Abstract
Purpose
Opioid-based intravenous patient-controlled analgesia (IV-PCA) is a popular method of postoperative analgesia, but many patients suffer from PCA-related complications. We hypothesized that PCA was not essential in patients undergoing major abdominal surgery by minimal invasive approach.
Methods
Between February 2013 and August 2013, 297 patients undergoing laparoscopic surgery for colorectal cancer were included in this retrospective comparative study. The PCA group received conventional opioid-based PCA postoperatively, and the non-PCA group received intravenous anti-inflammatory drugs (Tramadol) as necessary. Patients reported their postoperative pain using a subjective visual analogue scale (VAS). The PCA-related adverse effects and frequency of rescue analgesia were evaluated, and the recovery rates were measured.
Results
Patients in the PCA group experienced less postoperative pain on days 4 and 5 after surgery than those in the non-PCA group (mean [SD] VAS: day 4, 6.2 [0.3] vs. 7.0 [0.3], P = 0.010; and day 5, 5.1 [0.2] vs. 5.5 [0.2], P = 0.030, respectively). Fewer patients in the non-PCA group required additional parenteral analgesia (41 of 93 patients vs. 53 of 75 patients, respectively), and none in the non-PCA group required rescue PCA postoperatively. The incidence of postoperative nausea and vomiting was significantly higher in the non-PCA group than in the PCA group (P < 0.001). The mean (range) length of hospital stay was shorter in the non-PCA group (7.9 [6-10] days vs. 8.7 [7-16] days, respectively, P = 0.03).
Conclusion
Our Results suggest that IV-PCA may not be necessary in selected patients those who underwent minimal invasive surgery for colorectal cancer.
Keywords: Patient-controlled analgesia, Laparoscopy, Colorectal neoplasms
INTRODUCTION
Postoperative pain control is an important factor affecting patient recovery and satisfaction. Poorly controlled pain delays the return to normal bowel movement, reduces daily activity, and decreases ambulation [1]. Numerous clinical studies and trials have attempted to design the ideal analgesic strategy.
Intravenous patient-controlled analgesia (IV-PCA) has been used for postoperative analgesia due to its feasibility and safety in ensuring acute postoperative pain relief [2]. Currently, opioid-based IV-PCA is widely used for postoperative pain control due to its short action duration and strong analgesic effect. However, opioid-based IV-PCA is also associated with several adverse effects, for example, postoperative nausea and vomiting (PONV), dizziness, decreased blood pressure, and urinary retention [2,3,4,5,6]. These adverse effects are not easily avoided using multimodal preventive approaches. In extreme cases, opioid-based IV-PCA is discontinued preemptively due to these effects. Intractable opioid-induced adverse effects can be considered to indicate failure of IV-PCA as an analgesic technique [1,2].
In this study, we hypothesized that opioid-based IV-PCA was not essential in patients who underwent laparoscopic surgery for colorectal cancer. We investigated the necessity of routine IV-PCA after elective minimally invasive colorectal surgery and also compared the clinical outcomes between patients who received IV-PCA and those who did not.
METHODS
This study was the retrospective, comparative clinical study assessed patients who underwent minimally invasive colorectal cancer surgery at a single center from February 2013 until August 2013. Informed consent was obtained from all patients. This study was approved by the institutional Review Board of the Kyungpook National University Medical Center.
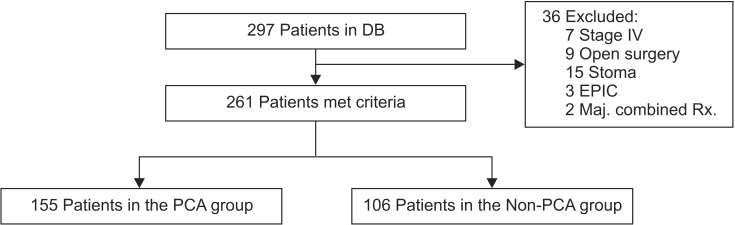
A total of 297 consecutive colorectal cancer patients were evaluated, of which 261 met the study criteria. The remaining 36 patients were excluded for the following reasons: distant metastasis, open surgery, presence of diverting stoma, early postoperative intraperitoneal chemotherapy, and undergoing combined resections. Postoperative pain was controlled by either a conventional PCA protocol (n = 155) or a non-PCA protocol (n = 106) according to the joint decision between the patient and physician (Fig. 1).
Fig. 1.
Patient allocation into intravenous patient-controlled analgesia (IV-PCA) group and non-PCA group. DB, data base; EPIC, early postoperative intraperitoneal chemotherapy; Maj. combined Rx., major combined resection.
All procedures were performed under general anesthesia by three experienced surgeons, who performed nearly identical techniques. Operations were performed under a CO2 pneumoperitoneum (12 mmHg) and 4-5 trocars (one 11-mm optic trocar, two or three 5-mm trocars, and one 12-mm trocar for the stapler) were used. The specimen was extracted through a vertical extension of the umbilical port. Generally, a 5- to 6-cm periumbilical longitudinal incision was used for the procedure. The routine postoperative protocol was standardized. The early recovery program for postoperative management was not applied during the study period. Generally, patients were discharged when the following criteria were fulfilled: tolerable condition, consuming a solid diet, stable vital signs, normal body temperature, and good wound healing without any other complications.
Postoperative pain management
In the PCA group, IV-PCA (Accumate 1100; WooYoung Medical, Seoul, Korea) was used to administer fentanyl. The PCA formulation contained 25-µg/kg fentanyl diluted to 100 mL in normal saline; the basal rate was set at 0.5 mL/hr, the bolus volume at 0.5 mL, and the lock-out time at 10 minutes. All IV-PCA were initiated in the postanesthesia care unit. The patients were instructed to push the button for PCA whenever they experienced pain. Rescue drug, usually tramadol, was injected intravenously if the patient asked for more analgesic. In the non-PCA group, postoperative analgesics were administered by the patients' need to control pain. Generally, patients who experienced prolonged pain of over 4.0 on a VAS were administered 50-mg tramadol intravenously as rescue analgesia until the pain was relieved (VAS < 3.0). Ketolac tromethamine (2 mg/kg) was added intravenously when pain was not relived even after tramadol injection. Any oral nonsteroidal anti-inflammatory drugs were not given routinely after surgery.
The following clinical parameters were recorded: patient age, body mass index, tumor location, tumor size, operative time, and the length of hospital stay. Pain was assessed at 24, 48, 72, and 90 hours after surgery, and the postoperative morbidity, including the PONV score, was assessed at 24, 48, and 72 hours after surgery. In addition, the total volume of rescue analgesia administered was recorded. Pain intensity was assessed at rest using the VAS from 0 to 10 (0, no pain; 10, the most severe pain), and the PONV score was evaluated separately on a VAS from 0 to 4 (0, no nausea; 4, vomiting). A PONV score greater than 2 was considered significant.
All statistical analyses were performed using the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Data were assessed according to the intention-to-treat principle. Nonparametric variables are expressed as the median and range and parametric variables as the mean ± 1 standard deviation. Variables expressed as proportions were compared using the chisquare test or Fisher exact test, where appropriate. Between-group differences in parametric and nonparametric variables were compared using the t-test or the Mann-Whitney U test for independent values, as appropriate. The differences were considered significant at P < 0.05.
RESULTS
During the study period, curative surgery was performed on 261 patients. Of these, 106 patients were not offered IV-PCA (non-PCA group). There were no significant differences in baseline clinical characteristics between the PCA and non-PCA groups (Table 1).
Table 1.
Baseline clinical characteristics in two groups
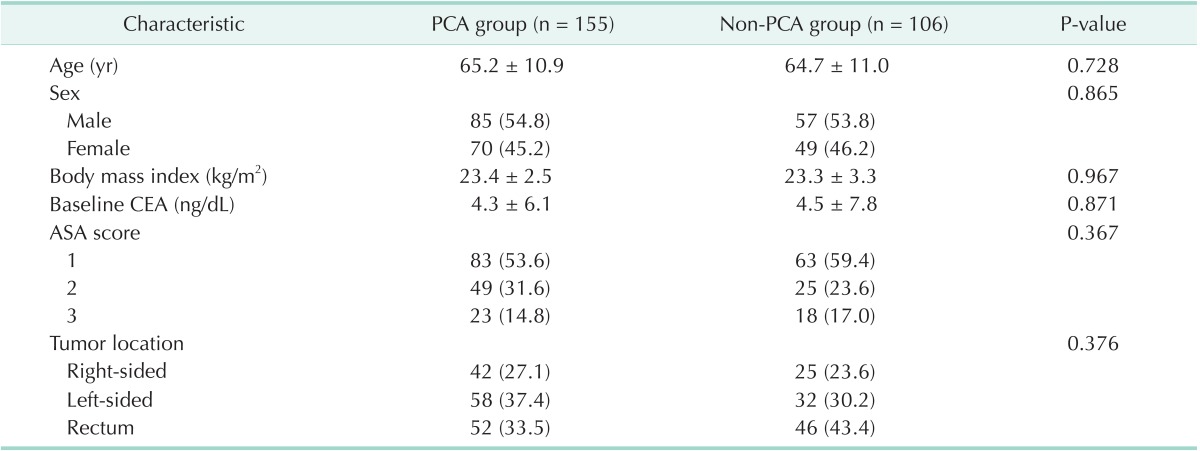
Values are presented as mean ± standard deviation or number (%).
PCA, patient-controlled analgesia; ASA, American Society of Anesthesiologists.
The operative results are summarized in Table 2. There were no differences in operative time and intraoperative blood loss between the groups. There was a trend toward a faster resumption of a regular diet in the non-PCA group. The duration of postoperative hospital stay was longer by a mean of 1.2 days in the PCA group (P = 0.081). Postoperative ileus was less frequent in the non-PCA group (1.9%) than in the PCA group (4.5%), but the difference was not statistically significant. The incidences of voiding difficulty requiring reinsertion of a Foley catheter and postoperative atelectasis, showed no differences between the groups. Adverse events related to PONV occurred in 23.2% of the patients in the PCA group and 1.9% of those in the non-PCA group, indicating that patients in the non-PCA experienced significantly fewer adverse effects (P < 0.001). In the non-PCA group, only one patient required rescue PCA after surgery due to the failure of analgesia. In the PCA group, 34 patients requested cessation of IV-PCA due to severe PONV. The IV-PCA was discontinued in 79.4% and 20.6% of patients 12-24, and 24-48 hours after surgery, respectively. In the PCA group, there were 8 morbidities related to IV-PCA, including seven cases of hypotension and one case of respiratory insufficiency.
Table 2.
Operative outcomes
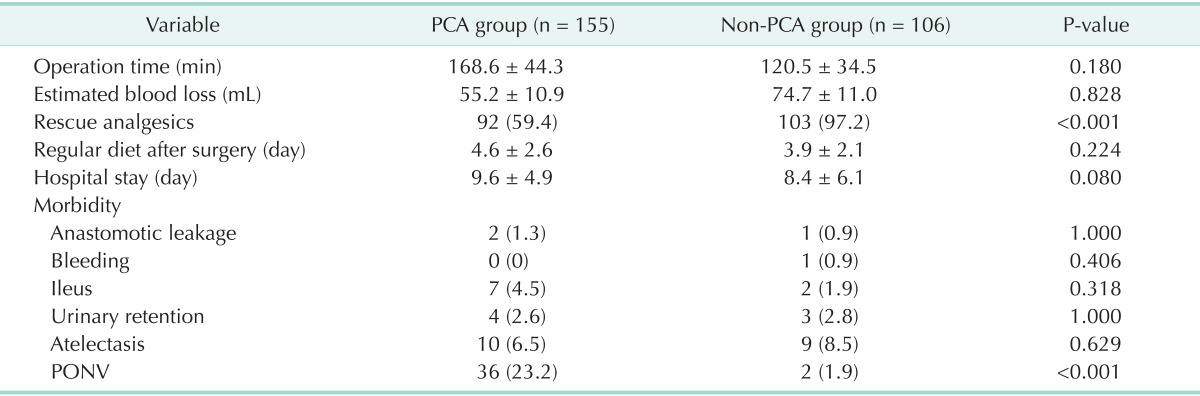
Values are presented as mean ± standard deviation or number (%).
PCA, patient-controlled analgesia; PONV, postoperative nausea/vomiting.
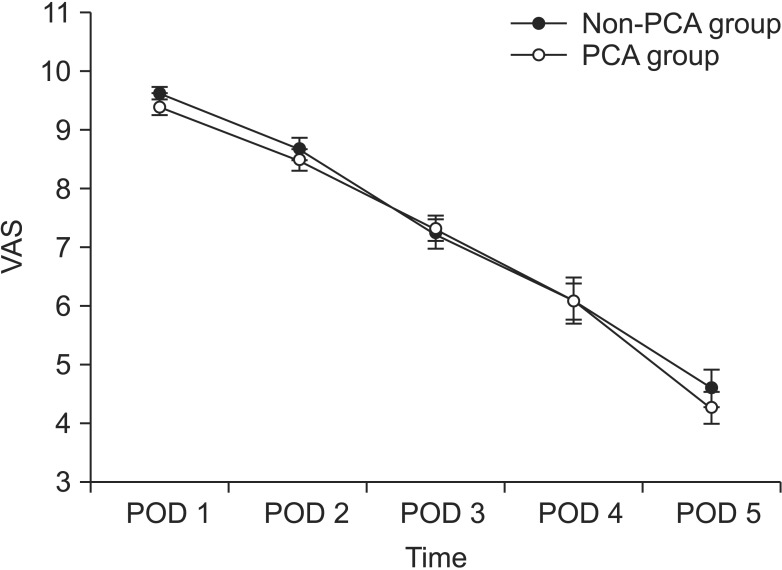
The primary outcome was the patient-reported pain score. The pain scores were collected from all patients, 241 patients on postoperative day 1 and 252 patients on postoperative day 2. The overall pain assessment card compliance rate was 93.8%. Fig. 2 shows the mean pain score of each group.
Fig. 2.
Visual analogue scale (VAS) assessing pain and postoperative nausea and vomiting (PONV) in the two groups. PCA, patient-controlled analgesia; POD, postoperative day.
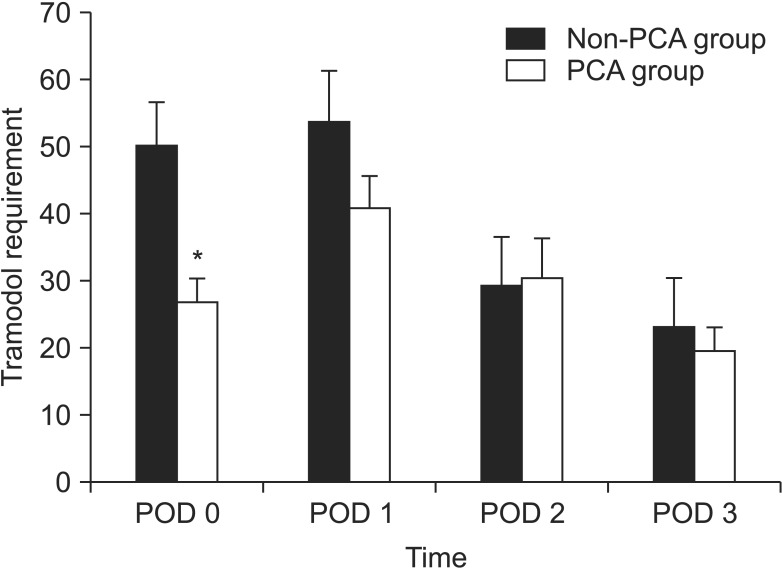
The postoperative pain score by VAS significantly decreased over time in both groups. Between postoperative day 1 and postoperative day 5, there was no significant difference in the VAS between the two groups. The mean pain scores on postoperative day 1 were 9.2 (3.1) in the PCA group and 9.3 (2.5) in the non-PCA group. However, patients in the non-PCA group requested more rescue analgesia (intravenous tramadol) during the first 24 hours (PCA, 26 ± 14 mg; non-PCA, 50.5 ± 21 mg; P = 0.001) (Fig. 3). There were no significant differences in the required dose of rescue drug between the two groups 48 hours after surgery.
Fig. 3.
Volume of rescue analgesia (intravenous tramadol) administered to patients in the two groups. PCA, patient-controlled analgesia; POD, postoperative day. *P < 0.05, significant difference.
In the evaluation of the total cost of hospitalization, which measured the costs of benefit services and nonbenefit services, the total cost in the PCA group was approximately 1,000 United States dollar (USD) more than that in the non-PCA group (P = 0.112) (Table 3). Anesthesia-related costs were higher in the PCA group than in the non-PCA group (556.7 USD vs. 444.2 USD, respectively, P = 0.103).
Table 3.
Costs
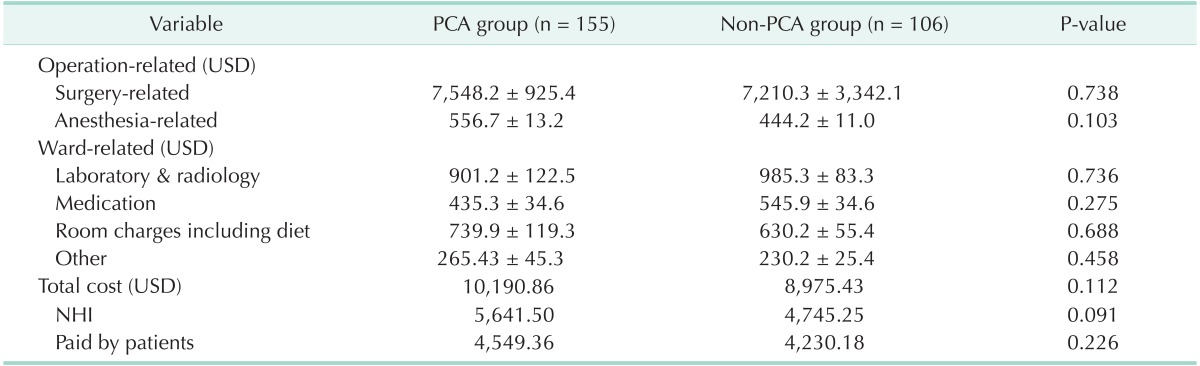
PCA, patient-controlled analgesia; NHI, National Health Insurance; USD, United States dollar.
Values are presented as mean ± standard deviation.
Costs were based on an exchange rate of 1,020 Korean won to 1 USD.
DISCUSSION
This retrospective, comparative study compared the clinical outcomes of IV-PCA and non-PCA (intravenous rescue analgesia on demand) groups to determine whether IV-PCA is essential for controlling postoperative pain in patients who underwent minimally invasive surgery for colorectal cancer. Patients in the PCA group experienced a nearly identical level of pain compared to the non-PCA group during the immediate postoperative period (Fig. 2). However, approximately 20% of patients ceased IV-PCA due to severe adverse effects including PONV (Table 2). In this study, the analgesic effect of routine IV-PCA was not remarkably superior to that of the non-PCA protocol. Therefore, our results showed that IV-PCA is not essential for controlling postoperative pain following minimally invasive surgery for colorectal cancer.
In 1968, PCA was first suggested by Sechezer (quoted from [6]) as a pain control technique. At that time, as with most abdominal surgeries, including colorectal cancer surgeries, were performed using an open method. Thus, IV-PCA has played an important role in the open surgery era for pain control.
In the 1990's, after laparoscopic surgery was introduced to many surgical fields, IV-PCA become the standard modality for postoperative pain control. However, the effectiveness and adverse effects of IV-PCA have not yet been analyzed in minimally invasive surgery using a 5- to 6-cm incision. The intensity and duration of postoperative pain following laparoscopic surgery is significantly less and shorter than that after the same procedure performed by open approach [7,8]. In one meta-analysis, early narcotic analgesia requirements were reduced by 36.9% and pain at rest by 34.8% in patients undergoing laparoscopic resection of colorectal cancer. Similarly, in one randomized controlled trial, the resting pain after laparoscopic surgery of colon cancer was significantly less than that after open colectomy, and pain during mobilization was less severe [8,9,10]. Therefore, a suitable clinical pathway should be designed for postoperative pain control following minimally invasive colorectal surgeries.
Opioid-based IV-PCA provides good pain relieving effects, but this modality has several well-known adverse effects, including PONV, urinary retention, hypotension, and respiratory depression. PONV is the most common and troublesome adverse effect of IV-PCA. According to one report, the frequency of PONV varies from 10% to 88% in patients using IV-PCA [3]. Pharmacologic strategies to reduce PCA-related PONV, for example using antiemetics and adjusting the ratio of analgesics and antiemetics, have been extensively investigated [2]. In the present study, 34 patients requested the cessation of IV-PCA due to severe PONV, and IV-PCA was discontinued in 79.4% and 20.6% of patients 12-24 hours and 24-48 hours after surgery, respectively (Table 2). Most opioid-based IV-PCA adverse effects improved after discontinuing the analgesia.
To reduce PCA-related adverse effects, opioid-free pain management regimens and prevention of adverse effects have been investigated in many studies. Several studies have attempted to compare analgesic methods, including epidural and spinal analgesia and IV-PCA, in patients undergoing laparoscopic colorectal surgery. One systemic review reported that the transverse abdominal plane (TAP) block is safe and could reduce opioid-related PONV after abdominal surgery (odds ratio, 0.41; P = 0.003) [11]. Recently, our center began using the TAP block to control postoperative pain; we expect that routine PCA use and PCA-related adverse effects will decrease as new pain control tools are offered to patients. Investigation of a postoperative recovery program in an established prospective clinical trial is warranted to confirm the present findings.
The cost analysis revealed little difference in the overall total cost between the groups (Table 3). However, the anesthesia-related charges were higher in the PCA group than in the non-PCA group although the cost of PCA is relatively inexpensive in Korea, which decreases the economic burden to patients far below that in other western countries [12,13].
There are several limitations of this study. First, this is a retrospective and single center study. Second, we did not assess patient satisfaction but instead evaluated the pain score in both groups. In addition, PCA-related adverse effects were recalled as intervals during the study period instead of being evaluated during the postoperative period. Finally, the postoperative pain control protocol was not standardized in the non-PCA group. Therefore, further studies using robust methods are required to address these limitations.
In conclusion, our study suggests that opioid-based IV-PCA does not offer significantly improved analgesia compared to intravenous analgesia on demand. If a standardized analgesic method could be established as a non-PCA protocol, we could potentially manage postoperative pain effectively without using IV-PCA in patients who undergo minimally invasive surgery for colorectal cancer.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No.2012R1A2A2A01045454).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Crisp CC, Bandi S, Kleeman SD, Oakley SH, Vaccaro CM, Estanol MV, et al. Patient-controlled versus scheduled, nurse-administered analgesia following vaginal reconstructive surgery: a randomized trial. Am J Obstet Gynecol. 2012;207:433.e1–433.e6. doi: 10.1016/j.ajog.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Shin YS, Oh YJ, Lee JR, Chung SC, Choi YS. Risk assessment of postoperative nausea and vomiting in the intravenous patient-controlled analgesia environment: predictive values of the Apfel's simplified risk score for identification of high-risk patients. Yonsei Med J. 2013;54:1273–1281. doi: 10.3349/ymj.2013.54.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101(5) Suppl:S44–S61. doi: 10.1213/01.ANE.0000177102.11682.20. [DOI] [PubMed] [Google Scholar]
- 4.Fero KE, Jalota L, Hornuss C, Apfel CC. Pharmacologic management of postoperative nausea and vomiting. Expert Opin Pharmacother. 2011;12:2283–2296. doi: 10.1517/14656566.2011.598856. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Jang SY, Kim MJ, Lee SY, Yoon JS. Comparison of pain-relieving effects of fentanyl versus ketorolac after eye amputation surgery. Korean J Ophthalmol. 2013;27:229–234. doi: 10.3341/kjo.2013.27.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen M. The genesis, development and current usage of patient-controlled analgesia (PCA) Anaesth News. 2009;267:9–11. [Google Scholar]
- 7.Kum CK, Wong CW, Goh PM, Ti TK. Comparative study of pain level and analgesic requirement after laparoscopic and open cholecystectomy. Surg Laparosc Endosc. 1994;4:139–141. [PubMed] [Google Scholar]
- 8.Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004;91:1111–1124. doi: 10.1002/bjs.4640. [DOI] [PubMed] [Google Scholar]
- 9.Veldkamp R, Gholghesaei M, Bonjer HJ, Meijer DW, Buunen M, Jeekel J, et al. Laparoscopic resection of colon Cancer: consensus of the European Association of Endoscopic Surgery (EAES) Surg Endosc. 2004;18:1163–1185. doi: 10.1007/s00464-003-8253-3. [DOI] [PubMed] [Google Scholar]
- 10.Kim WR, Baek SJ, Kim CW, Jang HA, Cho MS, Bae SU, et al. Comparative study of oncologic outcomes for laparoscopic vs. open surgery in transverse colon cancer. Ann Surg Treat Res. 2014;86:28–34. doi: 10.4174/astr.2014.86.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johns N, O'Neill S, Ventham NT, Barron F, Brady RR, Daniel T. Clinical effectiveness of transversus abdominis plane (TAP) block in abdominal surgery: a systematic review and meta-analysis. Colorectal Dis. 2012;14:e635–e642. doi: 10.1111/j.1463-1318.2012.03104.x. [DOI] [PubMed] [Google Scholar]
- 12.Tilleul P, Aissou M, Bocquet F, Thiriat N, le Grelle O, Burke MJ, et al. Cost-effectiveness analysis comparing epidural, patient-controlled intravenous morphine, and continuous wound infiltration for postoperative pain management after open abdominal surgery. Br J Anaesth. 2012;108:998–1005. doi: 10.1093/bja/aes091. [DOI] [PubMed] [Google Scholar]
- 13.Palmer P, Ji X, Stephens J. Cost of opioid intravenous patient-controlled analgesia: results from a hospital database analysis and literature assessment. Clinicoecon Outcomes Res. 2014;6:311–318. doi: 10.2147/CEOR.S64077. [DOI] [PMC free article] [PubMed] [Google Scholar]