Abstract
Trypanosoma cruzi infection may be caused by different strains with distinct discrete typing units (DTUs) that can result in variable clinical forms of chronic Chagas disease. The present study evaluates the immune response and cardiac lesions in dogs experimentally infected with different T. cruzi strains with distinct DTUs, namely, the Colombian (Col) and Y strains of TcI and TcII DTU, respectively. During infection with the Col strain, increased levels of alanine aminotransferase, erythrocytes, haematocrit and haemoglobin were observed. In addition, CD8+ T-lymphocytes isolated from the peripheral blood produced higher levels of interleukin (IL)-4. The latter suggests that during the acute phase, infection with the Col strain may remain unnoticed by circulating mononuclear cells. In the chronic phase, a significant increase in the number of inflammatory cells was detected in the right atrium. Conversely, infection with the Y strain led to leucopoenia, thrombopoenia, inversion of the ratio of CD4+/CD8+ T-lymphocytes and alterations in monocyte number. The Y strain stimulated the production of interferon-γ by CD4+ and CD8+ T-lymphocytes and IL-4 by CD8+ T-cells. In the chronic phase, significant heart inflammation and fibrosis were observed, demonstrating that strains of different DTUs interact differently with the host.
Keywords: Trypanosoma cruzi, strain, dog, immune response, cardiac inflammation
Chagas disease is caused by the flagellate protozoan Trypanosoma cruzi and is transmitted by blood-sucking insects of the subfamily Triatominae (Chagas 1909). The T. cruzi heterogenic nomenclature is based on grouping the populations into six discrete typing units (DTUs): TcI, TcII, TcIII, TcIV, TcV and TcVI (Zingales et al. 2009).
The parasite exhibits different wild and domestic behaviours. The distribution in South America indicates that TcI is the most abundant and dispersed DTU, as it is found from Central America to the north of South America, and includes chronic cases of cardiomyopathy and severe cases of meningoencephalitis. The DTUs TcIII and TcIV have been predominantly associated with the sylvatic cycle in South America, whereas TcV and TcVI are related to the domestic cycle in southern and central parts of South America. The majority of TcII has been found in South American countries, such as Brazil, Argentina and Chile, where it has been predominately associated with the domestic cycle and with severe chronic forms of Chagas disease, including cardiac and digestive manifestations (Miles et al. 1981, Zingales et al. 1998, 2012, Freitas et al. 2005, Lages-Silva et al. 2006).
Evaluating organs of the same patient, it was observed that different T. cruzi populations can parasitise distinct organs and this pattern might be related to the pathogenesis of chronic forms of the disease (Vago et al. 2000). In Colombian (Col) patients, TcII was detected in the heart tissue along with histological alterations characteristic of chronic chagasic cardiomyopathy (CCC). In contrast, TcI was detected in the muscular layer of oesophageal tissue and was accompanied by lymphocytic infiltrates and interstitial fibrosis (Mantilla et al. 2010).
The mouse was the first and remains the most extensively studied experimental model for Chagas disease. Although murine infection shares some aspects in common with human Chagas disease, such as immunological, pathological and physiological characteristics, there is poor correlation between the chronic alterations observed in mice and in humans (da Costa 1999). In particular, the murine model does not permit accurate and subtle determinations of cardiac dysfunction (Morris et al. 1991). Canine experimental infection with T. cruzi shares many of the characteristics of human Chagas disease, including the occurrence of an acute, an indeterminate asymptomatic and a symptomatic chronic phase. Additionally, they share clinical and serological aspects, including patent parasitaemia, parasitism of myocytes, myocardium inflammation, fibrosis and fatty replacement in the cardiac conduction system, with electrocardiographic alteration, evolution to cardiac dysfunction and congestive heart failure. Most importantly, canine infection reproduces diffuse fibrosis, as observed in human CCC (Andrade & Andrade 1980, Andrade et al. 1980, 19, 1984, Laranja & Andrade 1980, Tafuri et al. 1988, Morris et al. 1991, d, de Lana et al. 1992, Guedes et al. 2002, Diniz et al. 2010, Caldas et al. 2013).
The complex interaction between T. cruzi and the host, which determines the pathogenesis and diversity of clinical forms, remains to be elucidated. In this respect, evaluating the influence of different T. cruzi DTUs on the course of the disease can provide valuable insight into the host-parasite interaction. For this purpose, we characterised the biochemical, haematological and cardiac histopathological alterations in canine experimental infections with Y (TcII) or Col (TcI) T. cruzi strains.
MATERIALS AND METHODS
Ethics statement - Details of the project were submitted to and approved by the Ethical Committee on Animal Research of the Federal University of Ouro Preto (UFOP), Ouro Preto, state of Minas Gerais, Brazil (protocol 2012/14). All procedures in this study were done according to the guidelines set by the Brazilian Animal Experimental College (federal law 11794). Experimental animals were maintained in the kennel at UFOP.
T. cruzi stocks - Trypomastigote forms were obtained from Swiss mice infected with the Y strain (TcII) (Silva & Nussenzweig 1953, Zingales et al. 2009) or the Col strain (TcI) (Federici et al. 1964, Zingales et al. 2009).
Experimental animals and infection protocol - Ten four-month-old mongrel dogs were obtained from the kennel at UFOP. The animals were fed with commercial dog food and water ad libitum. Prior to the study, the animals were dewormed and vaccinated against several infectious diseases. Seven dogs were intraperitoneally inoculated with 2×103 blood trypomastigotes/kg of body weight (Guedes et al. 2002) of the Y strain (n = 3) or the Col strain (n = 4). Three age-matched uninfected dogs were used as negative controls (NI). After inoculation, 5 µL of blood was collected daily from the marginal ear vein of the animals to confirm infection (Brener 1962).
Haematological and biochemical analyses - Peripheral blood (5 mL) was collected from the jugular vein of each dog and transferred to tubes containing anticoagulant ethylenediamine tetraacetic acid. Blood samples were stored at room temperature (RT) for up to 12 h prior to processing. The absolute count of lymphocytes in each sample was obtained using a BC-2800 VET auto haematology analyser (Mindray, China) (Aguiar-Soares et al. 2014). The blood was centrifuged and the separated serum was used for biochemical determination of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-GT, urea and CK-NAC (Diagnostic Labtest SA, Brazil). These were evaluated using the Automatic Biochemical System (CELM SBA-200; CELM, Brazil) following the method described by the manufacturer.
Immunophenotyping and flow cytometry - Immunophenotyping analysis of canine peripheral blood was carried out via flow cytometry as follows: fresh whole blood samples were incubated at RT for 30 min in the dark in the presence of 50 µL of fluorochrome-labelled anti-canine cell surface marker monoclonal antibodies (mAbs), Anti-T CD3 (FITC, cat.: MCA1774), Anti-T CD4 (APC, cat.: MCA1038), Anti-T CD8 (Alexa Fluor, cat.: MCA1039647), Anti-B cell (PE, cat.: MCA1781) and Anti-CD14 (Cy5, cat.: MCA1568), all purchased from AbD Serotec (USA). The mAbs were previously diluted in phosphate-buffered saline (PBS) 20% foetal bovine serum (FBS) (PBS 0.15 M, pH 7.2, supplemented with 20% of FBS). After incubation, erythrocytes were lysed by adding 2 mL of lysis solution followed by incubation for 10 min at RT. Canine whole blood leucocytes were then washed twice with 2 mL of PBS and centrifuged at 400 g for 10 min at RT. The labelled cells were then fixed at RT with 200 µL of FACS FIX solution (10.0 g/L paraformaldehyde, 10.2 g/L sodium cacodylate and 6.65 g/L sodium chloride, pH 7.2) before analysis on the cytometer. The stained cells were stored at 4-8ºC up to 24 h before cytofluorometric analysis. Each assay included an internal control for autofluorescence, in which the cells were incubated in the presence of PBS 0.5% FBS. Flow cytometric measurements were performed on a FACSCalibur instrument (Becton Dickinson, USA). The Cell-Quest software package was used for data acquisition and analyses were performed with FlowJo software (TreeStar Inc, USA). A total of 20,000 events were acquired for each preparation.
T. cruzi epimastigote antigen preparation - Soluble epimastigote antigen was prepared from stationary phase Y and Col strain T. cruzi epimastigotes grown in liver infusion tryptose-medium, similar to the method described by Sathler-Avelar et al. (2006). After the third or fourth in vitro passage, epimastigotes were harvested, washed and resuspended in PBS, pH 7.2-7.4. The suspension was rapidly frozen at -70ºC and thawed at 37ºC three times, with a sonication procedure between each step. The crude lysate was centrifuged (37,000 g) for 90 min and the supernatant was collected, dialysed overnight against 15 mM PBS, pH 7.4, sterilised by filtration through a 0.22 μm pore membrane (Millex Filter; Millipore Products Division, USA) and stored at -70ºC until use. The protein content was assayed by the method described by Lowry et al. (1951).
Immunophenotyping of intracellular cytokines and flow cytometry - Peripheral blood samples were collected in sterile tubes containing heparin, as described by Leal et al. (2014), and 1 mL blood aliquots were incubated in the presence of 1 mL RPMI-1640 (GIBCO, USA) in 14 mL polypropylene tubes (Falcon; BD Pharmingen) (“unstimulated culture”). Epimastigote antigens of the Y or Col T. cruzi strains were added to the “stimulated culture” at a final concentration of 25 µg/mL. The tubes were incubated for 12 h at 37ºC in 5% CO2. Brefeldin A (Sigma, USA) was added to each tube at a final concentration of 10 mg/mL and the cultures were submitted to an additional period of 4 h of incubation in a 5% CO2 incubator at 37ºC. A tube containing phorbol myristate acetate at a final concentration of 25 ng/mL was used as a positive control after 4 h of incubation. At the end of the incubation period, 400 mL aliquots of suspension culture were immunostained with FITC labelled canine mAbs against CD4 (1:200, mouse IgG2a: clone YKIX322.3) and CD8 (1:100, mouse IgG2a: clone YKIX302.9), all purchased from Serotec (UK). After resuspension of labelled cells in U-bottom 96-well plates, intracellular cytokine staining was done with phycoerythrin-labelled anti-bovine mAbs against interferon (IFN)-γ (clone CC302) and interleukin (IL)-4 (clone CC303) that are cross-reactive with canine cytokines (Serotec). The microtubes were kept at 4ºC until the acquisition of counts on the flow cytometer FACScalibur (Becton Dickinson). Data acquisition and analysis were done with CELLQUEST software (Franklin Lakes, USA) based on 30,000 events/microtube. The cytokine analysis of CD4+ and CD8+ lymphocyte subsets was performed by first establishing a scatter gate on the lymphocyte population using SSC vs. FL1 dot plot distributions. After selecting the region of interest (R1), dot plots were constructed for FSC vs. IFN-γ/FL2 or IL-4/FL2 to determine the percentage of intracellular cytokine. The results were expressed as the index of positive cells, which was calculated as follows: mean percentage of positive cells of “stimulated culture”/mean percentage of positive cells of “unstimulated culture”. The cultures were stimulated with antigens corresponding to the strain used to inoculate the animals; thus, dogs infected with the Y strain were stimulated with Y epimastigote antigen, those infected with the Col strain were stimulated with Col epimastigote antigen and the uninfected dogs were stimulated with both Y and Col antigen.
Histopathology and morphometric analysis - Uninfected and infected dogs were euthanised 240 days post-infection and a fragment from the middle of the right atrial wall was taken for histopathological analysis. Tissue fragments were fixed in 10% formalin and embedded in paraffin. The blocks were cut into 4-µm-thick sections and stained with haematoxylin and eosin (H&E) for inflammatory analysis and with Masson trichrome for fibrosis quantitation.
Twenty fields stained with H&E and 30 fields stained with Masson trichrome were randomly chosen at 40X magnification, giving a total of 1.5×106 μm2 and 2×106 μm2 myocardium areas analysed, respectively. Images were obtained using a Leica DM 5000 B microscope with a coupled micro camera (model 2.4.0R1; Leica Application Suite; Leica Microsystems, Germany) and processed with Leica Qwin V3 image analyser software (Leica Microsystems).
Inflammation was estimated by subtracting the average number of cells found in the myocardium of control animals from the total number of cells present in the myocardium of each infected animal.
Fibrosis was determined using the image segmentation function. All pixels with blue hues in the Masson trichrome section were selected to build a binary image and fibrosis was subsequently calculated as the total area occupied by connective tissue in the myocardium of each infected animal minus the average area of the myocardium of control animals.
Statistical analysis - Statistical tests were conducted with GraphPad Prism 5 software (Prism Software, USA) using the two-way analysis of variance test, followed by the Bonferroni test, to compare the NI, Y and Col groups. For histopathology, one-way analysis of variance was used, followed by the Tukey test, to compare the NI, Y and Col groups. Differences were considered significant at p < 0.05.
RESULTS
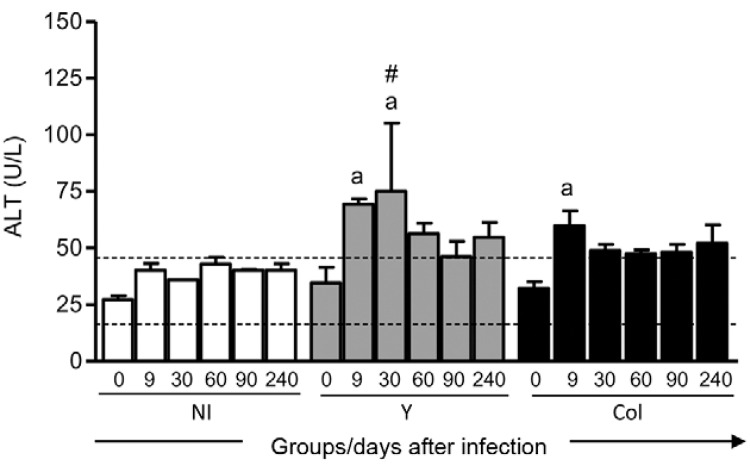
T. cruzi infection mainly alters hepatic enzymes - Our results demonstrate alterations in hepatic enzymes compared to values in non-infected dogs. This standard parameter was calculated as the mean value of all animals before infection with and without the value of two-times the standard deviation (SD) (mean ± 2 SD). The increased value of ALT in the Y and Col groups in relation to standard parameters was significant at nine and 30 days after infection (d.a.i.) for the Y group compared with day 0 and the NI group. For the Col group, this increase was statistically significant at 9 d.a.i. compared with day 0 (Fig. 1).
Fig. 1. : alanine aminotransferase (ALT) serum level before infection (0) and nine, 30, 60, 90 and 240 days after infection with Y strain or Colombian strain (Col) of Trypanosoma cruzi. The results are expressed as mean enzyme level ± standard error. Significant differences at p < 0.05 are indicated by letter a for comparisons with day 0. Dotted line indicates mean ± 2 standard deviations for all animals at day 0 as the standard parameter. #: differences between the non-infected group (NI) and infected groups (Y or Col).
Serum levels of AST, urea, gamma-GT, ALP and CK-NAC were evaluated, but no significant differences were observed (data not shown).
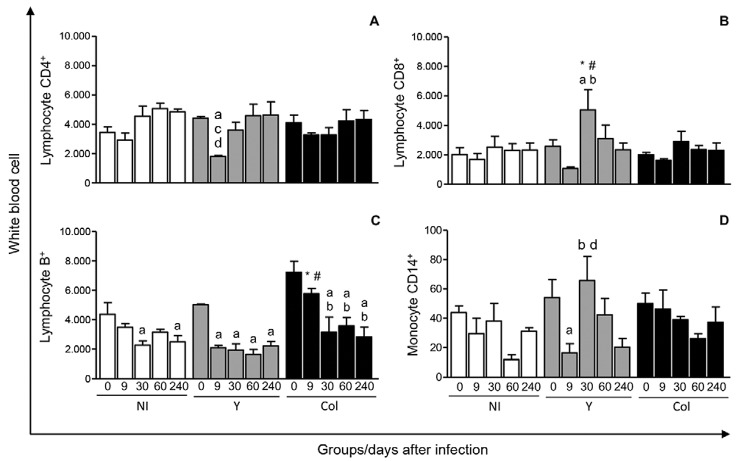
Haematological alterations resulting from T. cruzi infection - When analysing the haematological alterations, it was observed that the erythrocyte and haematocrit levels were increased in the Col group at 30, 90 and 240 d.a.i. compared to day 0. Haemoglobin was increased at 240 d.a.i. compared to day 0 for the NI and Y groups. In the Col group, this increase was observed at 30 and 240 d.a.i. compared to day 0. Platelets were decreased at 9 d.a.i. compared to day 0 in the Y group (Supplementary data, Table I). Infection with the Y strain resulted in leucopoenia at 9 d.a.i. compared to day 0. A reduction in peripheral blood monocytes was observed at nine and 240 d.a.i. with the Y strain compared to day 0, whereas this was observed at 90 d.a.i. for the Col group (Supplementary data, Table II). Y strain infection altered peripheral blood leucocytes in ex vivo analysis; T-lymphocyte CD4+ levels were reduced at 9 d.a.i. with the Y strain compared to day 0, 60 and 240 d.a.i. (Fig. 2A). T-lymphocyte CD8+ cells of the Y strain-infected group increased at 30 d.a.i. compared to day 0 and 9 d.a.i. and increased in comparison with the NI and Col groups at the same experimental time points (Fig. 2B). In terms of CD4+ and CD8+ T lymphocyte ratios, a reduction was observed during Y infection at 30 d.a.i. compared to the non-infected group (data not shown). The B+ lymphocyte frequency dropped dramatically between day 0-9 d.a.i. with the Y strain and remained constant throughout the rest of the experiment, whereas in the case of infection with the Col strain, the decrease in B+ lymphocytes was progressive from day 0-30 d.a.i. (Fig. 2C). CD14+ monocyte frequencies were highly variable and were drastically lower at 9 d.a.i. compared to day 0 in the Y group, but this percentage was higher at 30 d.a.i. (Fig. 2D).
Fig. 2. : ex-vivo quantification of CD4+ (A) and CD8+ T lymphocytes (B), B+ lymphocytes (C) and CD14+ monocytes (D) before infection (0) and nine, 30, 60 and 240 days after infection with Y strain (Y) or Colombian strain (Col) of Trypanosoma cruzi. The cell subsets were identified by flow cytometric immunostaining as described in Materials and Methods. Results are expressed as absolute cell counts in scatter plots ± standard error. Significant differences at p < 0.05 are indicated by letters a, b, c and d for comparisons with days 0, 9, 60 and 240, respectively. #: differences between the non-infected group (NI) and infected groups (Y or Col); *: differences between Y and Col groups at each time point.
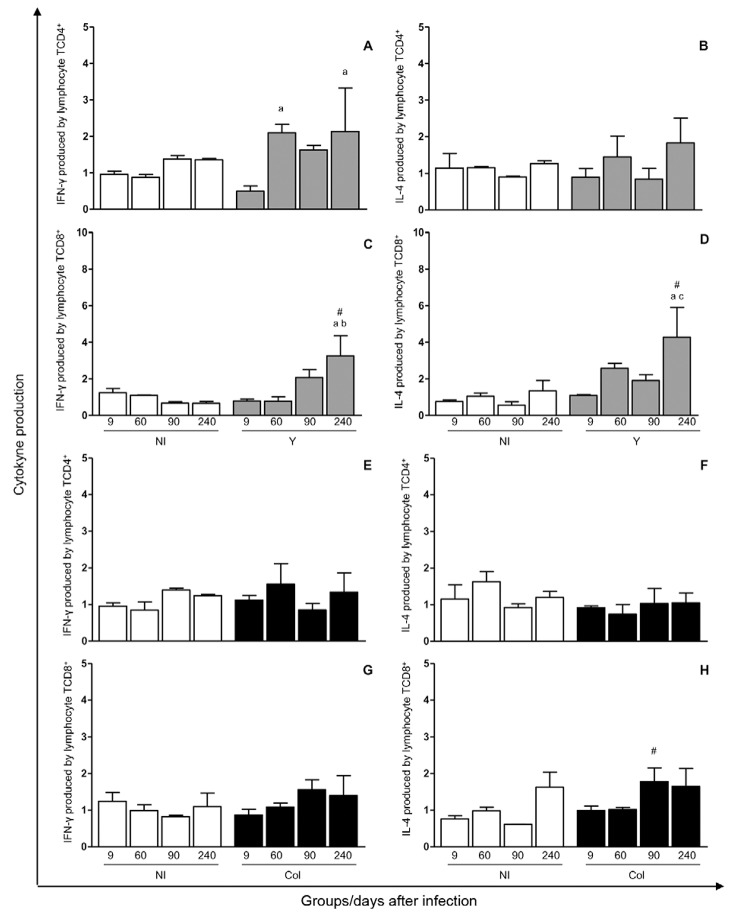
Each strain favours distinct cytokine profiles - Stimulation of NI groups with Y and Col strain epimastigote antigens did not yield any significant cytokine responses, indicating that the antigen was not able to stimulate the production of IFN-γ or IL-4 by CD4+ and CD8+ T-lymphocytes in NI dogs. In cultures stimulated with the Y antigen, the production o IFN-γ by CD4+ T lymphocytes was higher at 60 and 240 d.a.i. compared to 9 d.a.i. (Fig. 3A) and the production of IFN-γ by CD8+ T lymphocytes was higher at 240 d.a.i. compared to the NI group at this same time point and to that at 9 and 60 d.a.i. (Fig. 3C). IL-4 production by CD8+ T lymphocytes increased in cultures stimulated with the Y strain at 240 d.a.i. compared to 9 and 90 d.a.i. and in comparison with the NI group (Fig. 3D). In cultures stimulated with the Col antigen, IL-4 production was favoured by CD8+ T lymphocytes in comparison with the NI group at 90 d.a.i. (Fig. 3H).
Fig. 3. : immunophenotypic profile and cytokine patterns [interferon (INF)-γ and interleukin (IL)-4] of CD4+ and CD8+ T-lymphocyte present in peripheral blood before infection (0), nine, 60, 90 and 240 days after infection with Y strain (Y) or Colombian strain (Col) of Trypanosoma cruzi. T-cells subsets were identified by flow cytometric immunostaining as described in Materials and Methods. Data were expressed as the index, i.e., the mean percentage of positive cells of cultures stimulated/mean percentage of positive cells of unstimulated cultures, within gated lymphocytes. A-D: index of cytokine production by CD4+ or CD8+ in culture stimulated with epimastigote antigens of the Y strain; E-H: index of cytokine production by CD4+ or CD8+ in culture stimulated with epimastigote antigens of the Col strain. The results are expressed as index ± standard error. Significant differences at p < 0.05 are indicated by letters a, b and c for comparisons with days 9, 60 and 90, respectively. #: differences between the non-infected group (NI) and infected groups (Y or Col).
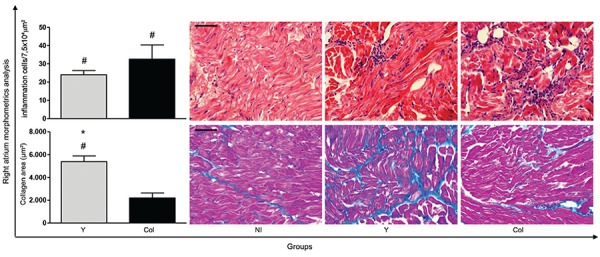
Cardiac inflammation and fibrosis - Infection promoted inflammatory cell migration to the right atrium (Fig. 4). During the chronic phase, a focal inflammatory infiltrate was observed, which consisted predominantly of mononuclear cells with lymphocyte morphology. The infection led to statistically significant increases in the number of cells in groups infected with either the Y or Col strain compared to the NI group. Moreover, the Y strain infection favoured collagen deposition and fibrosis formation was diffuse in the right atrium. This increase of areas occupied by fibrosis and the reduction of heart muscle were statistically significant in the Y group compared to the NI and Col groups (Fig. 4).
Fig. 4. : morphometric analysis and photomicrographs of the heart showing the number of inflammation cells (haematoxylin and eosin) and fibrosis area (Masson trichrome) at 240 days after infection with Y strain (Y) or Colombian strain (Col) of Trypanosoma cruzi. The results are expressed as mean number of cells or collagen area ± standard error. Significant differences at p < 0.05 are indicated by the symbols # for comparison between the non-infected group (NI) and infected groups (Y or Col) and * for comparison differences between Y and Col groups at each time point. Bar = 50 μm.
DISCUSSION
In Central and South America, different strains of T. cruzi with distinct DTUs are present, causing a variety of chronic forms of Chagas disease. Although various studies have tried to elucidate the relationship between T. cruzi strains and clinical alterations, there are no straightforward proven associations. This article exposes some aspects of acute and chronic Chagas disease with strains from different DTUs in a canine model to better understand the relationship between strains and clinical manifestations.
Parasite-host interactions during the initial events of T. cruzi infection reveal that the parasite is viable in the liver and that numerous diffuse cell infiltrates are present in the liver parenchyma, such as macrophages, CD4+ and CD8+ T-lymphocytes and natural killer cells. These cells combine to form focal inflammatory infiltrates in the liver parenchyma and perivascular spaces and are important for clearing parasites and for controlling parasitaemia, as previously observed in mice (Sardinha et al. 2010). We performed hepatic biochemical analyses and found that serum levels of ALT were significantly increased during the early stages of infection (at 9 d.a.i.) with the Y and Col strains, as observed in coatis (Nasua nasua) infected with Trypanosoma evansi (Herrera et al. 2002) and in dogs infected with T. cruzi (Barr et al. 1991).
Our results demonstrate that haematological parameters were minimally altered in dogs infected with the Y strain, whereas erythrocyte, haemoglobin and haematocrit numbers were increased during infection with the Col strain. This strain, which belongs to the TcI DTU, apparently has the capacity to alter haematological parameters in the chronic phase. In rats, T. cruzi infection resulted in increased plasma viscosity and haematocrit numbers, with morphological changes in red blood cells (Berra et al. 2005). Contradictory results were obtained by other authors during experimental canine infection with T. cruzi, as a decrease in erythrocyte frequency was observed in Beagle dogs infected with the Y, ABC or Be-78 strain (Guedes et al. 2012). However, no significant haematological changes were observed during out-bred canine infection with Be-62 and Be-78 strains (de Lana et al. 1992). Rhesus monkeys experimentally infected with metacyclic trypomastigotes of the Col strain did not show any statistically significant difference in erythrocyte numbers or haemoglobin levels during the acute phase (Bonecini-Almeida et al. 1990). Such variability in experimental data suggests that both the experimental model and the T. cruzi strain may influence the impact of T. cruzi infection on haematological characteristics.
Haemoglobin levels were significantly increased at 240 d.a.i. in all groups. This may be because an increase in haemoglobin levels normally occurs during the developmental stages in young dogs (Comazzi et al. 2004). However, anaemia was reported in dogs infected with the Sylvio X10/4 T. cruzi strain (Quijano-Hernández et al. 2012) and in Beagle dogs infected with the T. cruzi Y strain (Guedes et al. 2012).
The parasitaemia curve for dogs inoculated with Y or Col T. cruzi strains presented different pre-patent and patent periods. During Y infection, the patent period was between 11-21 d.a.i., the parasitaemia peak occurred at 15 d.a.i. (data not shown) and hepatic alterations occurred at 9 and 30 d.a.i.. The haematological alterations occurred at different times, with a decrease in platelets, leucocytes and monocytes at 9 d.a.i., whereas the increase in erythrocytes, haemoglobin, haematocrit and platelets occurred at 60 d.a.i (later than the parasitaemia peak). The animals infected with the Col strain presented higher parasitaemia levels and the patent period occurred between 21-38 d.a.i., which was later than during Y infection. Furthermore, the parasitaemia peak occurred at 33 d.a.i. (data not shown). The increase in ALT occurred at 9 d.a.i., the significant increase in erythrocyte and haematocrit levels was detected at 30, 90 and 180 d.a.i. and the haemoglobin increase was detected at 30, 60, 90 and 180 d.a.i. In conclusion, there was no clear correlation between the occurrence of the parasitaemia peak and that of the hepatic and haematological alterations.
We observed thrombocytopoenia during Y strain infection. This reduction in platelet count is characteristic of the acute phase in humans (Shikanai-Yasuda et al. 1990), in mice infected with T. cruzi (Cardoso & Brener 1980, Marcondes et al. 2000) and in dogs experimentally infected with T. evansi (La Rue et al. 1997). In the present study, we found that dogs presented leucocytopoenia during the early stages of Y strain infection, as described in the murine model (Marcondes et al. 2000). By evaluating other white blood cell parameters, we observed that infected animals presented monocytopoenia, similar to naturally infected dogs and dogs seropositive for T. cruzi (Cruz-Chan et al. 2009).
The role of CD4+ T-lymphocytes in controlling the parasite is not clear. The resistance to T. cruzi infection may be favoured by CD4+ cells, which can increase the production of IFN-γ, a cytokine that upregulates nitric oxide in macrophages, thus controlling parasite replication and promoting the synthesis of immunoglobulin isotypes (Brener & Gazzinelli 1997). Our identification of peripheral blood mononuclear cells in ex vivo analyses showed a reduction in CD4+ T lymphocytes and an increase in CD8+ T lymphocytes during infection with the Y strain. These same features were noted in dogs experimentally infected with the Be-78 strain (Carneiro et al. 2007). Importantly, Y and Be-78 are TcII strains, indicating that this cellular response may be related to DTU classification. Moreover, we observed an inversion of the ratio of CD4+/CD8+ T lymphocytes during Y strain infection, where the CD8+ T lymphocyte frequency was increased in the peripheral blood. This has been observed in studies of T. cruzi experimental infection, in which this ratio was considered an intrinsic immunological feature of infection (Carneiro et al. 2007).
The quantification of CD14+ cells showed significant reductions at 9 d.a.i. and sequential increases at 30 d.a.i during Y strain infection. Similarly, the lower level of monocytes during the acute phase of T. cruzi infection was the most relevant phenotypic alteration during experimental canine infection (Carneiro et al. 2007). Again, this feature depends on the T. cruzi strain.
In T. cruzi infection, B-cell apoptosis (Zuñiga et al. 2000) or polyclonal activation (Minoprio et al. 1989, Montes et al. 2002) is required for the establishment of parasitic infection. Moreover, T. cruzi infection reduces the levels of CD21+ lymphocytes (Carneiro et al. 2007), as was also described in the present study during infection with either the Y or Col strain.
Moreover, peripheral blood mononuclear cell cultures of patients with chronic Chagas disease in ex vivo analyses indicated cytokine profiles with high expression of IL-5, IL-10, IL-13 and IFN-γ compared to those without Chagas disease. However, when mononuclear cells of Chagas disease patients were incubated with T. cruzi antigens, IFN-γ expression was increased and IL-10 levels were reduced (Dutra et al. 1997). Another study comparing the indeterminate and cardiac forms observed high levels of IFN-γ and IL-10, respectively, but was unable to demonstrate a correlation between a particular cytokine expression profile and clinical manifestations (de Melo et al. 2012).
This contradictory pattern suggests that the role of IFN-γ in tissue lesion development is unclear. We postulate that the increased production of IFN-γ may have an impact on tissue inflammation, particularly in the case of infection with the Y strain, as observed with heart inflammatory infiltrates. However, this same strain favours IL-4 production, which can help control the tissue lesion. Similar observations were made during murine infection with Be-78 (TcII), which favours early production of IFN-γ and coincides with cardiac inflammation (Vieira et al. 2012). Petray et al. (1993) studied the influence of anti-IFN-γ and anti-IL-4 treatment on the course of experimental murine infection with two reticulotropic strains and the Col strain, a myotropic strain. They found that during infection with the Col strain, anti-IFN-γ and anti-IL-4 treatment did not influence the course of infection. In contrast, during infection with the reticulotropic strains, anti-IFN-γ treatment increased host susceptibility to the parasite and anti-IL-4 treatment increased resistance. We speculate that this TcI strain was silent in that it did not activate cytokine expression by T lymphocytes during the acute phase.
Histopathological analyses of the myocardium of NI animals or animals infected with the Y or Col strain indicated a correlation between the T. cruzi strain and the intensity of inflammation and fibrosis. Therefore, infection with both strains caused significant increases in inflammation, similar to that observed with canine experimental T. cruzi chronic infection (Guedes et al. 2009, Diniz et al. 2010). In addition, fibrosis was increased in dogs infected with the Y strain compared to NI animals and animals infected with the Col strain. The fibrosis area was characterised as intrafascicular collagen deposition (Guedes et al. 2009, Diniz et al. 2010), which caused disorganisation and isolation of the cardiomyocytes and likely contributed to the electrocardiographic alterations observed in canine models and in human CCC (Caliari et al. 2002). These anatomohistopathologic features of canine experimental infection by T. cruzi were described by de Lana et al. (1992), who thus considered the dog to be a suitable model for studying the acute and chronic phases of Chagas disease.
In summary, the Y strain triggers a more drastic immune response during the acute phase of infection in dogs in comparison with the Col strain. In the chronic phase, inflammation in the heart was balanced by tissue rearrangement and fibrosis, whereas Col infection at the chronic phase showed characteristics of inflammation. Sales-Campos et al. (2014) noted that in mixed infection with TcI and TcII, parasitism in the acute phase was similar in composition to that of the inoculum, whereas in the chronic phase, TcI was prevalent.
We hypothesise that the Col strain (TcI) can escape the host’s acute immune response, remain unnoticed by peripheral blood mononuclear cells and hence parasitise target organs faster. For the Y strain (TcII), the specific immune response begins at the acute phase. We think that this helps in controlling the myocardial lesion in the early chronic phase. We conclude that different DTU strains interact differently with the host, which is possibly related to variable expression of cell surface molecules depending on the DTU. More studies are necessary to further elucidate the parasite-host interaction.
ACKNOWLEDGEMENTS
To Renata Rocha Rezende Oliveira, for performing biochemical tests, to Maria Chaves dos Santos, for performing histopathological processing, to Gwenaelle Pound-Lana, for English revision, to UFOP, for the use of the facilities at Animal Science Centre, and to Mining Network Biotery (FAPEMIG), for support with the provision of experimental animals.
Footnotes
In memoriam
Financial support: FAPEMIG, UFOP, CNPq, CAPES
REFERENCES
- Aguiar-Soares RD, Roatt BM, Ker HG, Moreira ND, Mathias FA, Cardoso JM, Gontijo NF, Bruna-Romero O, Teixeira-Carvalho A, Martins OA, Filho, Corrêa-Oliveira R, Giunchetti RC, Reis AB. LBSapSal-vaccinated dogs exhibit increased circulating T-lymphocyte subsets (CD4+ and CD8+) as well as a reduction of parasitism after challenge with Leishmania infantum plus salivary gland of Lutzomyia longipalpis. 61Parasit Vectors. 2014;7 doi: 10.1186/1756-3305-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade ZA, Andrade SG. A patologia da doença de Chagas experimental no cão. Mem Inst Oswaldo Cruz. 1980;75:77–95. doi: 10.1590/s0074-02761980000200008. [DOI] [PubMed] [Google Scholar]
- Andrade ZA, Andrade SG, Sadigursky M. Damage and healing in the conducting tissue of the heart (an experimental study in dogs infected with Trypanosoma cruzi) J Pathol. 1984;143:93–101. doi: 10.1002/path.1711430204. [DOI] [PubMed] [Google Scholar]
- Andrade ZA, Andrade SG, Sadigursky M, Lima JA. Experimental Chagas disease in dogs. Morphologic and electrocardiographic relations in the acute phase of the infection. Arq Bras Cardiol. 1980;35:485–490. [PubMed] [Google Scholar]
- Barr SC, Gossett KA, Klei TR. Clinical, clinicopathologic and parasitologic observations of trypanosomiasis in dogs infected with North American Trypanosoma cruzi isolates. Am J Vet Res. 1991;52:954–960. [PubMed] [Google Scholar]
- Berra HH, Piaggio E, Revelli SS, Luquita A. Blood viscosity changes in experimentally Trypanosoma cruzi-infected rats. Clin Hemorheol Microcirc. 2005;32:175–182. [PubMed] [Google Scholar]
- Bonecini-Almeida MG, Galvão-Castro B, Pessoa MHR, Pirmez C, Laranja F. Experimental Chagas disease in rhesus monkeys. I. Clinical, parasitological, hematological and anatomo-pathological studies in the acute and indeterminate phase of the disease. Mem Inst Oswaldo Cruz. 1990;85:163–171. doi: 10.1590/s0074-02761990000200004. [DOI] [PubMed] [Google Scholar]
- Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- Brener Z, Gazzinelli RT. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas disease. Int Arch Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- Caldas IS, Guedes PMM, dos Santos FM, Diniz LF, Martins TA, do Nascimento AFS, Azevedo MA, de Lima WG, Neto RM, Torres RM, Talvani A, Bahia MT. Myocardial scars correlate with electrocardiographic changes in chronic Trypanosoma cruzi infection for dogs treated with benznidazole. Trop Med Int Health. 2013;18:75–84. doi: 10.1111/tmi.12002. [DOI] [PubMed] [Google Scholar]
- Caliari MV, Machado RP, de Lana M, Cajá RAF, Carneiro CM, Bahia MT, Santos CAB, Magalhães GA, Sampaio IBM, Tafuri WL. Quantitative analysis of cardiac lesions in chronic canine chagasic cardiomyopathy. Rev Inst Med Trop Sao Paulo. 2002;44:273–278. doi: 10.1590/s0036-46652002000500008. [DOI] [PubMed] [Google Scholar]
- Cardoso JE, Brener Z. Hematological changes in mice experimentally infected with Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1980;75:97–104. doi: 10.1590/s0074-02761980000200009. [DOI] [PubMed] [Google Scholar]
- Carneiro CM, Martins OA, Filho, Reis AB, Veloso VM, Araújo FM, Bahia MT, Lana M, Machado-Coelho GL, Gazzinelli G, Correa-Oliveira R, Tafuri WL. Differential impact of metacyclic and blood trypomastigotes on parasitological, serological and phenotypic features triggered during acute Trypanosoma cruzi infection in dogs. Acta Trop. 2007;101:120–129. doi: 10.1016/j.actatropica.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Chagas C. Nova tripanozomiaze humana. Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- Comazzi S, Pieralisi C, Bertazzolo W. Haematological and biochemical abnormalities in canine blood: frequency and associations in 1,022 samples. J Small Anim Pract. 2004;45:343–349. doi: 10.1111/j.1748-5827.2004.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Cruz-Chan JV, Bolio-González M, Colín-Flores R, Ramírez-Sierra MJ, Quijano-Hernández I, Dumonteil E. Immunopathology of natural infection with Trypanosoma cruzi in dogs. Vet Parasitol. 2009;162:151–155. doi: 10.1016/j.vetpar.2009.02.024. [DOI] [PubMed] [Google Scholar]
- da Costa SCG. Mouse as a model for Chagas disease: does mouse represent a good model for Chagas disease? Mem Inst Oswaldo Cruz. 1999;94(Suppl. I):269–272. doi: 10.1590/s0074-02761999000700045. [DOI] [PubMed] [Google Scholar]
- de Lana M, Chiari E, Tafuri WL. Experimental Chagas disease in dogs. Mem Inst Oswaldo Cruz. 1992;87:59–71. doi: 10.1590/s0074-02761992000100011. [DOI] [PubMed] [Google Scholar]
- de Melo AS, Lorena VMB, Braz SCM, Docena C, Gomes YM. IL-10 and IFN-γ gene expression in chronic Chagas disease patients after in vitro stimulation with recombinant antigens of Trypanosoma cruzi. Cytokine. 2012;58:207–212. doi: 10.1016/j.cyto.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Diniz LF, Caldas IS, Guedes PMM, Crepalde G, Lana M, Carneiro CM, Talvani A, Urbina JA, Bahia MT. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agents Chemother. 2010;54:2979–2986. doi: 10.1128/AAC.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra WO, Gollob KJ, Pinto-Dias JC, Gazzinelli G, Correa-Oliveira R, Coffman RL, Carvalho-Parra JF. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- Federici EE, Abelmann WH, Neva FA. Chronic and progressive myocarditis and myositis in c3h mice infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1964;13:272–280. doi: 10.4269/ajtmh.1964.13.272. [DOI] [PubMed] [Google Scholar]
- Freitas JM, Lages-Silva E, Crema E, Pena SD, Macedo AM. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int J Parasitol. 2005:411–417. doi: 10.1016/j.ijpara.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Guedes PM, Veloso VM, Mineo TW, Santiago-Silva J, Crepalde G, Caldas IS, Nascimento MS, Lana M, Chiari E, Galvão LM, Bahia MT. Hematological alterations during experimental canine infection by Trypanosoma cruzi. Rev Bras Parasitol Vet. 2012;21:151–156. doi: 10.1590/s1984-29612012000200015. [DOI] [PubMed] [Google Scholar]
- Guedes PM, Veloso VM, Tafuri WL, Galvão LM, Carneiro CM, de Lana M, Chiari E, Ataide Soares K, Bahia MT. The dog as model for chemotherapy of the Chagas disease. Acta Trop. 2002;84:9–17. doi: 10.1016/s0001-706x(02)00139-0. [DOI] [PubMed] [Google Scholar]
- Guedes PMM, Veloso VM, Afonso LCC, Caliari MV, Carneiro CM, Diniz LF, Marques-da-Silva EA, Caldas IS, Matta MAV, Souza SM, de Lana M, Chiari E, Galvão LMC, Bahia MT. Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-γ, TNF-α and low IL-10 production during the acute infection phase. Vet Immunol Immunopathol. 2009;130:43–52. doi: 10.1016/j.vetimm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Herrera HM, Alessi AC, Marques LC, Santana AE, Aquino LP, Menezes RF, Moraes MA, Machado RZ. Experimental Trypanosoma evansi infection in South American coati (Nasua nasua): hematological, biochemical and histopathological changes. Acta Trop. 2002;81:203–210. doi: 10.1016/s0001-706x(01)00204-2. [DOI] [PubMed] [Google Scholar]
- La Rue ML, Silva RAMS, De Carli GA. Coagulopathy in dogs infected with Trypanosoma (trypanozoon) evansi (Steel, 1885) Balbiani, 1888. Parasitol día. 1997;21:1–5. [Google Scholar]
- Lages-Silva E, Ramírez LE, Pedrosa AL, Crema E, Galvão LMC, Pena SD, Macedo AM, Chiari E. Variability of kinetoplast DNA gene signatures of Trypanosoma cruzi II strains from patients with different clinical forms of Chagas disease in Brazil. J Clin Microbiol. 2006;44:2167–2171. doi: 10.1128/JCM.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranja FS, Andrade ZA. Chronic cardiac form of Chagas disease in dogs. Arq Bras Cardiol. 1980;35:377–380. [PubMed] [Google Scholar]
- Leal GGA, Roatt BM, Aguiar-Soares RDO, Carneiro CM, Giunchetti RC, Teixeira-Carvalho A, Martins OA, Filho, Francisco AF, Cardoso JM, Mathias FA, Correa-Oliveira R, Carneiro M, Coura-Vital W, Reis AB. Immunological profile of resistance and susceptibility in naturally infected dogs by Leishmania infantum. 10.1016/j.vetpar.2014.08.022.Vet Parasitol. 2014 doi: 10.1016/j.vetpar.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough M, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mantilla JC, Zafra GA, Macedo AM, González CI. Mixed infection of Trypanosoma cruzi I and II in a Colombian cardiomyopathic patient. Hum Pathol. 2010;41:610–613. doi: 10.1016/j.humpath.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Marcondes MCG, Borelli P, Yoshida N, Russo M. Acute Trypanosoma cruzi infection is associated with anemia, thrombocytopenia, leukopenia and bone marrow hypoplasia: reversal by nifurtimox treatment. Microbes Infect. 2000;2:347–352. doi: 10.1016/s1286-4579(00)00333-6. [DOI] [PubMed] [Google Scholar]
- Miles MA, Cedillos RA, Povoa MM, de Souza AA, Prata A, Macedo V. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas disease? Lancet. 1981;1:1338–1340. doi: 10.1016/s0140-6736(81)92518-6. [DOI] [PubMed] [Google Scholar]
- Minoprio P, Itohara S, Heusser C, Tonegawa S, Coutinho A. Immunobiology of murine T. cruzi infection: the predominance of parasite-nonspecific responses and the activation of TCRI T cells. Immunol Rev. 1989;112:183–207. doi: 10.1111/j.1600-065x.1989.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Montes CL, Zuñiga EI, Vazquez J, Arce C, Gruppi A. Trypanosoma cruzi mitochondrial malate dehydrogenase triggers polyclonal B-cell activation. Clin Exp Immunol. 2002;127:27–36. doi: 10.1046/j.1365-2249.2002.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Barr S, Weiss L, Tanowitz H, Wittner M, Bilezikian JP. Myocardial beta-adrenergic adenylate cyclase complex in a canine model of chagasic cardiomyopathy. Circ Res. 1991;69:185–195. doi: 10.1161/01.res.69.1.185. [DOI] [PubMed] [Google Scholar]
- Petray PB, Rottenberg ME, Bertot G, Corral RS, Diaz A, Orn A, Grinstein S. Effect of anti-gamma-interferon and anti-interleukin-4 administration on the resistance of mice against infection with reticulotropic and myotropic strains of Trypanosoma cruzi. Immunol Lett. 1993;35:77–80. doi: 10.1016/0165-2478(93)90151-q. [DOI] [PubMed] [Google Scholar]
- Quijano-Hernández IA, Castro-Barcena A, Aparicio-Burgos E, Barbosa-Mireles MA, Cruz-Chan JV, Vázquez-Chagoyán JC, Bolio-González ME, Dumonteil E. Evaluation of clinical and immunopathological features of different infective doses of Trypanosoma cruzi in dogs during the acute phase. 635169Scientific WorldJournal. 20122012 doi: 10.1100/2012/635169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales-Campos H, Kappel HB, Andrade CP, Lima TP, Mattos ME, Jr, de Castilho A, Correia D, Giraldo LE, Lages-Silva E. A DTU-dependent blood parasitism and a DTU-independent tissue parasitism during mixed infection of Trypanosoma cruzi in immunosuppressed mice. Parasitol Res. 2014;113:375–385. doi: 10.1007/s00436-013-3665-z. [DOI] [PubMed] [Google Scholar]
- Sardinha LR, Mosca T, Elias RM, do Nascimento RS, Gonçalves LA, Bucci DZ, Marinho CR, Penha-Gonçalves C, Lima MR, Alvarez JM. The liver plays a major role in clearance and destruction of blood trypomastigotes in Trypanosoma cruzi chronically infected mice. PLoS Negl Trop Dis. 2010;4: doi: 10.1371/journal.pntd.0000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, Borges JD, de Lana M, Teixeira-Carvalho A, Dias JC, Elói-Santos SM, Martins AO., Filho Benznidazole treatment during early-indeterminate Chagas disease shifted the cytokine expression by innate and adaptive immunity cells toward a type 1-modulated immune profile. Scand J Immunol. 2006;64:554–563. doi: 10.1111/j.1365-3083.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- Shikanai-Yasuda MA, Lopes MH, Tolezano JE, Umezawa E, Amato V, Neto, Barreto ACP, Higaki Y, Moreira AAB, Funayama G, Barone AA, Duarte A, Odone V, Cerri GC, Sato M, Pozzi D, Shiroma M. Doença de Chagas aguda: vias de transmissão, aspectos clínicos e resposta à terapêutica específica em casos diagnosticados em um centro urbano. Rev Inst Med Trop Sao Paulo. 1990;32:16–27. doi: 10.1590/s0036-46651990000100004. [DOI] [PubMed] [Google Scholar]
- Silva LHP, Nussenzweig V. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clin Biol. 1953;20:191–203. [Google Scholar]
- Tafuri WL, de Lana M, Chiari E, Caliari MV, Bambirra EA, Rios-Leite VH, Barbosa AJ. Dogs as experimental models for the study of the natural course of Chagas disease. 77Rev Soc Bras Med Trop. 1988;21 doi: 10.1590/s0037-86821988000200010. [DOI] [PubMed] [Google Scholar]
- Vago AR, Andrade LO, Leite AA, Reis DA, Macedo AM, Adad SJ, Tostes S, Jr, Moreira MC, Brasileiro GB, Filho, Pena SD. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease. Am J Pathol. 2000;156:1805–1809. doi: 10.1016/s0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira PMA, Francisco AF, Machado EMM, Nogueira NC, Fonseca KS, Reis AB, Teixeira-Carvalho A, Martins OA, Filho, Tafuri WL, Carneiro CM. Different infective forms trigger distinct immune response in experimental Chagas disease. PLoS ONE. 2012;7: doi: 10.1371/journal.pone.0032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Zingales B, Souto RP, Mangia RH, Lisboa CV, Campbell DA, Coura JR, Jansen A, Fernandes O. Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exongene sequences. Int J Parasitol. 1998;28:105–112. doi: 10.1016/s0020-7519(97)00178-1. [DOI] [PubMed] [Google Scholar]
- Zuñiga E, Motran C, Montes CL, Diaz FL, Bocco JL, Gruppi A. Trypanosoma cruzi-induced immunosuppression: B cells undergo spontaneous apoptosis and lipopolysaccharide (LPS) arrests their proliferation during acute infection. Clin Exp Immunol. 2000;119:507–515. doi: 10.1046/j.1365-2249.2000.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]