is higher than that of CDC traps whether with or without attractants. Considering the importance of assessing the performance of MM in distinct climate and environmental conditions and also the need for testing new technologies for collecting mosquitoes, we compared the effectiveness of MMI to that of a CDC-A and a CDC trap with a light source, in lowland, rural areas within the Atlantic Forest biome in the municipality of Iguape, southeastern state of São Paulo (SP), Brazil.
MATERIALS AND METHODS
Mosquitoes were sampled in a rural area, on the Santa Rosa Farm (24.78951ºS 047.78068ºW, South American Datum 69), located at the Serra Azul neighbourhood, Prefeito Ivo Zanella State Highway, SP-222, km 87, Iguape (Fig. 1).
Fig. 1. : location of collection area in the municipality of Iguape, state of São Paulo, Brazil, 2012.
Mosquito collections were carried out monthly over the course of three summer months and three autumn months, totaling six months of field collections (from January-June 2012). A Latin square collection design was adopted to avoid potential biases caused by ecological and climatic peculiarities associated with the traps’ locations (Kline 2002) that might influence their performance - either positively or negatively of the traps. Three locations were randomly selected on the Santa Rosa farm, except for the distance among the traps that was somewhat fixed. Accordingly, each trap was separated from each other by approximately 200 m. Based on the published literature records, in order to avoid interference among the traps they should be kept separate by distances that normally vary from 30-50 m (Cilek & Hallmon 2005, Hiwat et al. 2011a). To ensure that there was no interference among the traps, we adopted a distance of approximately 200 m. All traps were installed and removed at the same time and period in day one and rotated in the consecutive two days. The CDC-LT and CDC-A were installed at approximately 1.5 m above ground level in a way that the light source, the Lurex and CO2 release point were at the same distance of the MMI inlet relative to the ground level. The traps were switched among the three locations over consecutive days and were left running for 12 h per day (from 06:00 pm-06.00 am), totaling 36 h per month for each trap.
The CDC-LT trap was chosen because it is widely used in entomological surveys. Another choice was the CDC trap without light source, but with attractive. According to Forattini et al. (1989) and Kline et al. (1990), the use of the CO2 and lactic acid as attractive in traps increases mosquitoes capture. The MMI was chosen to compare its effectiveness with other traps used in most entomological studies. Thus, in the present study, we evaluated a CDC-LT, a CDC-A and an MMI with CO2 plus Lurex3®. The CO2 used in the CDC was obtained from a compressed gas cylinder with a controlled flow rate of 450 mL CO2 per minute. The release of the CO2 in the trap was controlled by a low-pressure valve (Swagelok®, USA). The cartridge of the Lurex3® contained 4.88 g of lactic acid incorporated to 13.8 g of a gel matrix, thus releasing 230 mg/day of lactic acid (Hoel et al. 2007). The MMI simulates the human presence by releasing CO2, heat, humidity and lactic acid provided by the cartridge of the Lurex3®, identical to that used in the CDC-A.
The data obtained with the CDC-LT were employed as the baseline for comparative analyses. This procedure was adopted because the CDC-LT has been widely employed for surveying mosquito fauna (Hutchings et al. 2011), biodiversity studies (Cardoso et al. 2011) and entomological surveillance (Cardoso et al. 2010b, Mascheretti et al. 2013), thus facilitating comparisons. Mosquitoes were individually identified employing the morphological keys proposed by Lane (1953), Galindo et al. (1954), Correa and Ramalho (1956), Consoli and Lourenço-de-Oliveira (1998) and Forattini (2002).
The efficiency of traps was measured using two parameters: the diversity of species sampled by each trap and the performance of each trap, i.e., how each trap undertook the mosquito sampling. For this, the time spent working on the basis of abundance and species selectivity at the level of subfamily and tribe was analysed.
Statistical analyses were undertaken with the packages “Biodiversity” (Kindt & Coe 2005) and “Venneuler” (Chen & Boutros 2011) of the program R v.2.15.2. The homogeneity of variances was assessed by Levene’s test and the normality of abundance data was examined by the Shapiro-Wilk and Kolmogorov-Smirnov statistical tests. The diversity of species per trap was estimated by the diversity index obtained in the profile of the Rényi series (Melo 2008) that generalises total richness (α = 0), diversity (Shannon-Weiner α = 1; Simpson-Yule α = 2) and dominance (Berger-Parker α = inf). The Kruskal-Wallis (KW) test (p > 0.05) was used to assess differences among the Rényi diversity index estimated for each trap. The Bonferroni test was employed to perform multiple comparisons among the traps testing each pair of means. Bootstrap values were used to estimate the total species richness expected for each trap. Jaccard (Cj) and Sorensen (Cn) indexes were used to address the similarity of the species captured by the traps. The selectivity of traps estimated as part of trap performance was evaluated by the Pearson chi-squared test (χ2) using the SPSS v.17.0 program. Correspondence analysis (SPSS program v.15.0) which seeks to synthesise the mass of data with ease of implementation and interpretation was employed as a complement to the χ2 test and can graphically display the association observed, because of similar categories are placed more closely to each other (Hoffman & Franke 1986, Carvalho et al. 2002). This study showed the relationship among the selectivity of each trap relative to species grouped on the basis of the subfamilies and tribes they belong to and the trap employed.
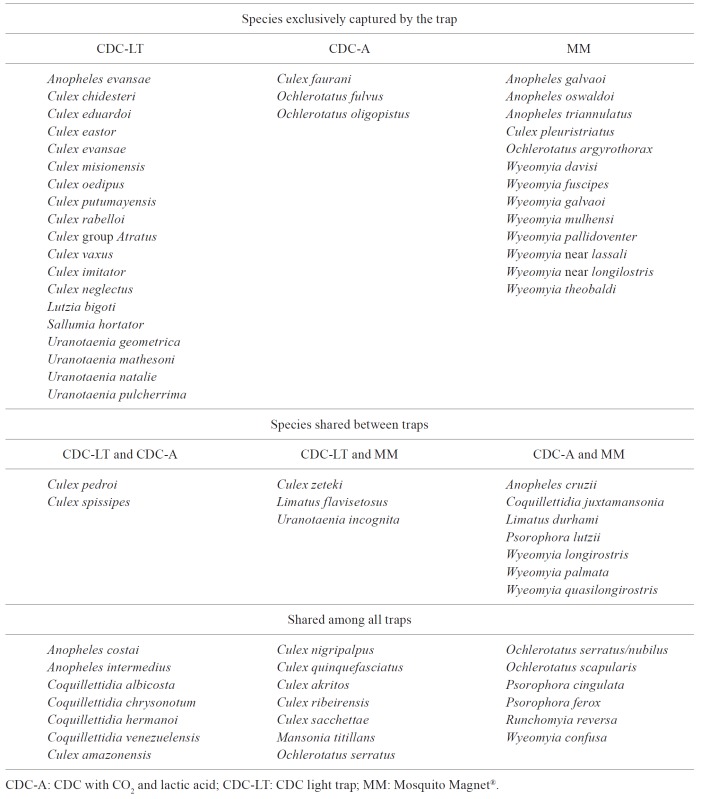
The Venn diagram was constructed to graphically illustrate the species shared by each pair of traps, by all the traps and those captured exclusively by each individual trap.
RESULTS
The three traps together captured 19,016 mosquitoes in 216 h of sampling effort. Of these, 860 individuals (4.25%) were damaged and were not identified. Consequently, 18,156 specimens were identified and grouped into 64 species and 11 taxonomic units (Table I). The traps showed distinct performances with regard to the collection of mosquitoes. The CDC-LT captured 18.82% (3,417) of all samples, with an average of 625 mosquitoes per month or 17 insects per hour. The CDC-A captured 26.10% (4,739) of the sample, with an average of 838 individuals per month, i.e., 23 mosquitoes/hour. The MMI trap captured 55.08% (10,000) of the insects representing an average of 1,706 specimens per month or 47 Culicidae/hour.
TABLE I. Species and taxonomic units abundance per sampling trap in rural areas in the tropical Atlantic rainforest, southeastern Brazil.
| Traps n (%) | ||||
|---|---|---|---|---|
| Species/taxonomic unit | A | B | C | Total (n) |
| Anophelinae | ||||
| Anopheles (Anopheles) costai | 3 (1.08) | 64 (23.02) | 211 (75.9) | 278 |
| Anopheles (Anopheles) intermedius | 1 (7.14) | 1 (7.14) | 12 (85.71) | 14 |
| Anopheles (Kerteszia) bellator | 0 (0) | 9 (13.85) | 56 (86.15) | 65 |
| Anopheles (Kerteszia) cruzii | 0 (0) | 54 (21.86) | 193 (78.14) | 247 |
| Anopheles (Nyssorhynchus) evansae | 1 (100) | 0 (0) | 0 (0) | 1 |
| Anopheles (Nyssorhynchus) galvaoi | 0 (0) | 0 (0) | 2 (100) | 2 |
| Anopheles (Nyssorhynchus) oswaldoi | 0 (0) | 0 (0) | 1 (100) | 1 |
| Anopheles (Nyssorhynchus) triannulatus | 0 (0) | 0 (0) | 4 (100) | 4 |
| Total | 5 (0.82) | 128 (20.91) | 479 (78.27) | 612 |
| Aedini | ||||
| Ochlerotatus (Protomacleaya) argyrothorax | 0 (0) | 0 (0) | 1 (100) | 1 |
| Ochlerotatus (Chrysoconops) fulvus | 0 (0) | 2 (100) | 0 (0) | 2 |
| Ochlerotatus (Ochlerotatus) scapularis | 4 (2.26) | 56 (31.64) | 117 (66.1) | 177 |
| Ochlerotatus (Protoculex) oligopistus | 0 (0) | 1 (100) | 0 (0) | 1 |
| Ochlerotatus (Protoculex) serratus | 11 (16.67) | 42 (63.63) | 13 (19.7) | 66 |
| Ochlerotatus (Protoculex) serratus/nubilus | 23 (11.79) | 112 (57.44) | 60 (30.77) | 195 |
| Psorophora (Grabhamia) cingulata | 1 (4.35) | 10 (43.48) | 12 (52.17) | 23 |
| Psorophora (Janthinosoma) ferox | 2 (15.38) | 3 (23.08) | 8 (61.54) | 13 |
| Psorophora (Janthinosoma) lutzii | 0 (0) | 7 (50) | 7 (50) | 14 |
| Sallumia hortator | 1 (100) | 0 (0) | 0 (0) | 1 |
| Total | 42 (8.52) | 233 (47.26) | 218 (44.22) | 493 |
| Culicini | ||||
| Culex (Aedes) amazonensis | 10 (30.30) | 15 (45.45) | 8 (24.24) | 33 |
| Culex (Culex) chidesteri | 3 (100) | 0 (0) | 0 (0) | 3 |
| Culex (Culex) eduardoi | 1 (100) | 0 (0) | 0 (0) | 1 |
| Culex (Culex) nigripalpus | 228 (13.02) | 381 (21.76) | 1,142 (65.22) | 1,751 |
| Culex (Culex) quinquefasciatus | 2 (8.33) | 1 (4.17) | 21 (87.5) | 24 |
| Culex (Culex) sp. | 30 (81.08) | 3 (8.11) | 4 (10.81) | 37 |
| Culex (Culex) sp. Coronator group | 6 (100) | 0 (0) | 0 (0) | 6 |
| Culex (Microculex) imitator | 1 (100) | 0 (0) | 0 (0) | 1 |
| Culex (Microculex) neglectus | 1 (100) | 0 (0) | 0 (0) | 1 |
| Culex (Microculex) pleuristriatus | 0 (0) | 0 (0) | 1 (100) | 1 |
| Culex (Melanoconion) akritos | 71 (55.9) | 5 (3.94) | 51 (40.16) | 127 |
| Culex (Melanoconion) eastor | 5 (100) | 0 (0) | 0 (0) | 5 |
| Culex (Melanoconion) evansae | 1 (100) | 0 (0) | 0 (0) | 1 |
| Culex (Melanoconion) faurani | 0 (0) | 1 (100) | 0 (0) | 1 |
| Culex (Melanoconion) misionensis | 1 (100) | 0 (0) | 0 (0) | 1 |
| Culex (Melanoconion) oedipus | 1 (100) | 0 (0) | 0 (0) | 1 |
| Culex (Melanoconion) pedroi | 39 (59.1) | 27 (40.9) | 0 (0) | 66 |
| Culex (Melanoconion) putumayensis | 2 (100) | 0 (0) | 0 (0) | 2 |
| Culex (Melanoconion) rabelloi | 6 (100) | 0 (0) | 0 (0) | 6 |
| Culex (Melanoconion) ribeirensis | 691 (43.51) | 628 (39.55) | 269 (16.94) | 1,588 |
| Culex (Melanoconion) sacchettae | 1,360 (32.04) | 1,862 (43.86) | 1,023 (24.1) | 4,245 |
| Culex (Melanoconion) sp. Atratus group | 1 (100) | 0 (0) | 0 (0) | 1 |
| Culex (Melanoconion) sp. Melanoconion section | 171 (72.15) | 46 (19.41) | 20 (8.44) | 237 |
| Culex (Melanoconion) spissipes | 4 (57.14) | 3 (42.86) | 0 (0) | 7 |
| Culex (Melanoconion) vaxus | 2 (100) | 0 (0) | 0 (0) | 2 |
| Culex (Melanoconion) zeteki | 1 (50) | 0 (0) | 1 (50) | 2 |
| Lutzia (Lutzia) bigoti | 1 (100) | 0 (0) | 0 (0) | 1 |
| Total | 2,639 (32.38) | 2,972 (36.46) | 2,540 (31.16) | 8,151 |
| Mansoniini | ||||
| Coquillettidia (Rhynchotaenia) albicosta | 4 (22.22) | 3 (16.67) | 11 (61.11) | 18 |
| Coquillettidia (Rhynchotaenia) chrysonotum | 227 (3.46) | 1,144 (17.45) | 5,186 (79.09) | 6,557 |
| Coquillettidia (Rhynchotaenia) hermanoi | 17 (13.39) | 34 (26.77) | 76 (59.84) | 127 |
| Coquillettidia (Rhynchotaenia) juxtamansonia | 0 (0) | 2 (50) | 2 (50) | 4 |
| Coquillettidia (Rhynchotaenia) venezuelensis | 352 (20.29) | 151 (8.7) | 1,232 (71.01) | 1,735 |
| Mansonia (Mansonia) titillans | 1 (20) | 1 (20) | 3 (60) | 5 |
| Total | 601 (7.11) | 1,335 (15.81) | 6,510 (77.08) | 8,446 |
| Sabethinii | ||||
| Limatus durhami | 0 (0) | 6 (28.57) | 15 (71.73) | 21 |
| Limatus flavisetosus | 1 (11.11) | 0 (0) | 8 (88.89) | 9 |
| Runchomyia (Runchomyia) reversa | 1 (5.26) | 6 (31.58) | 12 (63.16) | 19 |
| Wyeomyia (Phoniomyia) davisi | 0 (0) | 0 (0) | 4 (100) | 4 |
| Wyeomyia (Phoniomyia) fuscipes | 0 (0) | 0 (0) | 1 (100) | 1 |
| Wyeomyia (Phoniomyia) galvaoi | 0 (0) | 0 (0) | 18 (100) | 18 |
| Wyeomyia (Phoniomyia) longirostris | 0 (0) | 1 (20) | 4 (80) | 5 |
| Wyeomyia (Phoniomyia) mulhensi | 0 (0) | 0 (0) | 1 (100) | 1 |
| Wyeomyia (Phoniomyia) pallidoventer | 0 (0) | 0 (0) | 1 (100) | 1 |
| Wyeomyia (Phoniomyia) palmata | 0 (0) | 1 (50) | 1 (50) | 2 |
| Wyeomyia (Phoniomyia) pilicauda/incauda | 0 (0) | 0 (0) | 5 (100) | 5 |
| Wyeomyia (Phoniomyia) near lassali | 0 (0) | 0 (0) | 1 (100) | 1 |
| Wyeomyia (Phoniomyia) near longilostris | 0 (0) | 0 (0) | 3 (100) | 3 |
| Wyeomyia (Phoniomyia) quasilongirostris | 0 (0) | 2 (16.67) | 10 (83.33) | 12 |
| Wyeomyia (Phoniomyia) theobaldi | 0 (0) | 0 (0) | 8 | 8 |
| Wyeomyia confusa | 2 (1.1) | 55 (30.22) | 125 (68.68) | 182 |
| Wyeomyia felicia/pampeithes | 0 (0) | 0 (0) | 26 (100) | 26 |
| Wyeomyia mystes/finlayi | 0 (0) | 0 (0) | 3 (100) | 3 |
| Wyeomyia airosai/howardi/luteoventralis | 0 (0) | 0 (0) | 6 (100) | 6 |
| Total | 4 (1.22) | 71 (21.71) | 252 (77.06) | 327 |
| Uranotaeniini | ||||
| Uranotaenia (Uranotaenia) geometrica | 10 (100) | 0 (0) | 0 (0) | 10 |
| Uranotaenia (Uranotaenia) incognita | 1 (50) | 0 (0) | 1 (50) | 2 |
| Uranotaenia (Uranotaenia) mathesoni | 7 (100) | 0 (0) | 0 (0) | 7 |
| Uranotaenia (Uranotaenia) natalie | 5 (100) | 0 (0) | 0 (0) | 5 |
| Uranotaenia (Uranotaenia) pulcherrima | 103 (100) | 0 (0) | 0 (0) | 103 |
| Total | 126 (99.21) | 0 (0) | 1 (0.79) | 127 |
| Total specimens | 3,417 (18.82) | 4,739 (26.1) | 10,000 (55.08) | 18,156 |
| Total species/taxonomic unit | 47 | 35 | 50 | 75 |
A: CDC light trap; B: CDC with CO2 and lactic acid; C: Mosquito Magnet® Independence.
The statistical test to check the homoscedasticity of the variances of the observed data between the three traps showed a p value = 0.001 with a statistical significance of p > 0.05. Regarding the tests for assessing the normality of the data distribution, it was seen that for each of the trap the values presented by the Shapiro-Wilk and Kolmogorov-Smirnov tests were p = 0.000 for both tests, with a 5% level of significance, indicating that the mosquito abundance observed in the traps did not have a normal distribution. Thus the necessary conditions for the employment of the parametric tests were met; accordingly non-parametric statistics were adopted for the analysis of the data.
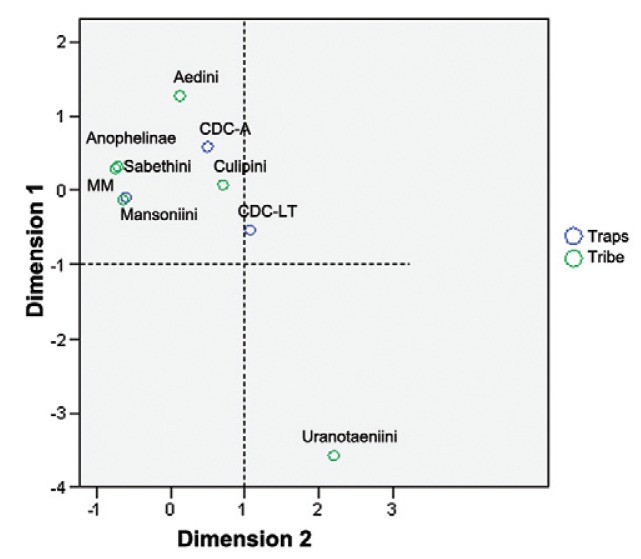
The results of the χ2 test showed the presence of a positive association between the CDC-LT and species of the Culicini and Uranotaeniini tribes. The species/taxonomic units belonging to the Culicini tribe represented 77.23% of the total abundance sampled by the trap (χ2 = 4594.040; p < 0.000). As compared with the other traps, the CDC-LT was highly selective for species of the Ura- notaeniini tribe, capturing 126 individuals, representing 99.21% of the total species of the genus Uranotaenia collected by the traps. Only one individual was captured by MMI. Regarding the CDC-A, there was a positive association between the trap and representatives of the Culicini and Aedini tribes. Thus 62.72% of the mosquitoes captured in the trap belonged to the Culicini (2,972 insects), whereas 4.91% were of the Aedini (233 individuals). The MMI showed a positive association with species of the Mansoniini and Sabethini tribes and the Anophelinae subfamily. Mosquitoes of the Mansoniini represented 65.51% (6,510 individuals) of the specimens obtained in the MMI, 252 mosquitoes were of the Sabethini and 479 belonged to the Anophelinae subfamily. The MMI was capable of capturing a greater number of Anopheles than the CDC-LT and the CDC-A (Table II), about 78% of Anopheles collected. The results of the correspondence analysis are given in Fig. 2, which shows the associations of the traps with the subfamilies and tribes.
TABLE II. Species and taxonomic units abundance grouped into tribes and subfamily per sampling trap in rural areas in the tropical Atlantic rainforest, southeastern Brazil.
| A n (%) | B n (%) | C n (%) | |
|---|---|---|---|
| Culicinae | |||
| Aedini | 42 (1.23) | 233 (4.91) | 218 (2.18) |
| Culicini | 2,639 (77.23) | 2,972 (62.72) | 2,540 (25.4) |
| Mansoniini | 601 (17.58) | 1,335 (28.17) | 6,510 (65.1) |
| Sabethini | 4 (0.12) | 71 (1.5) | 252 (2.52) |
| Uranotaeniini | 126 (3.69) | 0 (0) | 1 (0.01) |
| Anophelinae | 5 (0.15) | 128 (2.7) | 479 (4.79) |
| Total | 3,417 (100) | 4,739 (100) | 10,000 (100) |
A: CDC light trap; B: CDC with CO2 and lactic acid; C: Mosquito Magnet® Independence; χ2 = 4594.040; gl = 10; p = 0,000.
Fig. 2. : correspondence analysis graphically represents the associations of the traps (CDC-A: CDC with CO2 and lactic acid; CDC-LT: CDC light trap; MM: Mosquito Magnet®) and the tribes and subfamily.
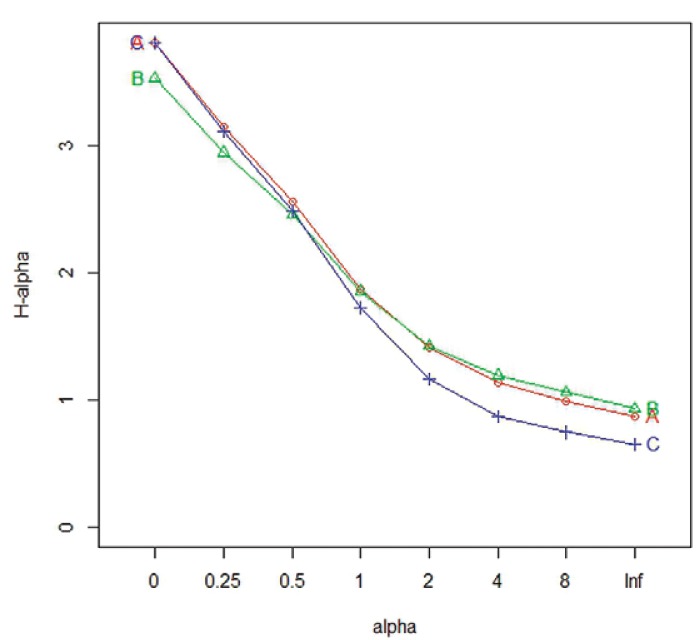
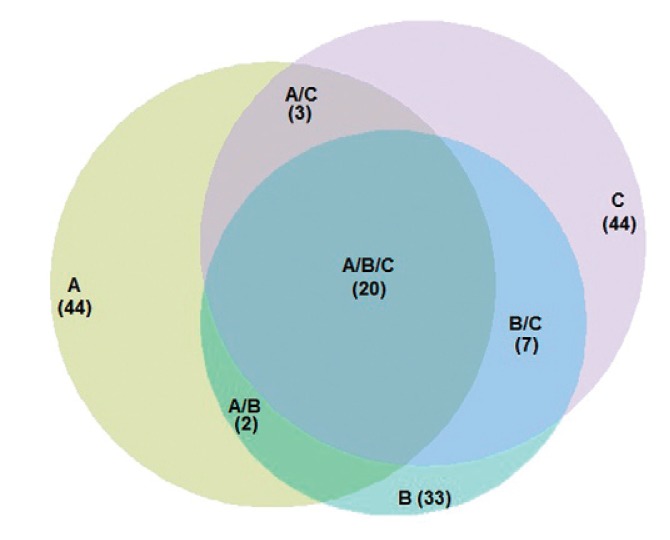
To address the diversity of culicids per trap, only individuals identified to the species level were considered. The results of the KW test indicated that the observed differences between the Rényi diversity index (Fig. 3) were statistically significant only as regards richness (KWχ2 = 19.338; p = 6.321 E-05). The results of the Bonferroni test indicated that the MMI presented a significant difference as compared to the CDC-LT (p = 6e-05) and the CDC-A (p = 0.0012). However, the CDC-A showed no significant difference (p = 0.6059) compared with the CDC-LT. The other Rényi diversity index were not statistically significant, Shannon-Weiner (KWχ2 = 0.9298; p = 0.6282), Simpson-Yule (KWχ2 = 2.3813; p = 0.304) and Berger-Parker dominance (KWχ2 = 3.4419; p = 0.1789). The expected values obtained by the bootstrap analyses were closer to the observed richness in the MMI data because the species captured represented 88.7% of the expected species (Sobserved = 44; bootstrap = 49.6). The CDC-LT was able to sample 83.5% (Sobserved = 44, bootstrap = 52.7) and the CDC-A 76.7% (Sobserved = 33, bootstrap = 43) of the expected species. The MMI and CDC-LT traps presented the same number of species, with some species common to both traps and other species that were captured by one specific trap. The Cj (0.57) and the Cn (0.72) values showed similarities between species captured by the CDC-A and MMI traps (Supplementary data 1). The Venn diagram (Fig. 4) illustrated species distribution in each trap, as well as those that were shared by two or three traps. The complete list of species is in the Supplementary data 2.
Fig. 3. : Rényi index illustrating differences in diversities estimated by the traps. A: CDC light trap; B: CDC with CO2 and lactic acid; C: Mosquito Magnet® Independence.
Fig. 4. : Venn diagram illustrating the similarity of the mosquito species captured and shared between traps in the municipality of Iguape, state of São Paulo, Brazil. A: CDC light trap; B: CDC with CO2 and lactic acid; C: Mosquito Magnet® Independence.
DISCUSSION
Results of the analyses conducted for the present study clearly demonstrated that the MMI is more efficient at capturing mosquitoes than the CDC-LT or the CDC-A. In this context, the MMI captured 2.0 and ~2.7 times more culicids than the CDC-A and the CDC-LT, respectively. Similar results were observed by Brown et al. (2008) and Dusfour et al. (2010) when comparing the performance of different models of MM with the CDC trap. Recently, de Sá and Sallum (2013) obtained similar results by employing the set of traps in different locations and with lower sampling effort.
The traps used in this study captured species of importance to public health. Among them it is worth mentioning the Coquillettidia venezuelensis that is competent to transmit the Oropuche virus and the eastern equine encephalitis virus (EEEV) (Forattini 1965, Rosa et al. 1996). Anopheles cruzii and Anopheles bellator are local vectors of Plasmodium sp. (Deane et al. 1984, Forattini et al. 1996) that can cause disease in humans. Ochlerotatus scapularis, captured in great abundance by MMI, is involved in the transmission of several viruses, as for instance, the Melon virus, Ilhéus virus, Rocio virus and Venezuelan equine encephalitis virus (Spence et al. 1962, Arnell 1976, Forattini et al. 1995a). Culex nigripalpus is a vector of the Saint Louis virus, West Nile virus and EEEV (Forattini et al. 1995b, Rutledge et al. 2003). The CDC-A captured a larger number of Ochlerotatus serratus and Oc. serratus/nubilus with vector competence for the yellow fever virus (YFV), Ilhéus, Oropuche, Aura, Tocara and San Luis viruses (Forattini 1965, Vasconcelos et al. 1998, Cardoso et al. 2010a). Culex ribeirensis with vector competence for the EEEV (Santos-Neto & Lozovei 2008) was captured with greater abundance by the CDC-LT.
Regarding selectivity, the CDC-LT showed a statistically significant positive association with species of the Culicini and Uranotaeniini tribes. As the Uranotaeniini species feed on the blood of amphibians (Cupp et al. 2004), the attractant factor was the light source of the CDC-LT that was absent from the CDC-A. The traps were linked to kairomones that attract insects that carry the blood obtained from mammals, including humans (Dugassa et al. 2013), thus the absence of mosquitoes of the genus Uranotaenia in the MMI was also observed by de Sá and Sallum (2013). The CDC-A showed a positive association with species of the Culicini and Aedini tribes. It is noteworthy that the CDC-A estimated the greatest abundance of Oc. serratus and Oc. serratus/nubilus. The presence of these mosquitoes in the CDC-A indicates the possibility of employing it for monitoring mosquito vector species of medical importance. For instance, for surveillance of species that is potentially involved in the introduction, establishment and dispersion of the YFV in new areas. According to Cardoso et al. (2010a), a new subtype of YFV had been isolated from specimens of Oc. serratus collected in rural areas of the state of Rio Grande do Sul. Results of a study conducted by Laporta and Sallum (2011) in a preserved area of the Atlantic Forest in southeastern SP, using CDCs traps associated with CO2 and 1-octeno-3-ol, showed that Oc. serratus was the second most abundant species sampled in the preserved forest area. Considering that Oc. serratus is present in both preserved forest areas as well as in rural areas surrounded by secondary forests we may be permitted to hypothesise that the species acts as a bridge vector for dispersing the YFV from forest to rural cycles.
The MMI was positively associated with species of the Mansoniini tribe and with Coquillettidia chrysonotum as the most abundant species. Cq. chrysonotum has not yet been incriminated in the transmission of pathogens to humans; however, its aggressive blood feeding behavior (Forattini 2002) is a cause of discomfort and annoyance to humans and domestic animals. As has been seen, the MMI trap performed well in sampling species of mosquitoes that have the potential to become pests in urban and rural environments. A second model of trap, the MM Professional (MMPro), has been employed for the control of Culicoides spp in the peridomestic environment in coastal areas of Florida, United States of America (Cilek & Hallmon 2005). The MMPro is capable of sampling several other groups of bloodsucking insects as well as mosquitoes. Furthermore, the MMPro has been utilised for assessing biodiversity parameters and species distribution in urban, rural and natural environments in Belgium (Versteirt et al 2013).
In relation to the subfamily Anophelinae, the MMI trap sampled the largest number of representatives of the genus Anopheles (479 individuals). This genus contains the mosquitoes that transmit malarial parasites to humans and other animals. Currently, almost 99.5% of human malaria cases reported in Brazil are the Amazon Region. Some cases do occur also outside the area of active transmission, in extra-Amazonian areas, where malaria is manifested as isolated cases or in small outbreaks occurring mainly under the conditions associated with the Atlantic Forest biome (Oliveira-Ferreira et al. 2010). In these areas, An. cruzii and An. bellator are local vectors of species of the genus Plasmodium sp. that cause malaria in humans. The transmission cycle of the pathogen may involve monkeys of the genus Cebus and Allouata (Oliveira-Ferreira et al. 2010, Yamasaki et al. 2011). In the study area, the MMI collected a higher number of An. bellator and An. cruzii than either the CDC-LT or the CDC-A. Thus, in the light of the results of this study within the context of entomological surveillance, the MMI was more effective for monitoring mosquito species of public health importance.
Other models of MM trap have been tested for surveillance of those Anopheles species that are vectors of Plasmodium. Recently, Rubio-Palis et al. (2012) examined the effectiveness of MM Liberty Plus (MMLP) regarding the method of capture by human attractant in an area where malaria is endemic, in the state of Bolivar, Venezuela. They argued that the MMLP can be employed for monitoring Anopheles populations. In a study conducted in the state of Mato Grosso, Brazil, Missawa et al. (2011) compared the MM Defender with human attraction for the sampling of Anopheles. The results showed that MM Defender was efficient because it captured the highest number of species and estimated the greater abundance of Anopheles triannulatus, Anopheles benarrochi, Anopheles oswaldoi, Anophles peryassui and Anopheles rangeli. Interestingly, this trap did not have the same positive effect in collecting Anopheles darlingi. Hiwat et al. (2011a), in a study in Suriname, found that the MM trap has potential for use as an alternative for collecting Anopheles aquasalis.
The various MM models have been developed to capture synanthropic mosquitoes in open domiciliary environments (Cilek & Hallmon 2005). However, multiple studies have been conducted to address the effectiveness of the trap in environments with distinct environmental, climatic and ecological conditions, including areas of tropical forest. Despite the presence of variations, the results suggest that the trap has potential for use in monitoring activities of mosquito fauna in different regions of the world, since it is able to capture diverse species compositions.
In this study, when the composition of species sampled by the MMI was compared with that observed in the CDC-A, greater similarity could be observed between them. This was proven by the similarity index, a result also observed by de Sá and Sallum (2013). The similarity is probably related to attractants (CO2 or Lurex3®) used in the traps, which permitted the collection of species that can carry blood from mammals and other vertebrates. Traps which have specific odours attractive to anthropophilic species, particularly combined with CO2, may provide improved results in the sampling of these species and have the potential to be effective as methods for capture, for example, in entomological monitoring and ecological studies of malaria vectors (Jawara et al. 2009, 2011, Hiwat et al. 2011b). It is noteworthy that the flow rate of CO2 might influence the mosquitoes collected.
The CDC-LT captured lower mosquito abundance than the other traps. However, it presented species richness similar to that of the MMI. On the other hand, the species composition was different (Supplementary data 2). The trap has been employed for many years in entomological research. However, this trap has been mainly employed in collections made during the night. The use of CDC-LTs for capturing diurnal mosquitoes is not appropriate because it depends on the attractiveness of a point source of light. To improve the performance of the collections made with the CDCs, chemicals attractants (Laporta & Sallum 2011) or bait animals (Lourenço-de-Oliveira 1984) are used. Associating ultraviolet (UV) light with the CDC increases the effectiveness of the trap, both for the species sampled and for obtaining males (Hutchings et al. 2011). However, other groups of insects may be also captured by a CDC-UV trap, what is not adequate in studies conducted in preserved or even rural environments. Insects outside the focus group may be unnecessarily removed from the environment, sometimes in great number. It is noteworthy that neither the MMI nor the CDC-A captured specimens of any other group of insects besides Culicidae - due to the use of specific attractants.
It is necessary to adopt different, new methods of capture if one is to achieve better sampling in order to assist study of the epidemiological surveillance of vectors. The MMI, despite requiring greater care in its installation, use and transport, presented good performance both in its ability to sequester a large number of mosquitoes from the environment, as in the capture of Culicidae of importance to public health.
ACKNOWLEDGEMENTS
To the field staff from Department of Epidemiology, for helping with field collections, and to A Fernandes, for assistance in mosquito identifications.
TABLE I. Jaccard (Cj) and Sorensen (Cn) indexes among species captured by the traps used to study in a rural area in the municipality of Iguape, state of São Paulo, Brazil

TABLE II. Species unique to each traps and shared between them
REFERENCES
- Arnell JH. Mosquito studies (Diptera, Culicidae). XXXIII. A revision of the scapularis group of Aedes (Ochlerotatus) Contrib Amer Ent Inst. 1976;13:1–144. [Google Scholar]
- Bisevac L, Franklin DC, Williamson GJ, Whelan PI. A comparison of two generic trap types for monitoring mosquitoes through an annual cycle in tropical Australia. J Am Mosq Control Assoc. 2009;25:58–65. doi: 10.2987/08-5814.1. [DOI] [PubMed] [Google Scholar]
- Brown HE, Paladini M, Cook RA, Kline DL, Barnard D. Effectiveness of mosquito trap in measuring species abundance and composition. J Med Entomol. 2008;45:517–521. doi: 10.1603/0022-2585(2008)45[517:eomtim]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cardoso JC, Almeida MA, Santos E, Fonseca DF, Sallum MAM, Noll CA, Monteiro HA, Cruz AC, Carvalho VL, Pinto EV, Castro FC, Nunes JP, Neto, Segura MN, Vasconcelos PF. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, southern Brazil, 2008. Emerg Infect Dis. 2010a;16:1918–1924. doi: 10.3201/eid1612.100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso JC, Paula MB, Fernandes A, Santos ED, Almeida MA, Fonseca DF, Sallum MAM. Novos registros e potencial epidemiológico de algumas espécies de mosquitos (Diptera, Culicidae), no estado do Rio Grande do Sul. Rev Soc Bras Med Trop. 2010b;43:552–556. doi: 10.1590/s0037-86822010000500016. [DOI] [PubMed] [Google Scholar]
- Cardoso JC, Paula MP, Fernandes A, Santos E, Almeida MAB, Fonseca DF, Sallum MAM. Ecological aspects of mosquitoes (Diptera: Culicidae) in an Atlantic Forest area on the north coast of Rio Grande do Sul state, Brazil. J Vector Ecol. 2011;36:175–186. doi: 10.1111/j.1948-7134.2011.00155.x. [DOI] [PubMed] [Google Scholar]
- Carvalho JRP, Vieira SR, Moran RCCP. Análise de correspondência - uma ferramenta útil na interpretação de mapas de produtividade. R Bras Ci Solo. 2002;26:435–443. [Google Scholar]
- Chaves LSM, Laporta GZ, Sallum MAM. Effectiveness of Mosquito Magnet in preserved area on the Coastal Atlantic Rainforest: implication for entomological surveillance. J Med Entomol. 2014;51:915–924. doi: 10.1603/me14050. [DOI] [PubMed] [Google Scholar]
- Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. 35BMC Bioinformatics. 2011;12 doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilek JE, Hallmon CF. The effectiveness of the Mosquito Magnet trap for reducing biting midge (Diptera: Ceratopogonidae) populations in coastal residential backyards. J Am Mosq Control Assoc. 2005;21:218–221. doi: 10.2987/8756-971X(2005)21[218:TEOTMM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Consoli RAGB, Lourenço-de-Oliveira R. Principais mosquitos de importância sanitária no Brasil. Editora Fiocruz; Rio de Janeiro: 1998. 228 [Google Scholar]
- Correa RR, Ramalho GR. Revisão de Phoniomyia Theobald, 1903 (Diptera, Culicidae, Sabethini) Folia Clin Biol. 1956;25:1–176. [Google Scholar]
- Cupp EW, Zhang D, Yue X, Cupp MS, Guyer C, Sprender TR, Unnash TR. Identification of reptilian and amphibian blood meals from mosquitoes in an eastern equine encephalomyelitis virus focus in central Alabama. Am J Trop Med Hyg. 2004;71:272–276. [PMC free article] [PubMed] [Google Scholar]
- de Sá ILR, Sallum MAM. Comparison of automatic traps to capture mosquitoes (Diptera: Culicidae) in rural areas in the tropical Atlantic rainforest. Mem Inst Oswaldo Cruz. 2013;108:1014–1020. doi: 10.1590/0074-0276130474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane LM, Ferreira JA, Neto, Lima MM. The vertical dispersion of Anopheles (Kerteszia) cruzi in a forest in southern Brazil suggests that human cases of malaria of simian origin might be expected. Mem Inst Oswaldo Cruz. 1984;79:461–463. doi: 10.1590/s0074-02761984000400011. [DOI] [PubMed] [Google Scholar]
- Dugassa S, Lindh JM, Oyieke F, Mukabana WR, Lindsay SW, Fillinger U. Development of a gravid trap for collecting live malaria vectors Anopheles gambiae s.l. PLoS ONE. 2013;8: doi: 10.1371/journal.pone.0068948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusfour I, Carinci R, Gaborit P, Issaly J, Girod R. Evaluation of four methods for collecting malaria vectors in French Guiana. J Econ Entomol. 2010;103:973–976. doi: 10.1603/ec09328. [DOI] [PubMed] [Google Scholar]
- Forattini OP. Genética. Vol. 3. EDUSP; São Paulo: 1965. Culicini: Haemagogus, Mansonia, Culiseta. Sabethini. Toxorhynchitini. Arboviroses. Filariose bancroftiana.416 [Google Scholar]
- Forattini OP. Culicidologia médica - Identificação, biologia e epidemiologia. Vol. 2. EDUSP; São Paulo: 2002. 864 [Google Scholar]
- Forattini OP, Gomes AC, Kakitani I, Marucci D. Observações sobre domiciliação de mosquitos Culex (Melanoconion) em ambiente com acentuadas modificações antrópicas. Rev Saude Publica. 1991;25:257–266. doi: 10.1590/s0034-89101991000400004. [DOI] [PubMed] [Google Scholar]
- Forattini OP, Gomes AC, Natal D, Kakitani I, Marucci D. Preferências alimentares e domiciliação de mosquitos no Vale do Ribeira, São Paulo, Brasil, com especial referência a Aedes scapularis e a Culex (Melanoconion) Rev Saude Publica. 1989;23:9–19. doi: 10.1590/s0034-89101989000100003. [DOI] [PubMed] [Google Scholar]
- Forattini OP, Kakitani I, Massad E, Marucci D. Studies on mosquitoes (Diptera: Culicidae) andanthropic environment. 9 - Synanthropy and epidemiological vector role of Aedes scapularis in south-eastern Brazil. Rev Saude Publica. 1995a;29:199–207. doi: 10.1590/s0034-89101995000300007. [DOI] [PubMed] [Google Scholar]
- Forattini OP, Kakitani I, Massad E, Marucci D. Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. 10 - Survey of adult behaviour of Culex nigripalpus and other species of Culex (Culex) in south-eastern Brazil. Rev Saude Publica. 1995b;29:271–278. doi: 10.1590/s0034-89101995000400003. [DOI] [PubMed] [Google Scholar]
- Forattini OP, Kakitani I, Massad E, Marucci D. Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. 11 - Biting activity and blood-seeking parity of Anopheles (Kerteszia) in south-eastern Brazil. Rev Saude Publica. 1996;30:107–114. doi: 10.1590/s0034-89101996000200001. [DOI] [PubMed] [Google Scholar]
- Galindo P, Blanton FS, Peyton EL. A revision of the Uranotaenia of Panama withnotes on other American species of the genus (Diptera, Culicidae). Ann Entomol Soc Am. 1954;47:105–177. [Google Scholar]
- Gomes AC, Rabello EX, Natal D. Uma nova câmara coletora para armadilha CDC-miniatura. Rev Saude Publica. 1985;19:190–191. doi: 10.1590/s0034-89101985000200009. [DOI] [PubMed] [Google Scholar]
- Hiwat H, Andriessen R, de Rijk M, Koenraadt CJM, Takken W. Carbon dioxide baited trap catches do not correlate with human landing collections of Anopheles aquasalis in Suriname. Mem Inst Oswaldo Cruz. 2011a;106:360–364. doi: 10.1590/s0074-02762011000300017. [DOI] [PubMed] [Google Scholar]
- Hiwat H, de Rijk M, Andriessen R, Koenraadt CJM, Takken W. Evaluation of methods for sampling the malaria vector Anopheles darlingi (Diptera, Culicidae) in Suriname and the relation with its biting behaviour. J Med Entomol. 2011b;48:1039–1046. doi: 10.1603/me10245. [DOI] [PubMed] [Google Scholar]
- Hoel DF, Kline DL, Allan SA, Grant A. Evaluation of carbon dioxide, 1-octen-3l and acid lactic as baits in Mosquito Magnet® Pro traps for Aedes albopictus in north central Florida. J Am Mosq Control Assoc. 2007;23:11–17. doi: 10.2987/8756-971X(2007)23[11:EOCDOA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Franke GR. Correspondence analysis: graphical representation of categorical data in marketing research. J Mark Res. 1986;23:213–227. [Google Scholar]
- Hutchings RS, Sallum MA, Hutchings RW. Mosquito (Diptera: Culicidae) diversity of a forest-fragment mosaic in the Amazon rainforest. J Med Entomol. 2011;48:173–187. doi: 10.1603/me10061. [DOI] [PubMed] [Google Scholar]
- Hutchings RSG, Hutchings RW, Sallum MAM. Zoologia. Vol. 30. Curitiba: 2013. Culicidae (Diptera: Culicomorpha) from the central Brazilian Amazon: Nhamundá and Abacaxis Rivers; pp. 1–14. [Google Scholar]
- Hutchings RSG, Sallum MAM, Ferreira RLM, Hutchings RW. Mosquitoes of the Jaú Park and their potential importance in Brazilian Amazonia. Med Vet Entomol. 2005;19:428–441. doi: 10.1111/j.1365-2915.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- Jawara M, Awolola TS, Pinder M, Jeffries D, Smallegange RCS, Takken W, Conway DJ. Field testing of different chemical combinations as odour baits for trapping wild mosquitoes in the Gambia. PLoS ONE. 2011;6: doi: 10.1371/journal.pone.0019676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawara M, Smallegange RC, Jeffries D, Nwakanma DC, Awolola TS, Knols BG, Takken W, Conway DJ. Optimizing odor-baited trap methods for collecting mosquitoes during the malaria season in The Gambia. PLoS ONE. 2009;4: doi: 10.1371/journal.pone.0008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Turell MJ, Sardelis MR, Watts DM, Coleman RE, Fernandez R, Carbajal F, Pecor JE, Calampa C, Klein TA. Seasonal distribution, biology and human attractant patterns of Culicine mosquitoes (Diptera: Culicidae) in a forest near Puerto Almendras, Iquitos, Peru. J Med Entomol. 2004;41:349–360. doi: 10.1603/0022-2585-41.3.349. [DOI] [PubMed] [Google Scholar]
- Kindt R, Coe R. Tree diversity analysis: a manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre; Nairobi: 2005. 207 [Google Scholar]
- Kitau J, Pates H, Rwegoshora RT, Rwegoshora D, Matowo J, Kweka EJ, Mosha FW, McKenzie K, Magesa SM. The effect of Mosquito Magnet® Liberty Plus trap on the human mosquito biting rate under semi-field conditions. J Am Mosq Control Assoc. 2010;26:287–294. doi: 10.2987/09-5979.1. [DOI] [PubMed] [Google Scholar]
- Kline DL. Evaluation of various models of propane-powered mosquito traps. J Vector Ecol. 2002;27:1–7. [PubMed] [Google Scholar]
- Kline DL, Takken W, Wood JR, Carlson DA. Field studies on the potential of butanone, carbon dioxide, honey extract, 1-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med Vet Entomol. 1990;4:383–391. doi: 10.1111/j.1365-2915.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Lane J. Neotropical Culicidae. Vol. 2. EDUSP; São Paulo: 1953. 1112 [Google Scholar]
- Laporta GZ, Sallum MAM. Effect of CO2 and 1-octen-3-ol attractants for estimating species richness and the abundance of diurnal mosquitoes in the southeastern Atlantic forest, Brazil. Mem Inst Oswaldo Cruz. 2011;106:279–284. doi: 10.1590/s0074-02762011000300005. [DOI] [PubMed] [Google Scholar]
- Lourenço-de-Oliveira R. Alguns aspectos da ecologia dos mosquitos (Diptera: Culicidae) de uma área de planície (Granjas Calábria) em Jacarepaguá, Rio de Janeiro. I. Frequência comparativa das espécies e métodos de coleta. Mem Inst Oswaldo Cruz. 1984;79:479–490. doi: 10.1590/s0074-02761986000300003. [DOI] [PubMed] [Google Scholar]
- Mascheretti M, Tengan CH, Sato HK, Suzuki A, Souza RP, Maeda M, Brasil R, Pereira M, Tubaki RM, Wanderley DM, Fortaleza CM, Ribeiro AF. Yellow fever: reemerging in the state of São Paulo, Brazil, 2009. Rev Saude Publica. 2013;47:881–889. doi: 10.1590/s0034-8910.2013047004341. [DOI] [PubMed] [Google Scholar]
- Melo AS. O que ganhamos “confundindo” riqueza de espécies e equabilidade em um índice de diversidade? Biota Neotrop. 2008;8:21–27. [Google Scholar]
- Missawa NA, Ribeiro AL, Maciel GB, Zeilhofer P. Comparison of capture methods for the diagnosis of adult anopheline populations from state of Mato Grosso, Brazil. Rev Soc Bras Med Trop. 2011;44:555–560. doi: 10.1590/s0037-86822011005000053. [DOI] [PubMed] [Google Scholar]
- Montes J. Fauna de Culicidae da Serra da Cantareira, São Paulo, Brasil. Rev Saude Publica. 2005;39:578–584. doi: 10.1590/s0034-89102005000400010. [DOI] [PubMed] [Google Scholar]
- Morrow MG, Johnson RN, Polanco J, Claborn DM. Mosquito vector abundance immediately before and after Tropical Storms Alma and Arthur, northern Belize, 2008. Rev Panam Salud Publica. 2010;28:19–24. doi: 10.1590/s1020-49892010000700003. [DOI] [PubMed] [Google Scholar]
- Oliveira-Ferreira J, Lacerda MVG, Brasil P, Ladislau JLB, Tauil PL, Daniel-Ribeiro CT. Malaria in Brazil: an overview. 115Malar J. 2010;9 doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci TM, Lynch J, Keiper JB. Insect composition of the Mosquito Magnet Pro® mosquito trap in northeastern Ohio. Gt Lakes Entomol. 2003;36:25–30. [Google Scholar]
- Rosa APAT, Rodrigues SG, Nunes MRT, Magalhães MTF, Rosa JFST, Vasconcelos PFC. Epidemia de febre do Oropouche em Serra Pelada, município de Curionpolis, Pará, 1994. Rev Soc Bras Med Trop. 1996;29:537–541. doi: 10.1590/s0037-86821996000600002. [DOI] [PubMed] [Google Scholar]
- Rubio-Palis Y, Moreno JE, Sánchez V, Estrada Y, Anaya W, Bevilacqua M, Cárdenas L, Martínez A, Medina D. Can Mosquito Magnet® substitute for human-landing catches to sample anopheline populations? Mem Inst Oswaldo Cruz. 2012;107:546–549. doi: 10.1590/s0074-02762012000400017. [DOI] [PubMed] [Google Scholar]
- Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- Santos LG, Neto, Lozovei AL. Aspectos ecológicos de Anopheles cruzii e Culex ribeirensis (Diptera, Culicidae) da Mata Atlântica de Morretes, Paraná, Brasil. Rev Bras Entomol. 2008;52:105–111. [Google Scholar]
- Spence L, Anderson CR, Aitke THG, Downs WZ. Bimiti virus: a new agent isolated from Trinidadian mosquitoes. Am J Trop Med Hyg. 1962;11:414–417. doi: 10.4269/ajtmh.1962.11.414. [DOI] [PubMed] [Google Scholar]
- Sudia WD, Chamberlain RW. Battery-operated light trap, an improved model. Mosq News. 1962;22:126–129. [PubMed] [Google Scholar]
- Vasconcelos PFC, da Rosa APAT, Pinheiro FP, Shope RE, Degallier N, da Rosa EST. Arboviruses pathogenic from man in Brazil. In: APAT da Rosa, Vasconcelos PFC, JFST da Rosa., editors. An overview of arbovirology in Brazil and neighbouring countries. Instituto Evandro Chagas; Belém: 1998. pp. 72–99. [Google Scholar]
- Versteirt V, Boyer S, Damiens D, Clercq EM, Dekoninck W, Ducheyne E, Grootaert P, Garros C, Hance T, Hendrickx G, Coosemans M, Van Bortel W. Nationwide inventory of mosquito biodiversity (Diptera: Culicidae) in Belgium, Europe. Bull Entomol Res. 2013;103:193–203. doi: 10.1017/S0007485312000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue RD, Doyle MA, Kline DL. Field evaluation of CDC and Mosquito Magnet® x traps baited with dry ice, CO2 sachet and octenol against mosquitoes. J Am Mosq Control Assoc. 2008;24:249–252. doi: 10.2987/5701.1. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Duarte AM, Curado I, Summa ME, Neves DV, Wunderlich G, Malafronte RS. Detection of etiological agents of malaria in howler monkeys from Atlantic Forest, rescued in regions of São Paulo city, Brazil. J Med Primatol. 2011;40:392–400. doi: 10.1111/j.1600-0684.2011.00498.x. [DOI] [PubMed] [Google Scholar]