Abstract
Advances in pharmacogenetic analysis technology have accelerated the movement to incorporate pharmacogenetic analysis data into medicine. Therefore, pharmacists will soon have to provide guidance and raise pharmaceutical questions regarding prescriptions based on patient pharmacogenomic information. The objective of this study was to clarify Japanese pharmacists’ awareness of pharmacogenetics. We conducted a postal questionnaire survey among 372 pharmacists belonging to Tsuruha Holdings. Available data were collected from 268 pharmacists (male [n=133], <40 years old [n=170], drugstore pharmacists [n=182]). Of the pharmacists, 19.0% of the population were aware of the Ethical Guidelines for Human Genome/ Gene Analysis Research in Japan, 31.0% of the population had heard either or both the terms “pharmacogenomics” and “pharmacogenetics”, and 16.8% of the population were aware that health insurance covered pharmacogenomic tests performed before prescription in Japan. Only 0.4% indicated that they could raise pharmaceutical questions regarding prescriptions based on patients’ pharmacogenomic information, and 61.2% of the population indicated a preference to undertake pharmacogenomic tests to predict the efficacy or adverse reactions of a drug. We found a need for actions to improve the awareness of pharmacists about pharmacogenetics and create an environment in which pharmacists are able to provide appropriate medical service based on pharmacogenomic information.
Keywords: pharmacogenomics/pharmacogenetics, awareness, pharmacist
Introduction
When paired with epidemiology, pharmacogenetic analysis is a truly promising tool for evaluating the risk of multifactorial diseases affected by lifestyle and environmental factors, as well as multiple genetic factors, for characterizing the mechanisms of disease onset and for establishing personalized prophylaxis and therapies. Advances in pharmacogenetic analysis technology have accelerated the movement to incorporate pharmacogenetic analysis data into medicine. Many cases have been reported in which analyses of gene polymorphisms related to drug-metabolizing enzymes would be useful for predicting efficacy and adverse reactions.1 In Shinshu University Hospital (Japan), gene diagnostics are done with mitochondrial deoxyribonucleic acid for preventing deafness caused by aminoglycoside antibiotics, and drug cards are distributed to the relevant patients in clinical settings (http://www.shinshu-u.ac.jp/faculty/medicine/chair/ent/nonsyndromic/Mit1555.html, http://www.pharm.tohoku.ac.jp/~seikatsu/mysite1/sub3.html). In Japan, a UGT1A1 gene polymorphism diagnostic agent for use before irinotecan was allowed under insurance coverage in 2009 (http://www.info.pmda.go.jp/go/pack/4240404A1091_1_08/).
These developments mean that pharmacists will soon have to provide guidance and raise pharmaceutical questions regarding prescriptions based on patient pharmacogenomic information. Recently, Johansen Taber and Dickinson reported that physicians’ familiarity with pharmacogenomics was low.2 However, little is known about Japanese pharmacists’ awareness of pharmacogenetics. The objective of this study was to clarify Japanese pharmacists’ awareness of pharmacogenetics.
Materials and methods
Pharmacists employed by the Tsuruha Group, a Japanese drugstore chain, were surveyed using a self-administered questionnaire on their awareness of the Japanese Ethical Guidelines for Human Genome/Gene Analysis Research (http://www.meti.go.jp/policy/mono_info_service/mono/bio/Seimeirinnri/shishinnhonbun.pdf), awareness of the terms “pharmacogenomics” and “pharmacogenetics”, the status of the clinical use of insurance-covered pharmacogenomic tests when dispensing drugs in Japan, their ability to perform such duties as raising pharmaceutical questions regarding prescriptions based on patients’ pharmacogenomic information, and preferences for undertaking pharmacogenomic tests for predicting efficacy and adverse reactions. Tsuruha headquarters disseminated these questionnaires to 372 pharmacists, who were asked to complete and return them. Completion and submission were optional. A total of 287 pharmacists responded, giving a response rate of 77.2%. The 268 pharmacists who provided valid responses to the questions on basic characteristics and pharmacogenomics were included in the analysis population. The responses were tabulated and analyzed following stratification according to sex, age (<40 years, ≥40 years), location of workplace (stand-alone pharmacy, pharmacy within a drugstore), and duration of employment (<10 years, ≥10 years). Statistical analysis was performed by the χ2 test and Fisher’s exact probability test using the SAS package (version 9.3; SAS Institute, Cary, NC, USA).
Results
Of the 268 pharmacists in the analysis population, 86 worked in a stand-alone pharmacy and 182 worked in a pharmacy within a drugstore (Table 1).
Table 1.
Characteristics of pharmacists (n=268)
| Sex | |
| Female, n (%) | 135 (50.4) |
| Male, n (%) | 133 (49.6) |
| Age | |
| <30 years, n (%) | 83 (40.0) |
| 30–39 years, n (%) | 87 (32.5) |
| 40–49 years, n (%) | 37 (13.8) |
| ≧50 years, n (%) | 61 (22.8) |
| Location of employment | |
| Stand-alone pharmacy, n (%) | 86 (32.1) |
| Pharmacy within a drugstore, n (%) | 182 (67.9) |
| Duration of employment | |
| <10 years, n (%) | 148 (55.2) |
| ≥10 years, n (%) | 120 (44.8) |
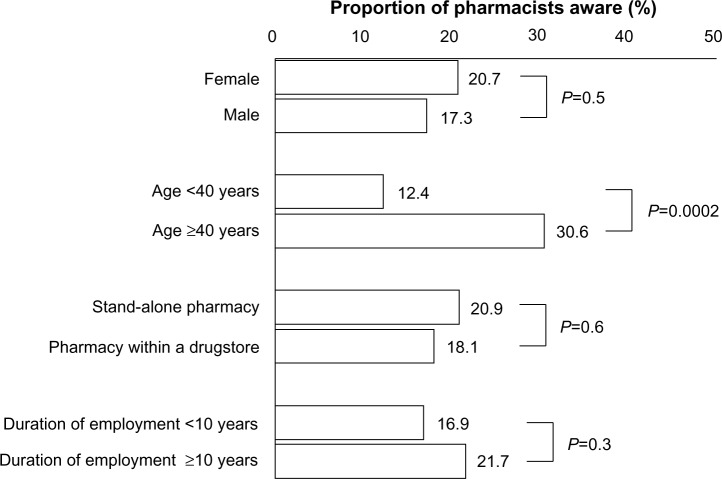
A total of 19.0% of the population were aware of the Ethical Guidelines for Human Genome/Gene Analysis Research by the Japanese Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare, and Ministry of Economy, Trade and Industry. Awareness was higher among those at least 40 years of age in comparison to those younger than 40 years (Figure 1).
Figure 1.
Awareness of Ethical Guidelines for Human Genome/Gene Analysis Research.
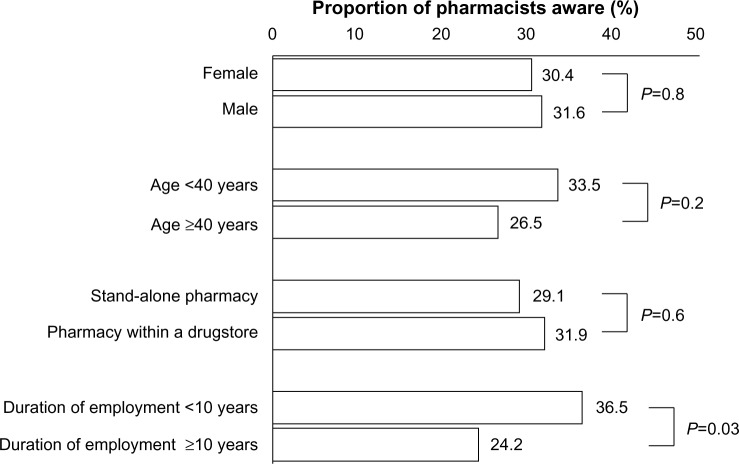
Thirty-one percent of the population had heard the term “pharmacogenomics” or “pharmacogenetics”. A higher proportion of the pharmacists who had worked as pharmacists for less than 10 years were aware of these terms than the pharmacists who had worked for at least 10 years (Figure 2).
Figure 2.
Awareness of the terms “pharmacogenomics”/”pharmacogenetics”.
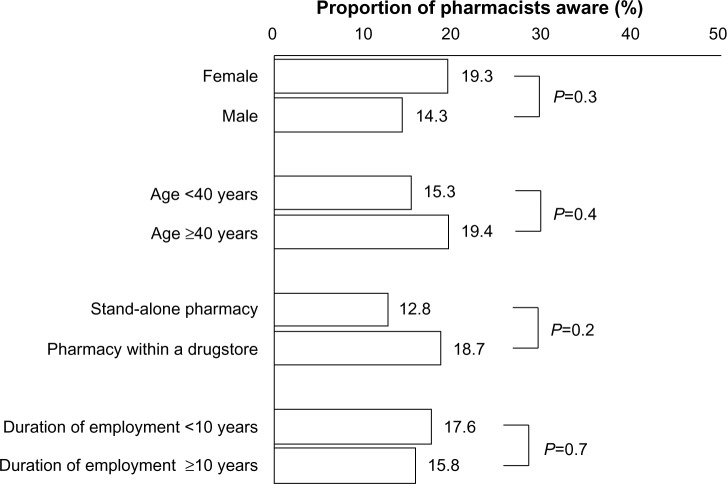
A total of 16.8% of the population were aware that health insurance covered pharmacogenomic tests performed before prescription in Japan. Responses to this question did not differ according to sex, age, location of workplace, or duration of employment (Figure 3).
Figure 3.
Awareness of use of insurance-covered pharmacogenomic tests before prescription in Japan.
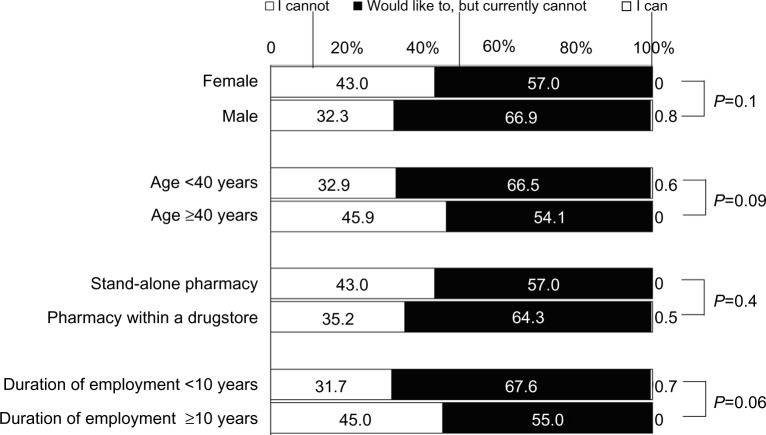
When asked about the possibility of raising pharmaceutical questions regarding prescriptions based on patients’ pharmacogenomic information, 37.7% of the population indicated they would be unable, 61.9% indicated they would like to but currently cannot, and 0.4% indicated they could. Responses to this question did not differ according to sex, age, location of workplace, or duration of employment (Figure 4).
Figure 4.
Awareness regarding ability to raise pharmaceutical questions regarding prescriptions based on pharmacogenomic information.
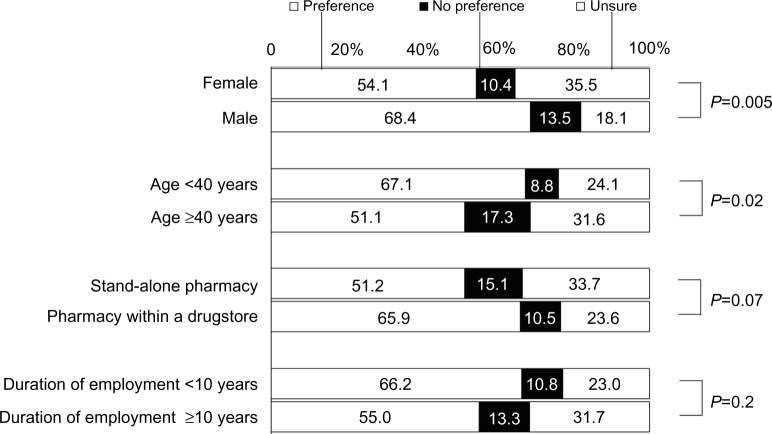
When asked about personal preferences to undertake pharmacogenomic tests able to predict the efficacy or adverse reactions of a drug based on available pharmacogenomic information, 61.2% of the population indicated a preference to undertake pharmacogenomic tests, 11.9% indicated a preference not to undertake pharmacogenomic tests, and 26.9% indicated they were unsure. A higher proportion of women indicated they were unsure, and a higher proportion of pharmacists younger than 40 years of age indicated a preference to undertake pharmacogenomic tests (Figure 5).
Figure 5.
Personal preference with regard to undergoing pharmacogenomic tests for own medication.
Discussion
Awareness of ethical guidelines and terminology
The survey indicated that 19.0% of respondents were aware of the Ethical Guidelines for Human Genome/Gene Analysis Research and 31% were aware of the term “pharmacogenomics” or “pharmacogenetics”. Awareness of the ethical guidelines and related terminology must increase to allow pharmacists to deal with the increasing use of pharmacogenetic analysis in medicine. The higher awareness of the ethical guidelines among pharmacists at least 40 years of age and higher and awareness of the terms among pharmacists who had worked as a pharmacist for less than 10 years indicates the need to provide information and education to students in pharmacy school, in addition to providing information to pharmacists of all ages.
Awareness of clinical application of pharmacogenomic tests before medication prescription
A total of 16.8% were aware of the clinical application of pharmacogenomic tests in Japan. As with the ethical guidelines and terminology, pharmacists must be made more aware of how much pharmacogenomic tests are used in the clinic. To better develop pharmacogenomic test technologies beyond the level of basic research to clinical application, a new research environment must be built that makes researchers in basic pharmacology more mindful of clinical pharmacy and practicing pharmacists more aware of basic science.3
Roles of pharmacists
A similar survey of 303 American pharmacists indicated that while more than 70% of the respondents thought that pharmacists should be knowledgeable about pharmacogenetics and should play an important role in drug therapy based on pharmacogenomic information, but only “14.2% agreed that they are confident in their ability to accurately apply the results of a pharmacogenetic analysis to drug selection”.4 In our survey, less than 1% of Japanese pharmacists indicated an ability to raise pharmaceutical questions regarding prescriptions based on patients’ pharmacogenomic information in light of the possibility of such inquiries in the future. The difference between American and Japanese pharmacists in this point may be caused not only by Japanese pharmacists’ poor knowledge of pharmacogenetics but also poor literacy regarding which pharmacists could propose patients’ prescriptions based on pharmaceutical aspects. Therefore, the system for pharmacists must be better developed to cope with the gradually increasing clinical application of pharmacogenetic analysis. However, as about 60% of the respondents answered that they would like to but currently cannot, many are accepting of this new duty of pharmacists. Providing better information about pharmacogenetics to practicing pharmacists should better prepare pharmacists for the new duty of pharmacists based on patients’ pharmacogenomic information.
Personal opinions about pharmacogenomic tests
About 60% of the responding pharmacists indicated a personal preference to undertake pharmacogenomic tests able to predict drug efficacy or adverse-reaction profiles. No previous study has characterized the level of preference by pharmacists or members of other populations to undertake pharmacogenetic analysis. The proportion of pharmacists preferring to undertake pharmacogenetic analysis was expected to be high, given the pharmacists’ ability to adequately understand efficacy and adverse reactions.
About 40% of the population of our study, however, indicated being unsure or a preference not to undertake pharmacogenomic tests to predict efficacy and adverse reactions. Possible reasons for this reluctance are the fact that there remains a limitation concerning the precision of pharmacogenetic analysis and the possibility of unexpected outcomes, such as when a test shows a parent–child relationship different from the assumed relationship. Debate on the ethical, legal, and social implications of pharmacogenetic analysis in particular has not kept pace with the rapid progress of pharmacogenetic analysis technology. Guidelines and systems must continue to be developed on an international level as well. For instance, the publication of conflicting reports supporting5 and questioning6 the usefulness of genotype-guided dosing of warfarin for safe and effective drug treatment suggests that dosing guided by pharmacogenomic information may not be as beneficial as initially thought. The clinical usefulness of pharmacogenomic tests must be assessed carefully.
Study limitations
The survey was limited to pharmacists employed by the Tsuruha Group. The response rate was 77%, and many of the responding pharmacists may have had a higher-than-average interest in the topic of the survey. A more generalizable group of pharmacists should be surveyed next.
Conclusion
Our survey clarified the current status of awareness of pharmacists about pharmacogenetics. The results indicated the need for actions to improve the awareness of pharmacists about pharmacogenetics and create an environment in which pharmacists are able to provide appropriate medical service based on pharmacogenomic information.
Supplementary material
Questionnaire
Acknowledgments
We thank all the pharmacists who took the time to complete the questionnaire. This work was supported by Grants by Tsuruha Holdings Inc., Japan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pirmohamed M. Personalized pharmacogenomics: predicting efficacy and adverse drug reactions. Annu Rev Genomics Hum Genet. 2014;15:349–370. doi: 10.1146/annurev-genom-090413-025419. [DOI] [PubMed] [Google Scholar]
- 2.Johansen Taber KA, Dickinson BD. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmgenomics Pers Med. 2014;7:145–162. doi: 10.2147/PGPM.S63715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiratsuka M. Pharmacogenomics in personalized drug therapy. Jpn J Pharm Health Care Sci. 2013;39(6):327–337. [Google Scholar]
- 4.McCullough KB, Formea CM, Berg KD, et al. Assessment of the pharmacogenomics educational needs of pharmacists. Am J Pharm Educ. 2011;75(3):51. doi: 10.5688/ajpe75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirmohamed M, Burnside G, Eriksson N, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369(24):2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 6.Kimmel SE, French B, Kasner SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369(24):2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.