Abstract
Characteristics of stroke cases, acute stroke care, and outcomes after stroke differ according to geographical and cultural background. To provide epidemiological and clinical data on stroke care in South Korea, we analyzed a prospective multicenter clinical stroke registry, the Clinical Research Center for Stroke-Fifth Division (CRCS-5). Patients were 58% male with a mean age of 67.2±12.9 years and median National Institutes of Health Stroke Scale score of 3 [1-8] points. Over the 6 years of operation, temporal trends were documented including increasing utilization of recanalization treatment with shorter onset-to-arrival delay and decremental length of stay. Acute recanalization treatment was performed in 12.7% of cases with endovascular treatment utilized in 36%, but the proportion of endovascular recanalization varied across centers. Door-to-IV alteplase delay had a median of 45 [33-68] min. The rate of symptomatic hemorrhagic transformation (HT) was 7%, and that of any HT was 27% among recanalization-treated cases. Early neurological deterioration occurred in 15% of cases and were associated with longer length of stay and poorer 3-month outcomes. The proportion of mRS scores of 0-1 was 42% on discharge, 50% at 3 months, and 55% at 1 year after the index stroke. Recurrent stroke up to 1 year occurred in 4.5% of patients; the rate was higher among older individuals and those with neurologically severe deficits. The above findings will be compared with other Asian and US registry data in this article.
Keywords: Stroke registry, South Korea, Case profile, Hyperacute treatment, Thrombolysis, Outcome, Recurrent event
Introduction
In general, the term clinical registry refers to a observational database of patients' characteristics, treatments, and outcomes without specified inclusion or exclusion criteria and therapeutic approaches unrelated to purposes of the registry.1 Well-designed, organized and performed clinical registry may provide relevant data from real world experience and patients' characteristics as well as outcomes and safety of cases. Purposes of clinical registries may be summarized as describing natural history of disease, determining effectiveness, measuring or monitoring safety and harm, and measuring quality.2 Recently, clinical registries started to adapt data elements for care quality and patients' safety, and thus these data may be utilized in activities for quality of care monitoring and quality improvement. Such qualities of clinical registries make it valuable source of clinical researches because case characteristics in large clinical trials are only partially representative of patients' in clinical practice.3 However, to incorporate clinical registries into research purposes, ensuring high-quality of stored data is of paramount importance, including meticulous documentation of data elements and capture processes, completeness of individual cases and data, regular monitoring and auditing processes including prespecified query algorithm, update and correction of erroneous entries after public distribution of dataset. Likewise, linking clinical registry database to secondary data sources such as governmental statistics and health insurance reimbursement database provides broader ranges of epidemiological data and current status of under-represented subgroups.4
In this context, the authors summarize the structure and organization of the Clinical Research Center for Stroke-fifth division (CRCS-5) registry, which is a prospective, multicenter, quality-improvement, clinical stroke registry in South Korea.
Brief history and overview of the CRCS-5 registry
Organization of CRCS-5 registry
The Clinical Research Center for Stroke (CRCS; director, Dr. Byung-Woo Yoon, Seoul National University Hospital), supported by the Korea Healthcare Technology R&D Project, Ministry of Health, Republic of Korea (HI10C2020), was established in 2006 to facilitate multi-center collaborative clinical research and to develop a series of clinical practice guidelines based on data from the Korean population. Six divisions comprise the CRCS and the fifth division, CRCS-5 (principal investigator, Dr. Hee-Joon Bae, Seoul National University Bundang Hospital), is designated to develop clinical guidelines for secondary prevention of stroke through clinical epidemiological research.
To this end, CRCS-5 tried to establish a prospective, multi-center clinical registry for stroke cases from the start of CRCS. Before the commencement of CRCS, the Korean Stroke Registry (KSR; director, Dr. Jae-Kyu Roh, Professor Emeritus at Seoul National University Hospital) was operated through a web-based database to which more than 30 hospitals submitted acute stroke cases.5 The CRCS decided to continue with the baseline structure of database tables from the KSR. CRCS-5 also maintained the overall KSR database structure and added a few tables and fields for its own purposes. To ensure standardization of registration process, CRCS-5 workbook for data collection and variable definitions was established and disseminated throughout the participating center. The CRCS-5 steering committee also regularly held a database workshop to help investigators and registrars being accustomed to the database structure and data entry system at least once a year. Participating researchers in the CRCS-5 registry tried to integrate the registration, auditing, and reporting of the clinical registry into daily clinical practice, and thus established "quality of stroke care monitoring and improvement" as a primary objective of the registry.
Expansion of the CRCS-5 registry
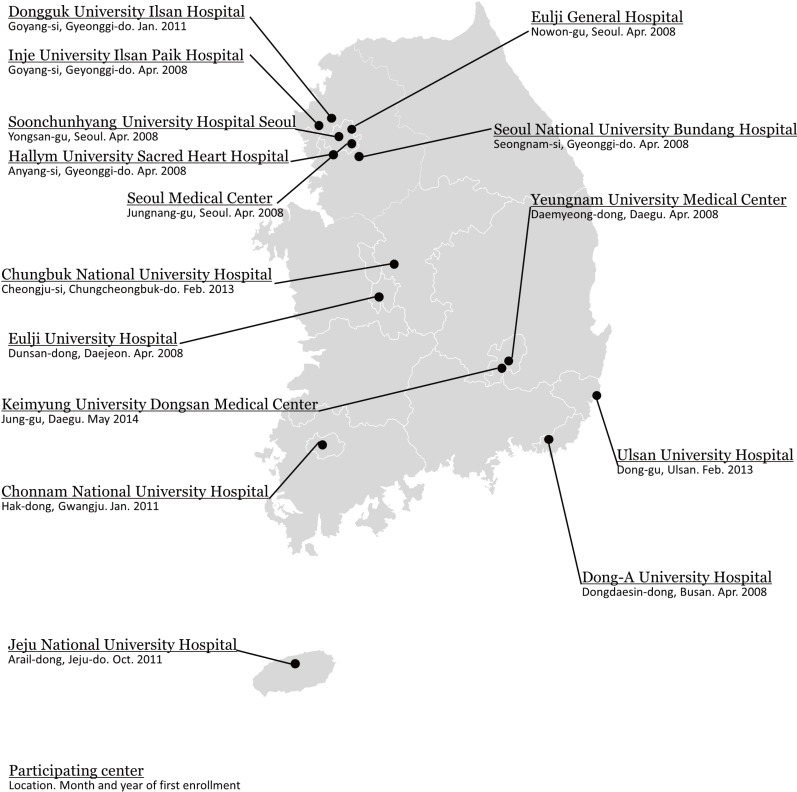
The CRCS-5 registry was initiated in April 2008 with nine hospitals. In April 2008, participating centers were mostly located in the Seoul metropolitan area, with one from the central area and one from the southeastern area of South Korea. Such geographical preponderance was slowly alleviated with the participation of Chonnam National University (located in the southwestern part of South Korea) and Dongguk University Ilsan Hospital in January 2011. Jeju National University Hospital joined the CRCS-5 registry in October 2011, and in February 2013, two hospitals joined the CRCS-5 registry-Chungbuk National University Hospital and Ulsan University Hospital. Keimyung University Dongsan Medical Cetner, located in Daegu, began enrolling acute stroke cases in the CRCS-5 registry in May 2014. As of September 2014, 15 hospitals, covering most areas of South Korea, are actively registering stroke cases in the CRCS-5 registry (Figure 1).
Figure 1.
CRCS-5 centers and their locations.
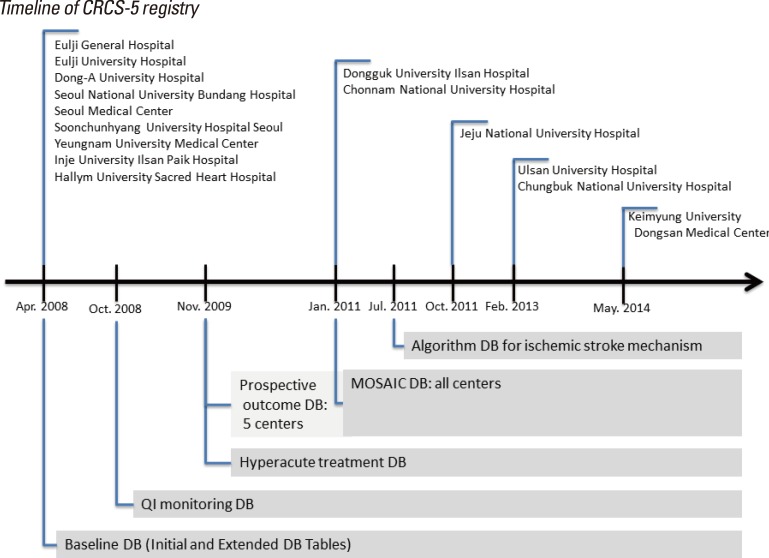
The database tables of the CRCS-5 registry have expanded since its initiation. The initial KSR database included demographic information locations and parenchymal lesions, arterial occlusion, stroke mechanism, National Institute of Health Stroke Scale (NIHSS) score at arrival, laboratory values, and treatment information. The CRCS-5 registry maintained the structure of the KSR database as "initial and extended tables" with some minor modifications. In October 2008, a clinical practice-monitoring database table was introduced for quality improvement of stroke care. In November 2009, the CRCS-5 registry set up a new database table dedicated to hyperacute treatment and acute management information. The CRCS-5 registry steering committee has long been in need of prospective stroke outcomes, and thus it implemented a systematic capture strategy for prospective outcomes, including functional status and vascular events in November 2009, selecting five centers with sufficient resources (Eulji General Hospital, Seoul National University Bundang Hospital, Seoul Medical Center, Soonchunhyang University Hospital Seoul, and Inje University Ilsan Paik Hospital). With a piloting operation duration of 14 months, the prospective outcome capture strategy was expanded to all participating CRCS-5 registry centers in January 2011. Prospective outcome information included functional and event outcomes at 3 months and 1 year after stroke onset as well as early neurological deterioration (END; details will be discussed later). Further, temporary database tables were set up for any specific research purposes (Figure 2).
Figure 2.
Timeline of the CRCS-5 registry. DB, database. QI, quality indicator.
The structure of the CRCS-5 registry and the epidemiological profile of 14,792 stroke cases enrolled up to January 2012 have been discussed previously.6 In the current article, the case profile is up-to-date as of August 2014, and details on hyperacute treatment and outcome information after the index stroke will be discussed.
Data management and analysis of CRCS-5 registry
Acute stroke cases were registered to the CRCS-5 web-based database (http://www.stroke-crc.or.kr/ecrf), and the registration process was required to be started within 48 hours of arrival. The central data manager monitors the number of registered cases from each hospital biweekly, and when the number deviates from the usual level, an inquiry is made to the check for any bias or missing data. The data manager also checks the integrity and completeness of the database bimonthly. Pre-specified queries are used, and peripheral registrars revise erroneous entries upon inquiries. The CRCS-5 registry steering committee has monthly gatherings to review and supervise the process. Details have previously been presented as supplemental data.6 The overall process of case registration, monitoring, inquiry and correction of erroneous data, and outcome capture usually takes 6-7 months to be completed, and then, the up-to-date CRCS-5 database is opened to participating researchers.
As of August 2014, the most recent available data are for cases from April 2008 to November 2013. The figures in the current article are derived from this database. Two-tailed significance values were set as P<0.05. Values are presented as frequencies (percentages), means±standard deviations, or medians [interquartile ranges], as appropriate. Statistical analyses were performed using STATA/MP 13.1 for Mac (STATACorp, College Station, TX, USA).
Overview of included cases
Baseline characteristics
The most recent database of the CRCS-5 registry as of August 2014 contained stroke cases admitted between April 2008 and November 2013. During these 5.5 years, a total of 28,348 cases were enrolled in the registry, and 27,851 ischemic stroke or transient ischemic attack (TIA) cases were available to be analyzed. In Korea, hemorrhagic stroke cases are usually managed by neurosurgeons, and thus the 497 hemorrhagic stroke cases included in the CRCS-5 registry are not representative of Korean cases. Accordingly, they were removed for the present analyses.
The profile of the analyzable cases is presented in Table 1. The demographic information and profile of vascular risk factors were relatively comparable to previous reports, in spite of a longer inclusion period and more participating centers.6 The overall profile was similar to that from the report of 34,000 cases from the Japan Standard Stroke Registry Study (JSSRS).7 Regarding stroke mechanisms, the CRCS-5 registry showed a higher proportion of cardioembolism than that observed in Taiwan,8 and also a higher proportion of undetermined etiologies in comparison to the JSSRS.7 The discordant rates of stroke mechanisms may originate from differences in work-up intensity and requirements for etiologic evaluation. Onset (defined as last seen normal) to arrival delay was median 12.7 hours in the CRCS-5 registry as of August 2014, a decrease from 14.2 hours observed in the previous report.6 Median NIHSS score decreased by 1 point to 3 points in the current analysis.
Table 1.
Profile of ischemic stroke or transient ischemic attack cases registered to CRCS-5 between April 2008 and November 2013 (N = 27,851)
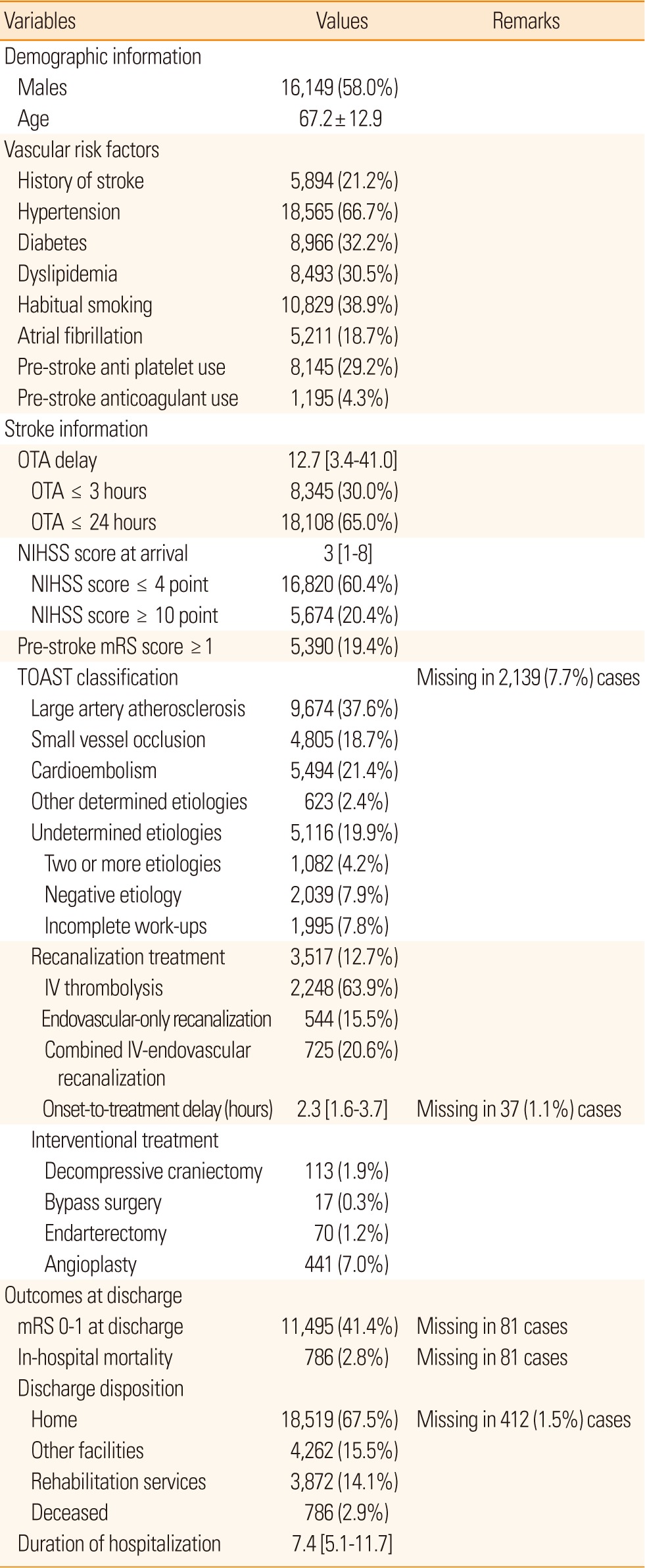
Values are presented as frequency (percentage), mean ± standard deviation, or median [interquartile range], as appropriate. Onset was defined as last seen normal. Percentages in the subcategories of undetermined etiologies are based on the total number of TOAST-available cases. Percentages in the detailed methods of recanalization are based on the cases with recanalization treatment. Interventional treatment was counted when performed during admission due to the index stroke. OTA, onset to arrival; NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale.
Among the 27,851 cases, 13% received hyperacute recanalization treatment, higher than that observed in the US, Taiwan,8,9 and the South Korean general population.10 However, our data should be interpreted with caution, given that the participating centers in the CRCS-5 registry mainly consist of tertiary academic hospitals, meaning that the figure is likely not representative of the primary care environment. The median delay between stroke onset and the initiation of recanalization treatment was 2.3 hours.
On discharge, the proportion of cases with a modified Rankin Scale (mRS) score of 0-1 was 41%, similar to the number from JSSRS.7 The median duration of hospitalization was 7.4 days, and 68% of cases were discharged to their home. Discharge disposition is dependent on cultural background. Specifically, the proportion observed here was lower than data from Taiwan (89%), but much higher than the US (46%) and France (47%).11,12 In-hospital mortality was 2.8%.
Temporal trends
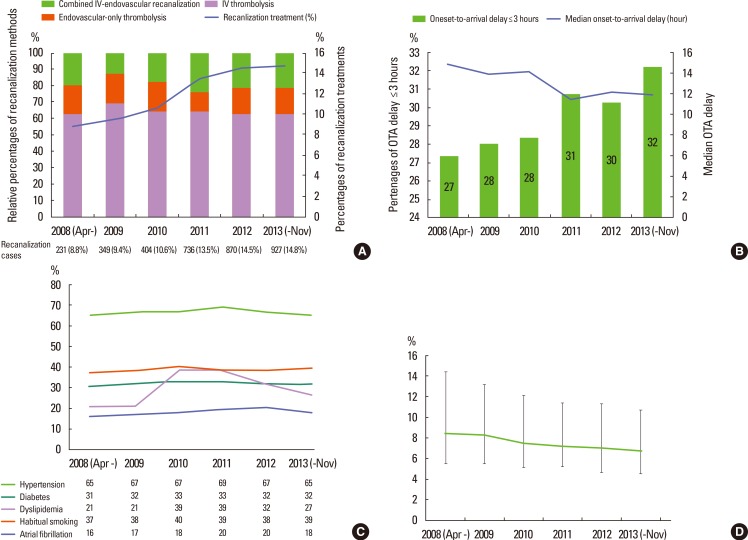
Over the 6 years, a few interesting trends were noted in the CRCS-5 registry. The proportion of acute recanalization treatment of any sort increased from 8.8% in 2008 to 14.8% in 2013 (P-for-trend over the 6 years <0.01; Figure 3A). Increasing utilization of acute treatment was also detected in the US,13 but the dramatic increase by 6% over 6 years observed here was exceptional. During the 6 years, the proportion of endovascular recanalization treatment with or without preceding IV alteplase remained unchanged at around 30%-37% (P-for-trend, 0.13). Even after the introduction of the Solitaire neurothrombectomy stent device (ev3 Inc., Plymouth, MN, USA) to South Korea in 2011, the proportion did not change. Such improvement in the utilization of alteplase would result from earlier arrival of acute stroke cases. The proportion of stroke cases who arrived within 3 hours of onset was 27% in 2008 (median onset-to-arrival delay of the whole population, 14.8 hours) but elevated to 32% in 2013 (median 11.9 hours; P-for-trend of arrival within 3 hours from onset <0.01; Figure 3B). However, the vascular risk factors showed relatively consistent profiles over the 6 years (Figure 3C), contrasting the increased prevalence and attributable risk from obesity and hypertension observed in the US over the last 10 years.13,14 One interesting finding was that the length of hospital stay has been consistently decreasing in CRCS-5 registry cases (Figure 3D). The duration of hospitalization is usually considered a major source of healthcare costs for stroke patients, and it can be inferred from the trends that economical pressure for cost reduction is growing in the stroke care system in South Korea.15,16,17
Figure 3.
Temporal trends of selected variables over 5.5 years of the CRCS-5 registry. Temporal trends of recanalization treatments in the CRCS-5 registry over the 6 years (A). The relative proportions of IV thrombolysis (purple bar), endovascular-only recanalization (orange bar) and combined IV-endovascular recanalization (green bar) remained stable during the inclusion period (bar graph). However, the proportion of recanalization-treated cases consistently increased in the registry from 8.8% (231 cases) in 2008 to 14.8% (927 cases) in 2013 (line graph). Temporal trends of onset (last seen normal) to arrival delay over the 6 years in the CRCS-5 registry (B). The proportions of early arrivals within 3 hours of onset steadily increased (bar graph) and the median onset to arrival delay were lowered from 14.8 hours in 2008 to 11.9 hours in 2013 for the entire population of CRCS-5 registry. Temporal trends of vascular risk factors in CRCS-5 registry (C). Overall, the percentages of risk factors did not demonstrate noticeable changes over the recruitment period. Temporal trends of median hospitalization duration over the 6 years, decreasing from 8.4 [5.5-14.4] days in 2008 to 6.7 [4.5-10.7] days in 2013 (D). The upper and lower error bars represent the 75th and 25th percentiles, respectively. OTA, onset-to-arrival.
Hospital variability
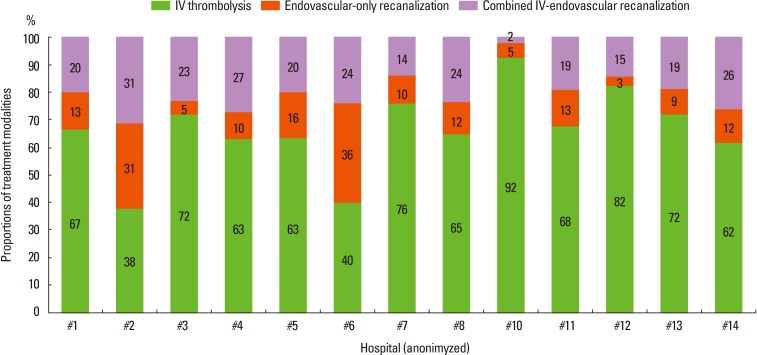
The demographic and vascular risk factor profiles of admitted stroke cases to 14 participating centers were relatively comparable, but the acute stroke treatment showed noticeable variability across hospitals (Table 2). Among the differences, arrival-to-IV alteplase injection time and the utilization of endovascular recanalization treatment were most discordant across centers. Median in-hospital delay ranged from 29 minutes (hospital #13) to 55 minutes (hospital #3), and the proportion of endovascular treatment was between 6% (hospital #10) and 64% (hospital #2). Still, real-world evaluation based on the Korean population is not available to determine whether such center disparity affects outcomes and quality of stroke care. Thus, this issue warrants further investigation.
Table 2.
Profiles of stroke cases, treatment, and outcomes according to CRCS-5 center (anonymized)
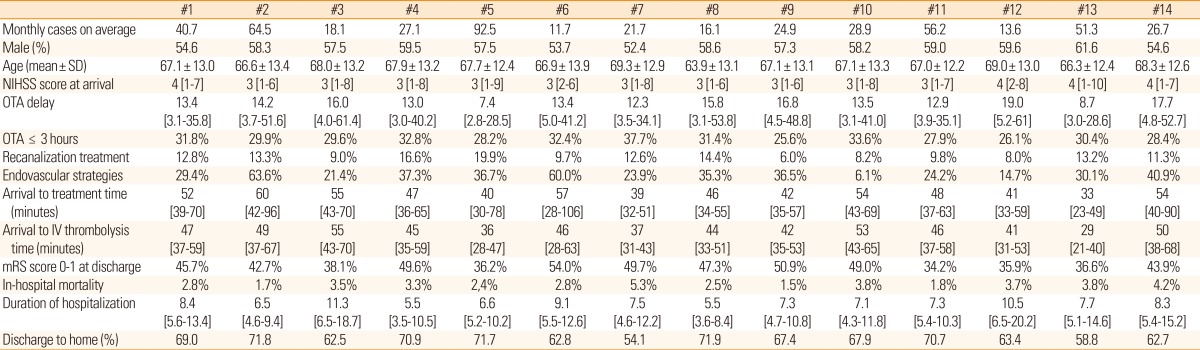
Values are presented as frequency (percentage), mean ± standard deviation, or median [interquartile range], as appropriate.
OTA, onset to arrival; NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale.
Hyperacute recanalization treatment
Overview of recanalization treatment in the CRCS-5 registry
In November 2009, a separate database table was established in the CRCS-5 registry dedicated to acute stroke and recanalization treatment information, collecting NIHSS scores 24 hours after recanalization treatment, occlusion and recanalization status of cerebral artery, hemorrhagic transformation, utilization of stroke unit, and detailed information on hyperacute recanalization treatment modalities. In this section of the article, a total of 2,724 stroke cases receiving hyperacute recanalization treatment across 13 hospitals will be analyzed (Table 3).
Table 3.
Characteristics of hyperacute recanalization treatment cases (N = 2724)
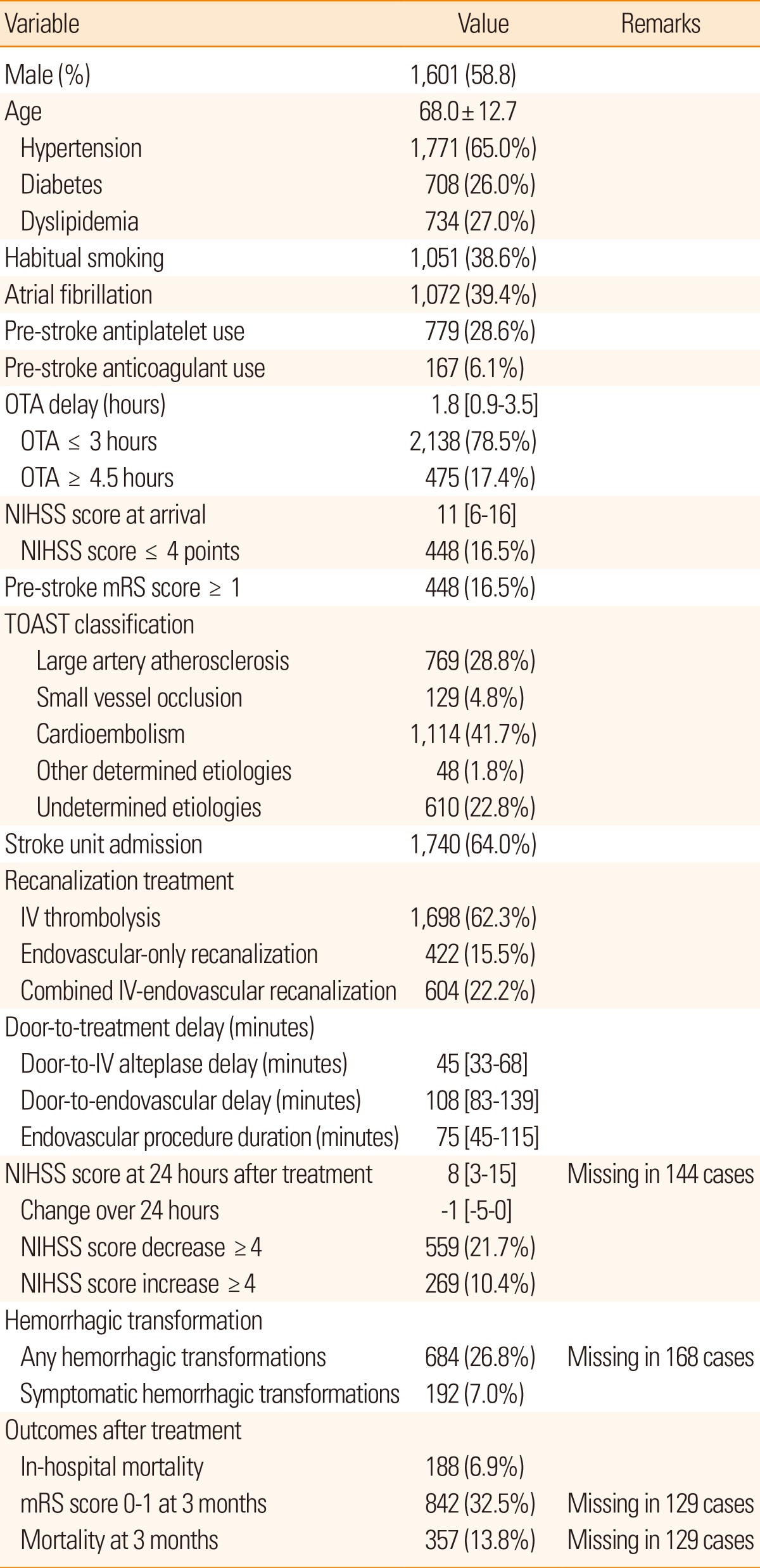
Values are presented as frequency (percentage), mean±standard deviation, or median [interquartile range], as appropriate.
Door-to-IV alteplase delay was based on IV thrombolysis or combined IV and endovascular treatments.
Door-to-endovascular delay was based on endovascular treatments with or without preceding IV alteplase.
OTA, onset to arrival; NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale.
The demographic and vascular risk factor profiles of the 2724 cases were relatively similar to those of the overall CRCS-5 registered stroke cases. Despite similar age, the prevalence of atrial fibrillation was higher among the treated cases and thus NIHSS score at arrival was elevated and more cases were designated as having cardioembolic stroke. The median arrival-to-IV alteplase injection delay was 45 minutes, which was lower than that from the Get With The Guidelines registry data.13 Median procedure time of endovascular treatment was 75 minutes, and 17% of cases were treated beyond the 4.5-hours time window for IV alteplase, 37% (176 cases) of which only had IV alteplase injection without endovascular treatment. Considering recent advances in IV alteplase treatment that note the possibility of extending the IV time window beyond 4.5 hours,18,19 such off-label use of alteplase requires further investigation.
Symptomatic hemorrhagic transformation, associated with NIHSS score increment ≥4 points, occurred in 7% of the treated cases, and any hemorrhagic transformation (including asymptomatic ones) was detected in 27% of the cases. In-hospital mortality was 7%, and at 3 months after stroke, mRS scores of 0-1 were observed in 33%, and mortality was 14%. The prevalence of hemorrhagic transformation was higher in the CRCS-5 registry than it was in the data from Taiwan,20 which may be due to the treatment beyond the conventional time window and the high utilization of endovascular recanalization treatment.
Treatment modalities for hyperacute recanalization
Endovascular recanalization treatment became widely available in clinical practice due to its intuitive outcomes and its function as a rescue treatment.21 Two separate phase II clinical trials in 2,012 reported superior efficacy of newer thrombectomy stent devices, and thus supported wider acceptance of endovascular modalities.22,23,24 Among the 2,724 recanalization-treated cases in the CRCS-5 registry, endovascular treatment with or without preceding IV alteplase was utilized in 38% of cases (Table 4). Combined IV-endovascular recanalization treatment was usually performed for cases arriving within the time window of IV thrombolysis and for those who had higher prevalence of atrial fibrillation, higher NIHSS scores, and lower pre-stroke disability. The arrival-to-initiation of IV alteplase injection was comparable between IV alteplase-only and combined IV and endovascular treatment groups. Between endovascular-only and combined treatment groups, preceding IV alteplase did not cause a significant delay in the intrahospital logistics of stroke cases. Interestingly, a lower dose of IV alteplase (0.6 mg/kg) was utilized in 29% (653) of the 2,724 cases, but the proportion was much higher in the combined IV and endovascular groups at 55% (P-for difference <0.01). In the current descriptive analyses, the combined IV and endovascular treatment group showed a larger degree of NIHSS score decrement over the first 24 hours following treatment, but the prevalence of hemorrhagic transformation was elevated. Mortality rate at discharge and at 3 months after stroke was comparable between the IV-only and endovascular-treated groups, but the proportion of mRS scores of 0-1 was lower in the endovascular group. Although the efficacy of endovascular recanalization was suggested in the prior phase II trials, the scientific evidence supporting the treatment modality is still lacking, as demonstrated in phase III trials with older-generation modalities.25,26,27 Such confusion is prevalent among CRCS-5 registry centers (Figure 4). The real-world effectiveness of hyperacute endovascular intervention warrants further investigation, and the clinical registry with disparity between centers on this issue would be a valuable source for clinical analyses.
Table 4.
Baseline comparison of treatment modalities for hyperacute recanalization
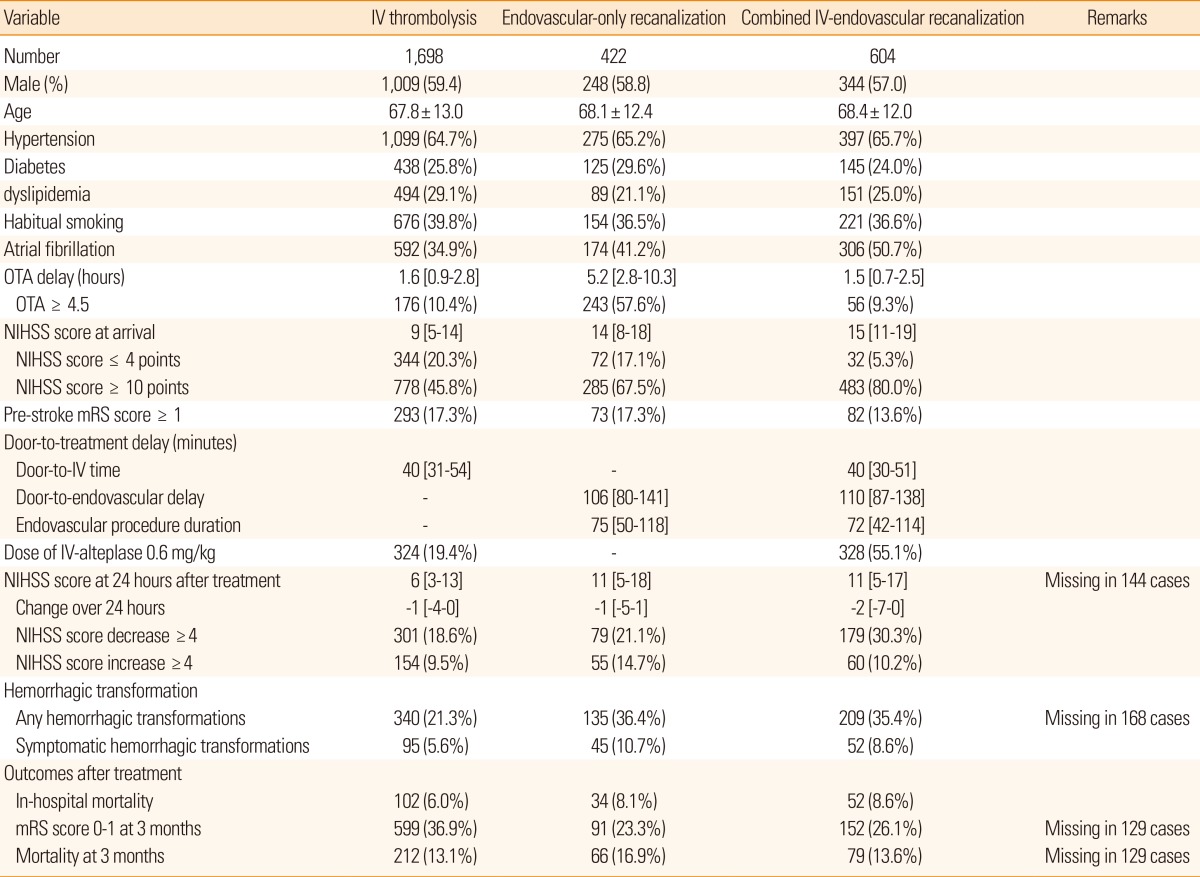
Values are presented as frequency (percentage), mean ± standard deviation, or median [interquartile range], as appropriate.
OTA, onset to arrival; NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale.
Figure 4.
Hospital variability in hyperacute treatment modality.
Outcomes after stroke
Definitions of outcome variables in CRCS-5 registry
The CRCS-5 registry initiated prospective outcome collection in November 2009 with a unified definition of stroke outcomes and capture strategy. The detailed definitions of major outcome variables are presented in Table 5. Long-term recurrent events and functional outcomes have been collected since November 2009 for selected centers, and this was expanded to all participating centers in January 2011 with the introduction of END. The CRCS-5 registry steering committee checks and audits the occurrence of END and captures the rate of long-term outcomes at monthly intervals.
Table 5.
Definitions of outcome variables in the CRCS-5 registry
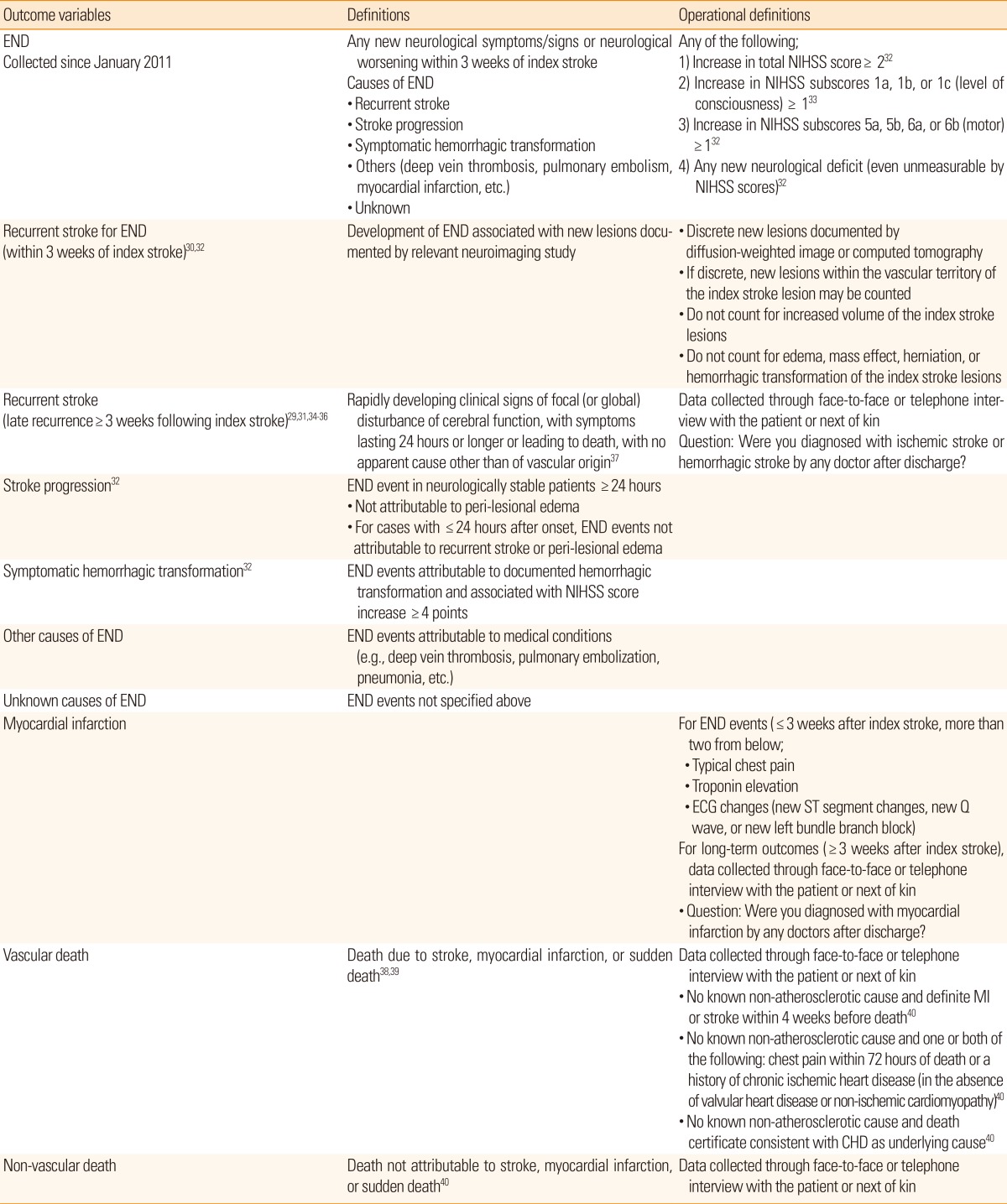
END, early neurological deterioration; NIHSS, National Institute of Health Stroke Scale; ECG, electrocardiography; MI, myocardial infarction; CHD, coronary heart disease.
Early neurological deteriorations
A total of 14,730 cases admitted after January 2011 were available to analyze for ENDs. Among them, END occurred in 2,615 cases (15%), including two ENDs in 342 cases and three separate END events in 47 cases. The total number of END events was 3,004. The following causes led to ENDs: 300 recurrent strokes (10%), 2059 stroke progressions (69%), 171 symptomatic hemorrhagic transformations (6%), 147 other (5%), 300 unknown (10%), and 22 transient ischemic attacks (1%; missing information in 5 cases). Ischemic strokes characterized 92% (275 cases) of recurrent strokes, and 15 cases were found to have hemorrhagic stroke. The median time from stroke onset to END occurrence was 35 hours [interquartile range, 15-80].
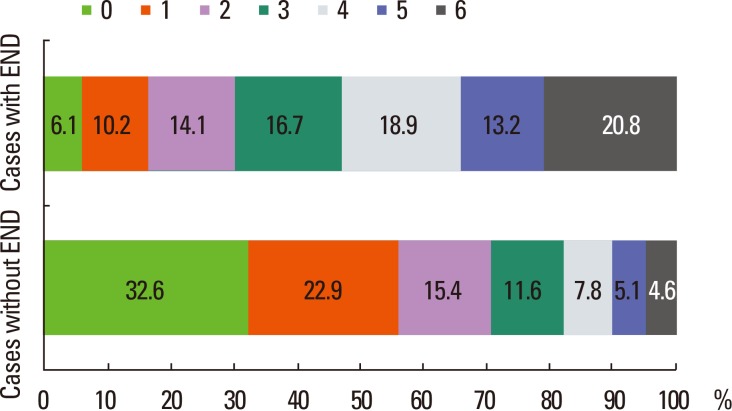
END cases tended to be older, have higher prevalence of vascular risk factors, and were dependent before the index stroke (Table 6). They had higher NIHSS scores at arrival and arrived earlier, with higher proportions of large artery atherosclerosis and cardioembolism. Stroke cases with END often had poor outcomes, with a longer length of stay and higher in-hospital mortality, as well as poor 3-month outcomes (Figure 5).
Table 6.
Characteristics of cases with or without early neurological deterioration (N=17,345)
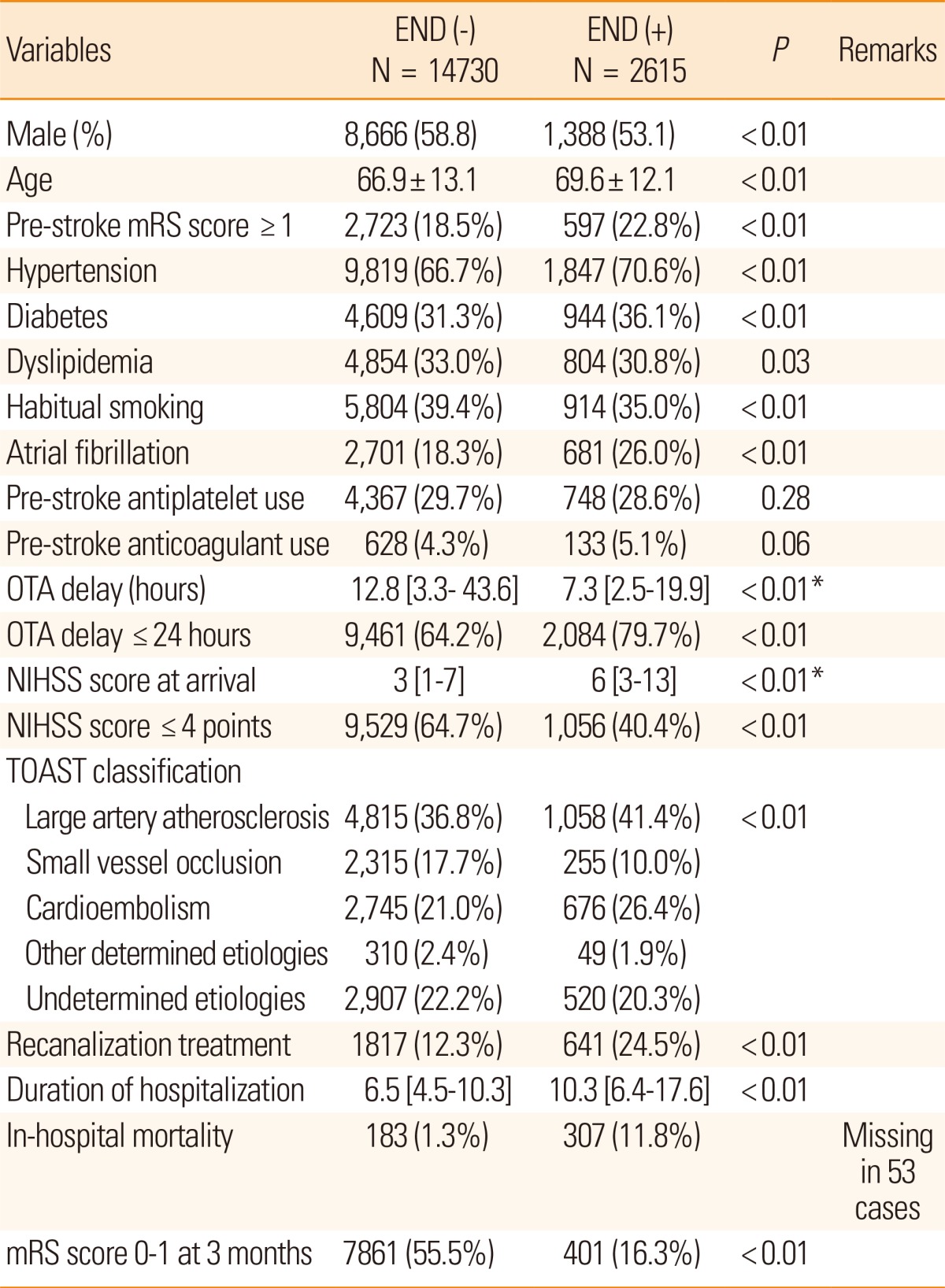
Values are presented as frequency (percentage), mean±standard deviation, or median [interquartile range], as appropriate.
*P values were calculated adjusting for unequal variance.
Figure 5.
mRS score at 3 months according to early neurological deterioration occurrence. END, early neurological deterioration.
Functional outcomes at 3 months and 1 year after stroke
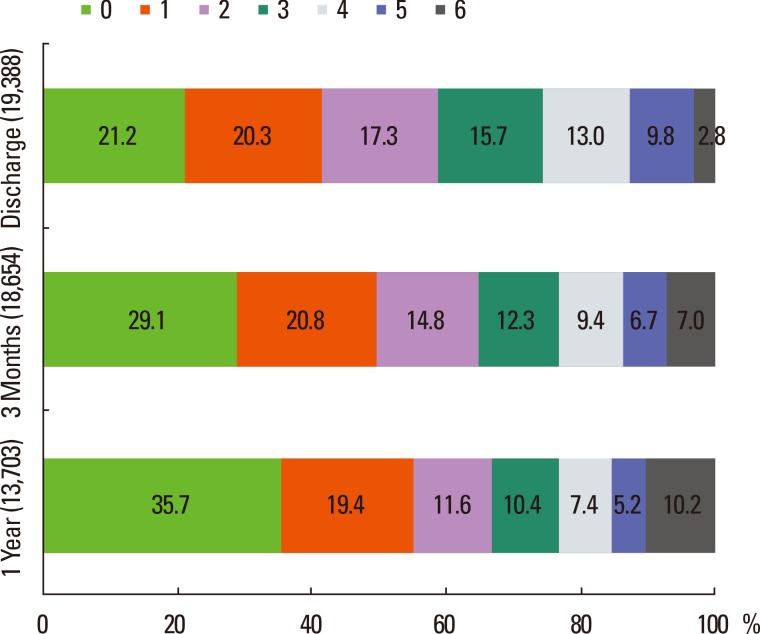
Prospective collection of long-term functional and vascular event outcomes was performed for 19,441 cases. Discharge outcome was available in 19,388 of these cases (99.7%), 3-month outcome in 18,564 cases (96%), and 1-year outcome in 13,703 cases (71%). The availability of 1-year outcome will increase, as the long-term outcome capture is an ongoing process. Cross tables comparing mRS scores at different time points after stroke are presented in Table 7. Overall, the proportion of acute stroke cases with mRS scores 0-1 was 42% at discharge, 50% at 3 months, and 55% at 1 year after stroke (Figure 6). Mortality rates also increased as time passed. In the China National Stroke Registry data, the proportion of mRS scores of 3-5 (dependent but not dead) at 3 months was 25.6% and 19.4% at 1 year. In comparison, the CRCS-5 registry had higher values (37% and 29%, respectively).28
Table 7.
Cross tables comparing mRS scores at discharge versus 3 months and at 3 months versus 1 year after stroke
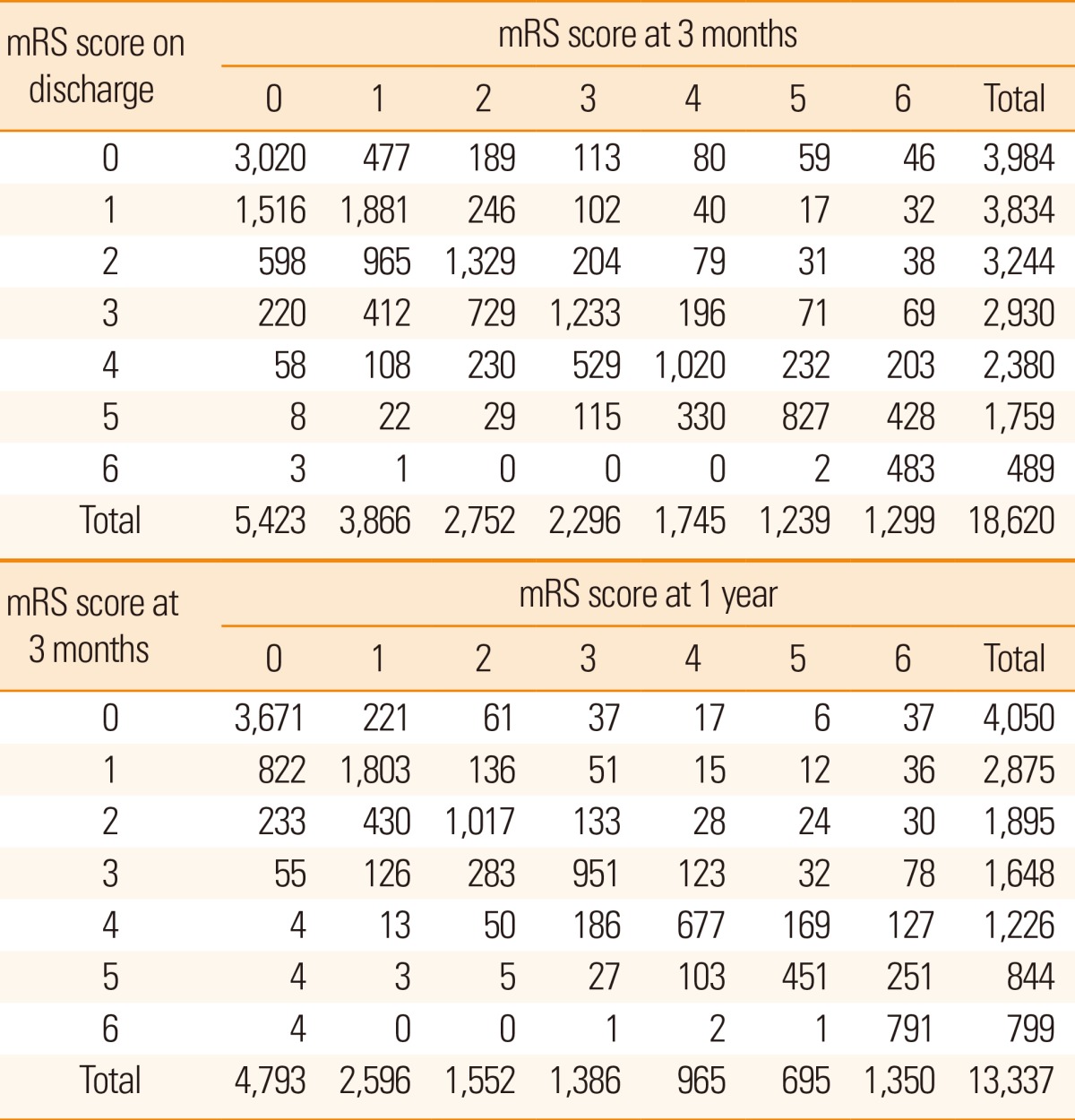
mRS, modified Rankin Scale.
Figure 6.
Distribution of mRS score at discharge, 3 months, and 1 year after stroke.
Event outcomes at 3 months and 1 year after stroke
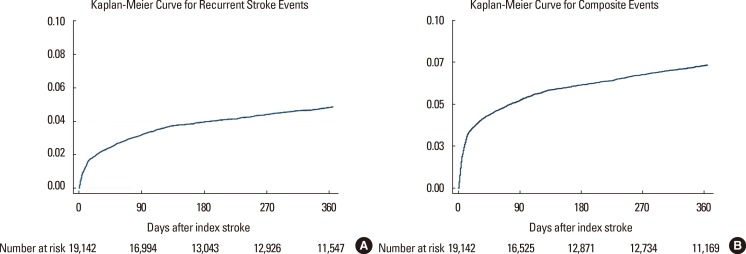
Among the 19,441 cases whose long-term outcomes were available, recurrent stroke occurred in 4.5%, vascular death in 3.0%, and composite events of recurrent stroke, myocardial infarction and vascular death in 6.8% within 365 days of the index stroke (Table 8). The median time between stroke onset and the first event was 34 [7-116] days for recurrent stroke, 78 [21-237] days for myocardial infarction, 8 [3-44] days for vascular death, and 18 [5-90] days for composite events. The median observation period was 365 [116-382] days. Overall, the rate of vascular events after stroke was higher in the early period after stroke but decreased thereafter, for both recurrent stroke and composite events (Figure. 7).
Table 8.
Event outcome rates after stroke (N=19,186)
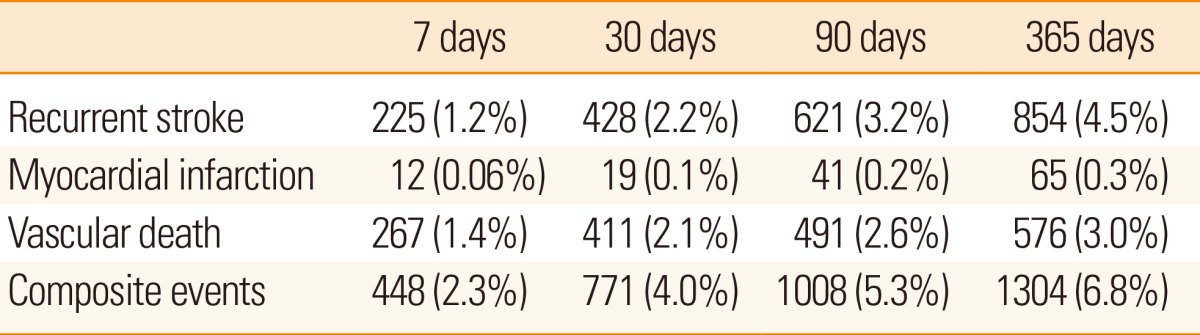
Composite events include recurrent stroke, myocardial infarction, and vascular death.
Figure 7.
Failure curves for recurrent stroke events (A) and composite outcomes (B) after the index stroke.
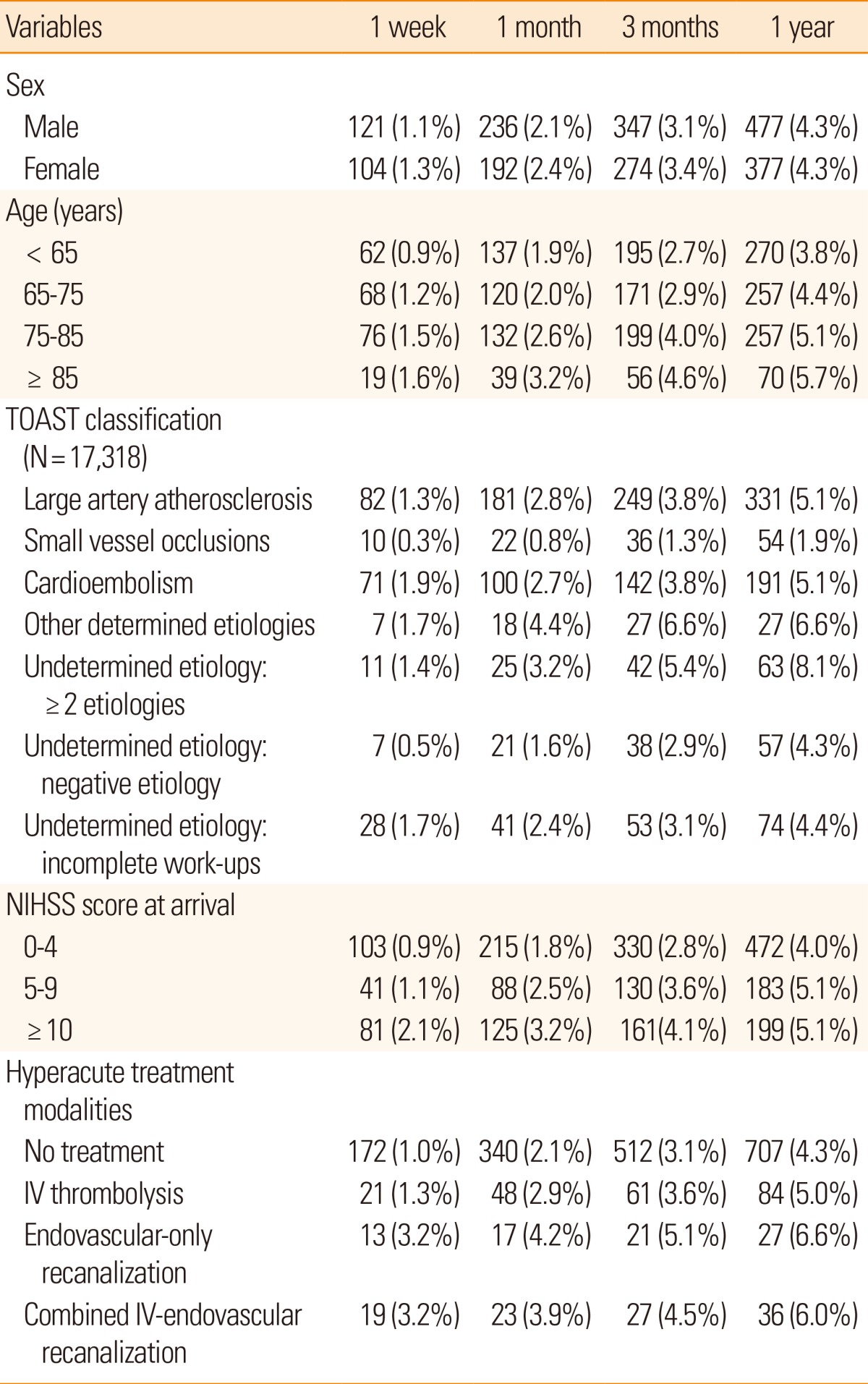
The stroke recurrence rate was higher among older individuals and cases with aggravated initial stroke severity (Table 9). It also differed according to ischemic stroke subtypes; the lowest recurrent rate was detected in small vessel occlusions, and similar rates were observed in large artery atherosclerosis and cardioembolism. Interestingly, the recurrence rate of stroke was lower in cryptogenic stroke cases. This discrepancy across ischemic stroke subtypes was documented previously,29,30,31 but the absolute recurrent event rates from the CRCS-5 registry were lower than previously reported. This might be explained by advances in the management of stroke cases and secondary prevention of ischemic stroke as well as ethnic or cultural differences, on which further investigations with regional collaborations among East Asian countries are warranted for clarification.
Table 9.
Recurrent stroke rates after stroke according to the selected variables (N = 19,186)
Discussion
We summarized and analyzed a prospective multicenter clinical stroke registry including 15 hospitals in South Korea to provide a global overview of the case profiles, the status of hyperacute recanalization treatment, and outcome information after index stroke. After the prior report involving stroke cases who admitted until January 2012,6 CRCS-5 registry collected about thirteen thousand patient and their demographic and vascular risk factor profiles were not much changed. In contrast, as discussed previously, we experienced rapid changes in acute treatment and logistics of the stroke cases, including shorter onset to arrival delay and increased utilization of acute recanalization treatment. However, there still is a long road ahead before make a toast for the improvement, as the majority of our data is based on the tertiary academic centers and the information on quality of care and treatment opportunities in local hospitals are not available yet.
The authors made comparison between the current CRCS-5 registry and selected hospital-based acute stroke registries from East Asian and United States (Table 10). Stroke cases from East Asian registries share similar sex and age distributions. At least half of the East Asian ischemic stroke cases showed NIHSS score ≤4 at arrival. However, there was noticeable difference in profiles of vascular risk factors between stroke registries; the proportions of diabetes, dyslipidemia and atrial fibrillation were quite low in China National Stroke Registry data. It was not easy to compare the recanalization treatments for hyperacute ischemic stroke patients and the stroke outcomes between various registries due to the scarcity of published data. Interestingly, in spite of off-label use, lower than standard dose of alteplase (≤0.9 mg/kg) was popular in East Asian countries, between 29% and 100%. Good functional recovery was documented in almost half of the cases at 3 months after stroke.
Table 10.
Current status of acute treatment and outcomes after acute ischemic stroke in hospital-based registries from selected countries
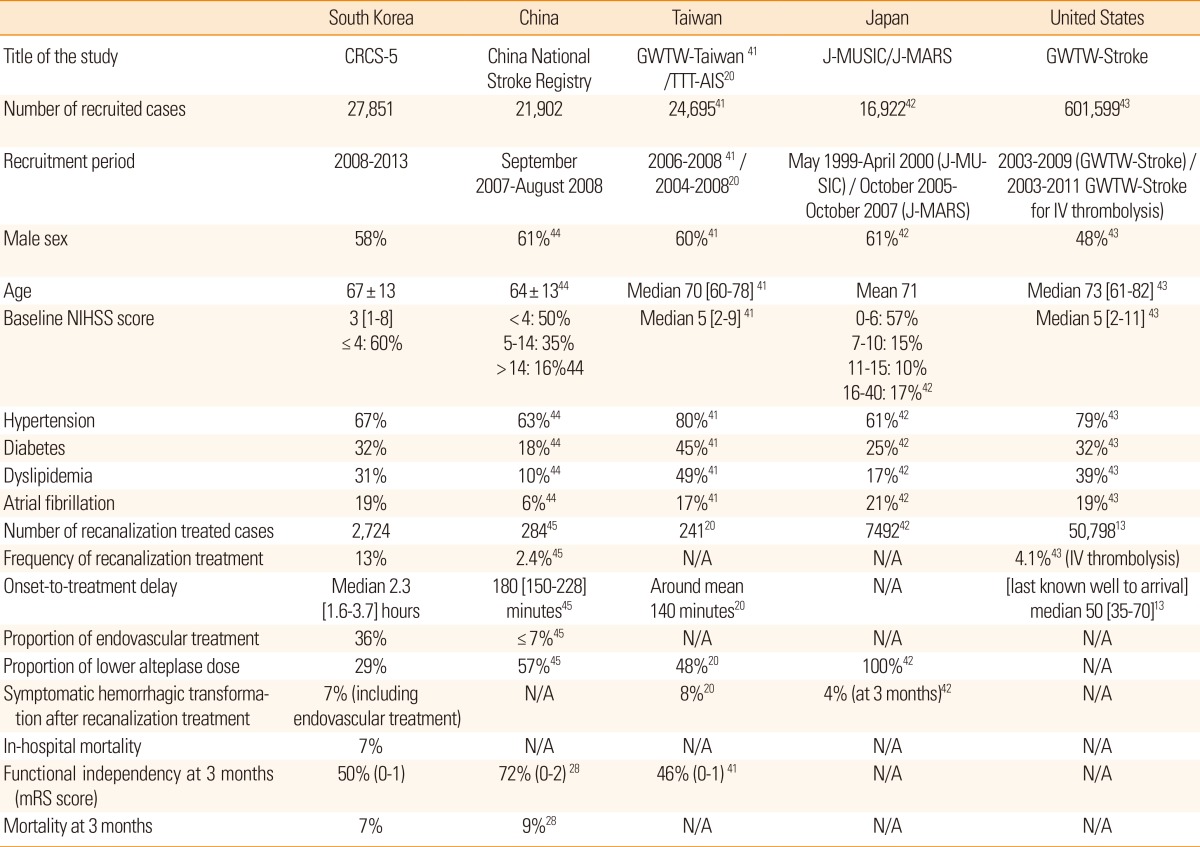
Numbers for eligible cases may vary according to the information. Refer to the specific references.
N/A, not available.
Limitations of the study include the representativeness of the source population, as most participating centers are tertiary academic centers. The locations of the CRCS-5 participating centers are still concentrated in the Seoul metropolitan area, and thus the CRCS-5 needs to expand its geographic coverage. Rural population is under-represented in CRCS-5 registry.
The current analyses documented the utilization of hyperacute recanalization treatment, heterogeneity of recanalization treatment modality, relatively frequent occurrence of ENDs and their clinical impact, and relatively low rates of recurrent vascular events. Comparison of international hospital-based stroke registries distinguished the need to publicly discuss about current status of in-hospital treatment and outcomes. CRCS-5 registry has provided a nationwide, in-depth aspect for the current status of in-hospital treatment and outcomes after ischemic stroke as well as secular change of stroke care over the past six years. CRCS-5 registry will be used as important basis for future clinical research design, policy prioritization, and international collaboration.
Footnotes
This study was supported from the Korea Healthcare Technology R&D Project, Ministry of Health, Republic of Korea (HI10C2020).
The authors have no financial conflicts of interest.
References
- 1.Bufalino VJ, Masoudi FA, Stranne SK, Horton K, Albert NM, Beam C, et al. The American Heart Association's recommendations for expanding the applications of existing and future clinical registries: a policy statement from the American Heart Association. Circulation. 2011;123:2167–2179. doi: 10.1161/CIR.0b013e3182181529. [DOI] [PubMed] [Google Scholar]
- 2.Gliklich RE, Dreyer NA. Registries for Evaluating Patient Outcomes: A User's Guide. 2nd ed. Rockville (MD): Agency for Healthcare Research and Quality (US); 2010. [PubMed] [Google Scholar]
- 3.Maasland L, van Oostenbrugge RJ, Franke CF, Scholte Op Reimer WJM, Koudstaal PJ, et al. Patients enrolled in large randomized clinical trials of antiplatelet treatment for prevention after transient ischemic attack or ischemic stroke are not representative of patients in clinical practice: the Netherlands Stroke Survey. Stroke. 2009;40:2662–2668. doi: 10.1161/STROKEAHA.109.551812. [DOI] [PubMed] [Google Scholar]
- 4.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BC, Roh JK. International Experience in Stroke Registries. Am J Prev Med. 2006;31:S243–S245. doi: 10.1016/j.amepre.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Kim BJ, Han M-K, Park TH, Park S-S, Lee KB, Lee B-C, et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int J Stroke. 2014;9:514–518. doi: 10.1111/ijs.12199. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Toyoda K, Minematsu K, Kobayashi S. Effects of Sex Difference on Clinical Features of Acute Ischemic Stroke in Japan. J Stroke Cerebrovasc Dis. 2013;22:1070–1075. doi: 10.1016/j.jstrokecerebrovasdis.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh FI, Chiou HY. Stroke: morbidity, risk factors, and care in taiwan. J Stroke. 2014;16:59–64. doi: 10.5853/jos.2014.16.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong D, Reeves MJ, Hernandez AF, Zhao X, Olson DM, Fonarow GC, Schwamm LH, Smith EE. Times From Symptom Onset to Hospital Arrival in the Get With The Guidelines-Stroke Program 2002 to 2009: Temporal Trends and Implications. Stroke. 2012;43:1912–1917. doi: 10.1161/STROKEAHA.111.644963. [DOI] [PubMed] [Google Scholar]
- 10.Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke Statistics in Korea: Part I. Epidemiology and Risk Factors: A Report from the Korean Stroke Society and Clinical Research Center for Stroke. J Stroke. 2013;15:2–20. doi: 10.5853/jos.2013.15.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Béjot Y, Troisgros O, Gremeaux V, Lucas B, Jacquin A, Khoumri C, et al. Poststroke disposition and associated factors in a population-based study: the Dijon Stroke Registry. Stroke. 2012;43:2071–2077. doi: 10.1161/STROKEAHA.112.658724. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MFK. Discharge destination as a surrogate for Modified Rankin Scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Phys Med Rehabil. 2012;93:1408–1413. doi: 10.1016/j.apmr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. Temporal Trends in Patient Characteristics and Treatment With Intravenous Thrombolysis Among Acute Ischemic Stroke Patients at Get With the Guidelines-Stroke Hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. doi: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S, Claggett B, Correia AW, Shah AM, Gupta D. Temporal Trends in the Population Attributable Risk for Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820–828. doi: 10.1161/CIRCULATIONAHA.113.008506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur P, Kwatra G, Kaur R, Pandian JD. Cost of stroke in low and middle income countries: a systematic review. Int J Stroke. 2014;9:678–682. doi: 10.1111/ijs.12322. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y, Jiao Y, Hu R, Wang Y, Wang Y, Wang Y, et al. Reduction of length of stay and costs through the implementation of clinical pathways for stroke management in China. Stroke. 2014;45:e81–e83. doi: 10.1161/STROKEAHA.114.004729. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, et al. Characteristics, Performance Measures, and In-Hospital Outcomes of the First One Million Stroke and Transient Ischemic Attack Admissions in Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2010;3:291–302. doi: 10.1161/CIRCOUTCOMES.109.921858. [DOI] [PubMed] [Google Scholar]
- 18.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IST-3 collaborative group. Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao AC, Hsu HY, Chung CP, Liu CH, Chen CH, Teng MMH, Peng GS, et al. Outcomes of Thrombolytic Therapy for Acute Ischemic Stroke in Chinese Patients: The Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) Study. Stroke. 2010;41:885–890. doi: 10.1161/STROKEAHA.109.575605. [DOI] [PubMed] [Google Scholar]
- 21.Hassan AE, Chaudhry SA, Grigoryan M, Tekle WG, Qureshi AI. National trends in utilization and outcomes of endovascular treatment of acute ischemic stroke patients in the mechanical thrombectomy era. Stroke. 2012;43:3012–3017. doi: 10.1161/STROKEAHA.112.658781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokin M, Dumont TM, Veznedaroglu E, Binning MJ, Liebman KM, Fessler RD, et al. Solitaire Flow Restoration Thrombectomy for Acute Ischemic Stroke: Retrospective Multicenter Analysis of Early Postmarket Experience After FDA Approval. Neurosurgery. 2013;73:19–26. doi: 10.1227/01.neu.0000429859.96652.57. [DOI] [PubMed] [Google Scholar]
- 25.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular Therapy after Intravenous t-PA versus t-PA Alone for Stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular Treatment for Acute Ischemic Stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A Trial of Imaging Selection and Endovascular Treatment for Ischemic Stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Li J, Wang C, Yao X, Zhao X, Wang Y, et al. Gender Differences in 1-Year Clinical Characteristics and Outcomes after Stroke: Results from the China National Stroke Registry. PLoS ONE. 2013;8:e56459. doi: 10.1371/journal.pone.0056459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petty GW, Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic Stroke Subtypes : A Population-Based Study of Functional Outcome, Survival, and Recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 30.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 31.Jackson C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005;128:2507–2517. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong KS, Kang DW, Koo JS, Yu KH, Han MK, Cho YJ, et al. Impact of neurological and medical complications on 3-month outcomes in acute ischaemic stroke. Eur J Neurol. 2008;15:1324–1331. doi: 10.1111/j.1468-1331.2008.02310.x. [DOI] [PubMed] [Google Scholar]
- 33.Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, Diener H-C, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. 2005;62:393–397. doi: 10.1001/archneur.62.3.393. [DOI] [PubMed] [Google Scholar]
- 34.Petty GW, Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction A population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 35.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, et al. Long-Term Risk of First Recurrent Stroke in the Perth Community Stroke Study. Stroke. 1998;29:2491–2500. doi: 10.1161/01.str.29.12.2491. [DOI] [PubMed] [Google Scholar]
- 36.Hillen T. Cause of Stroke Recurrence Is Multifactorial: Patterns, Risk Factors, and Outcomes of Stroke Recurrence in the South London Stroke Register. Stroke. 2003;34:1457–1463. doi: 10.1161/01.STR.0000072985.24967.7F. [DOI] [PubMed] [Google Scholar]
- 37.WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 38.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MSV. Recurrent stroke and cardiac risks after first ischemic stroke: The Northern Manhattan Study. Neurology. 2006;66:641–646. doi: 10.1212/01.wnl.0000201253.93811.f6. [DOI] [PubMed] [Google Scholar]
- 40.Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau C-S, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: An international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J. 2006;151:786.e1–786.e10. doi: 10.1016/j.ahj.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh FI, Lien LM, Chen ST, Bai CH, Sun MC, Tseng HP, et al. Get With The Guidelines-Stroke Performance Indicators: Surveillance of Stroke Care in the Taiwan Stroke Registry: Get With The Guidelines-Stroke in Taiwan. Circulation. 2010;122:1116–1123. doi: 10.1161/CIRCULATIONAHA.110.936526. [DOI] [PubMed] [Google Scholar]
- 42.Kimura K, Kazui S, Minematsu K, Yamaguchi T. Analysis of 16,922 Patients with Acute Ischemic Stroke and Transient Ischemic Attack in Japan. Cerebrovasc Dis. 2004;18:47–56. doi: 10.1159/000078749. [DOI] [PubMed] [Google Scholar]
- 43.Fonarow GC, Reeves MJ, Smith EE, Saver JL, Zhao X, Olson DW, et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes. 2010;3:291–302. doi: 10.1161/CIRCOUTCOMES.109.921858. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6:355–361. doi: 10.1111/j.1747-4949.2011.00584.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Liao X, Zhao X, Wang DZ, Wang C, Nguyen-Huynh MN, et al. Using Recombinant Tissue Plasminogen Activator to Treat Acute Ischemic Stroke in China: Analysis of the Results From the Chinese National Stroke Registry (CNSR) Stroke. 2011;42:1658–1664. doi: 10.1161/STROKEAHA.110.604249. [DOI] [PubMed] [Google Scholar]