Abstract
BACKGROUND
We sought to determine which systolic blood pressure (SBP) characteristics are associated with reduced brain integrity and whether these associations are stronger for white or gray matter. We hypothesized that exposure to higher and variable SBP will be associated with lower structural integrity of both gray and white matter.
METHODS
Neuroimaging, SBP, and cognition were obtained in 311 community-dwelling adults in 2006–2008 (average age = 83 years; 58% women; 40% black). Antihypertensive medications, SBP, and health-related factors were collected from 1997 to 1998 to time of neuroimaging. SBP values obtained from 1997 to 1998 to time of neuroimaging were used to compute mean; pulse pressure; coefficient of variation; and peak, load, and group-based trajectories.
RESULTS
Higher mean SBP was associated with lower white matter integrity in uncinate and superior lateral fasciculi bilaterally, independent of age, stroke history, antihypertensive medication use (odds ratio of having white matter hyperintensities greater than or equal to the median for 10mm Hg of SBP = 10.4, 95% confidence interval = 10.2–10.6, P = 0.0001; standardized beta for fractional anisotropy = −13.54, SE = 4.58, P = 0.003). These neuroimaging markers attenuated the association between higher SBP and lower digit symbol substitution test. Results were similar for trajectories of SBP and stronger for those with previously higher and variable SBP even if SBP was normal at neuroimaging. Results were similar for those without stroke. Associations with gray matter measures were not significant.
CONCLUSIONS
If confirmed, these data suggest a history of higher and variable SBP for very old adults may be useful to alert clinicians to potential lower integrity in selected tracts, whereas cross-sectional SBP measurements may obscure the risk of underlying white matter hyperintensities. Whether lowering and/or stabilizing SBP levels in very old adults without a remarkable cardiovascular history would have neuroprotective effects and reduce dementia risk needs further study.
Keywords: blood pressure, diffusion tensor imaging, hypertension, systolic blood pressure, white matter.
Hypertension and long-term exposure to higher systolic blood pressure (SBP) are risk factors for dementia and disability among older adults living in the community.1–3 Although the beneficial effects of antihypertensive treatments have been shown for major cardiovascular events,4 the effects of antihypertensive strategies to reduce the excess risk of dementia attributable to higher SBP have yielded mixed results.5–8 Moreover, several studies have shown lower blood pressure (BP), but not high BP, relates to cognitive impairment.9,10 The strongest evidence that lowering SBP can reduce cerebral small vessel disease is for selected cohorts of stroke patients or for adults aged <75 years.6 Overall, it is controversial whether lowering SBP in adults aged >80 years who are otherwise without a remarkable cardiovascular history would have neuroprotective effects.7,8,11
We have limited knowledge of which SBP characteristics may be most detrimental to the central nervous system. Similarly, the brain areas and parenchyma that are most vulnerable to higher SBP in older age are not entirely identified. Although several cross-sectional studies of older adults report that higher SBP is associated with generalized white matter hyperintensities and gray matter atrophy,6,12–15 there are few longitudinal studies of repeated SBP measures combined with neuroimaging, and associations with specific brain networks are not consistent across reports.14–18 Longitudinal variations in SBP, in addition to higher absolute levels, are emerging as risk factors for brain structural abnormalities, but evidence is derived largely from studies of patients with clinically overt vascular conditions16,19,20 rather than older adults living in the community.
Quantifying the associations between longitudinal measures of SBP and the spatial distribution of brain abnormalities can help understand the mechanisms underlying the effects of SBP on risk of dementia and disability. Careful characterization and estimates of these associations can also provide biomarkers of brain health to monitor the possible neuroprotective effects of SBP management. This knowledge could lend insight and help in designing intervention trials targeting very old adults, a topic that is especially timely in light of the growing number of adults surviving past age 80 years.
We hypothesized that, if higher SBP is related to higher risk of dementia and disability, as prior works suggest, then that the harmful SBP-related effects on the central nervous system should be primarily localized in brain areas most related to memory and executive function—that is, medial temporal, prefronto-parietal, and basal ganglia. Because of their watershed vascularization, these areas are also most vulnerable to the perfusion changes and small vessel disease that accompany higher SBP.21
In this study, careful characterization of SBP and antihypertensive medications over 10 years were examined in relationship to neuroimaging markers of brain integrity at the macro- and microstructural level in multiple regions and tracts. The relationship between higher variability of SBP over time and neuroimaging markers was also explored using coefficient of variation and trajectories analyses.
METHODS
Participants
Health characteristic data were collected at regular intervals from time of study entry in 1997–1998 to time of brain magnetic resonance imaging (MRI) in 2006–2008 in participants of the Health Aging and Body Composition (HEALTH ABC) study. The study was based on a random sample of Medicare-eligible adults provided by the Health Care Financing Administration. The identified adult and other household members aged 70–79 years were included.22 Main inclusion criteria in the study were no difficulty getting around without an assistive device, ability to walk one-quarter of a mile or climb 10 steps without resting, remain in the study area for 3 years, having no active therapy for life-threatening cancers, and not participating in any research study involving medications or modification of eating or exercise habits. In 2006–2008, 819 of the 1,527 participants who had entered the study at the Pittsburgh site in 1997–1998 returned for an in-person visit. At this visit they were invited to participate in the Healthy Brain Project ancillary study. Of these 819, 311 were eligible for neuroimaging at 3.0 Tesla, had concurrent SBP measurements, and were still able to walk without an assistive device.23 All subjects provided written informed consent. The University of Pittsburgh Institutional Review Board approved the protocol.
SBP measurements
Standard protocols were followed, and quality assurance and control protocols were regularly implemented. Briefly, measures were obtained by centrally trained and certified staff using a standard mercury sphygmomanometer after 5 minutes of quiet rest while sitting. The same measurement protocol was used each year, and examiners were recertified for each clinic visit. BP was measured yearly from study entry in 1997–1998 through time of neuroimaging in 2006–2008, with the exception of 2003–2004 and 2005–2006. SBP was computed as the average of 2 measurements of sitting SBP in millimeters of mercury. SBP was defined as normal (<120mm Hg), moderate (120–140mm Hg), high (>140 and ≤160mm Hg), or very high (>160mm Hg) based on the 2003 National Institute of Heart, Lung and Blood Institute (NHLBI) classification of “high stages 1 and 2.”24
Characterization of SBP
Overall SBP mean and SDs across the study visits were computed for each person using all available SBP data points. The coefficient of variation was computed as the ratio of the overall SBP SD to the overall SBP mean.
SBP load was computed to estimate the length of exposure to high SBP, which was defined as the percentage of visits in which SBP was >160mm Hg. SBP peak was computed as the absolute highest SBP during the visits between study entry and time of neuroimaging.
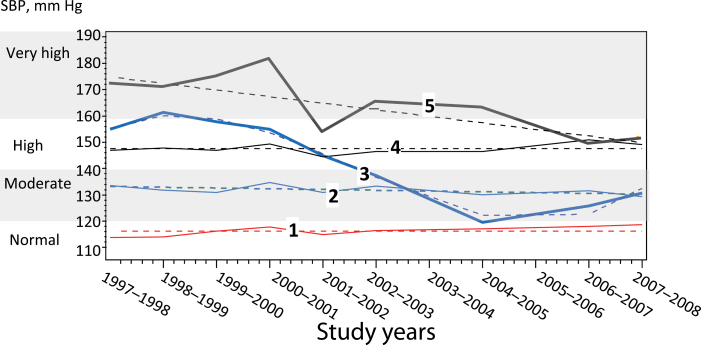
Group-based trajectory models25,26 using PROC TRAJ in SAS9 were applied to identify SBP trajectories. Groups were chosen using the Bayesian information criteria without a priori assumptions regarding the order of the trajectory curves as long as the estimated probabilities of group membership were significant and the time coefficients were of statistical significance. Five groups were identified (Figure 1): 3 had stable SBP levels, and 2 had higher and varying levels in the years preceding neuroimaging.
Figure 1.
Systolic blood pressure (SBP) measures of the 5 groups identified using the group-based trajectory models of repeated SBP measures from study entry in 1997–1998 to time of neuroimaging in 2006–2008. Groups identified using group-based trajectory modeling: group 1 (n = 56; 19%): normal SBP at time of neuroimaging (<120mm Hg), previously stable; group 2 (n = 158; 50%): moderate SBP at time of neuroimaging (120–140 mm Hg), previously stable; group 3 (n = 68; 22%): moderate SBP at time of neuroimaging, previously higher and varying; group 4 (n = 15; 5%): high SBP (>140 and <120), previously stable; group 5 (n = 14; 4%): high SBP at time of neuroimaging (>140 and ≤120), previously higher and varying. Note that SBP measurements were not obtained in 2003–2004 and 2005–2006. Dashed lines indicate estimated values. Solid lines indicate interpolate measured values.
A medication inventory was completed at each annual visit, as previously described.27 Antihypertensive medication use was recorded, and a variable was computed to report patients having taken these medications at least 50% of the time over the entire follow-up period between study entry and time of neuroimaging.
Hypertension at time of neuroimaging was computed using self-report information of having been treated for hypertension, confirmed by use of medication combined with actual BP level. Pulse pressure was computed at time of neuroimaging and at study entry by subtracting diastolic BP from SBP.
Neuroimaging measurements
A previously published protocol was applied23 to quantify fractional anisotropy (FA) and brain tissue volumes (gray matter, white matter, cerebrospinal fluid) on skull-stripped T1-weighted images in native anatomical space. Gray matter atrophy was computed by dividing the volume of the gray matter by intracranial volume. White matter hyperintensity (WMH) volume was estimated by summing all voxels classified as WMH and further normalized by brain volume.28 A radiologist examined the magnetic resonance images and excluded unexpected findings. We examined 6 white matter tracts of interest identified using the S. Mori atlas, uncinate fasciculus, superior longitudinal fasciculus and cingulum, anterior thalamic radiations, fornix, and corticospinal tracts. Additionally, we examined 7 gray matter regions of interest identified using the Automated Anatomical Labeling atlas:23 hippocampus, parahippocampus, entorhinal cortex, cingulate cortex, posterior parietal lobule, basal ganglia, and middle frontal gyrus.
Other variables of interest
Demographic and education data were collected at study entry. Cognition was assessed using the Digit Symbol Substitution Test (DSST) and the Mini-Mental State Exam (MMSE). Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies-Depression Scale. History of myocardial infarction, diabetes, and stroke were determined based on self-report of physician diagnoses and recorded medications23 at each in-person visit from study entry to time of neuroimaging. Other factors known to be related to BP control that were examined included body mass index (based on height and weight measurements at time of neuroimaging), physical activity, smoking (self-reported), and high-density lipoprotein blood levels.
Statistical analysis
Because of the highly skewed distribution of WMH in this cohort, WMH volumes of total brain and tracts were transformed into dichotomous variables greater than or equal to the median. Gray matter atrophy and FA were used as continuous measures. Χ2 or analysis of variance (ANOVA) were used to investigate age-adjusted associations of neuroimaging measures and SBP-related measures with other measures of interest. Associations between SBP characteristics and neuroimaging measures were tested in multiple regression models (logistic for WMH and linear for FA and gray matter atrophy) adjusted for age, antihypertensive medication use, and stroke. Longitudinal trends in antihypertensive medication use (percentage using antihypertensive medications at each time point) or in SBP load (SBP >160mm Hg) from study entry through time of neuroimaging were compared between those with and without WMH greater than or equal to the median (or FA greater than or equal to the median) using repeated measures multivariable logistic regression generalized estimating equations adjusted for age, antihypertensive medication use, and stroke; these models included a separate baseline rate term plus time “slope” for each WMH group.
Further adjustments were made for race, sex, and other characteristics that were significantly associated with WMH or FA in univariate models.
RESULTS
Characteristics of the parent cohort (n = 819) in relationship to the subgroup with brain MRI have been previously published.23 Briefly, compared with those who refused or were ineligible for the MRI study, those included in this analysis were younger (P = 0.002), more likely to be white (P = 0.02), more likely to be men (P = 0.04), and had a higher score on the MMSE (P = 0.02) and Digit Symbol Substitution tests (P = 0.0001) at the time of neuroimaging. Average BP measurements and prevalence of cardiovascular-related conditions or diseases at time of neuroimaging were similar. In this group of 311 participants, a total of 273 (86.8%) had SBP measures at each time point between study entry and time of neuroimaging (Table 1); 30 (9.6%) had SBP measures missing at 1 time point. Of the 8 remaining participants, 3 (1%), 3 (1%), and 2 (<1%) had missing SBP measures at 2, 3, and 5 time points, respectively. At time of neuroimaging, use of calcium channel blockers (30%), angiotensin-converting enzyme inhibitor (27%), and beta-blocker medications (25%) or thiazide (22%) was more common than use of angiotensin II receptor blockers (16%) or potassium, alpha-blockers, or loop diuretics (<10% for all).
Table 1.
Characteristics of the study sample (n = 311)
| Demographic characteristics | No. (%) or mean ± SD |
|---|---|
| Age, y, | 82.9±2.8 |
| Sex, male | 130 (41.8) |
| Race, white | 185 (59.5) |
| Education greater than high school | 159 (51.3) |
| Modified Mini-Mental score, points | 92.8±8.3 |
| Stroke, prevalent | 25 (8.0) |
| Diabetes, present | 81 (26.0) |
| Cardiovascular diseases, absent | 222 (71.4) |
| Characteristics of SBP | |
| Hypertension, prevalent | 217 (69.3) |
| Antihypertensive medication usea | 193 (62.1) |
| SBP at study entry, mm Hg | 137.0±20.0 |
| SBP at time of neuroimaging, mm Hg | 134.7±19.5 |
| Overall mean SBP,b | 135.8±13.8 |
| Overall coefficient of variation of SBPb | 0.10±0.03 |
| SBP load,b visits with SBP >160mm Hg | 0.80 (10.2) |
| Peak SBP,b mm Hg | 157.5±20.4 |
| Pulse pressure, mm Hg | 64.7±16.2 |
| Group-based trajectories of SBPb | |
| 1: Normal (<120mm Hg), previously stable | 58 (18.6) |
| 2: Moderate (120–140mm Hg), previously stable | 157 (50.5) |
| 3. Moderate (120–140mm Hg), previously higher/varying | 15 (4.8) |
| 4: High (>140 and ≤160mm Hg), previously stable | 67 (21.5) |
| 5. High (>140 and ≤160mm Hg), previously higher/varying | 14 (4.5) |
Abbreviations: MRI, magnetic resonance imaging; SBP, systolic blood pressure.
aDefined as having taken these medications at least 50% of the time over the entire follow-up period between study entry and time of neuroimaging (multiplied by 100 for reporting).
bComputed using SBP values obtained from study entry to time of MRI.
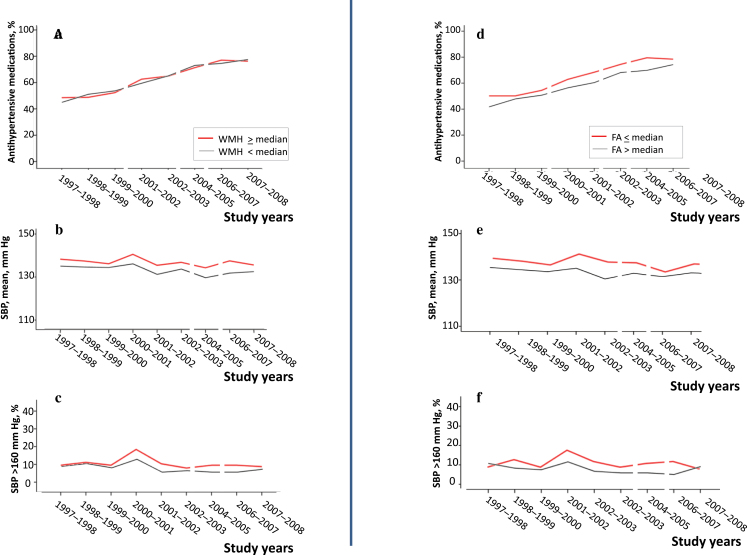
Among the SBP characteristics examined, overall higher mean and group trajectories of SBP were associated with higher WMH prevalence (P = 0.002 and P = 0.048, respectively) and with lower FA of total brain (P = 0.001 and P = 0.02, respectively). Pulse pressure at study entry was also associated with higher WMH prevalence and with lower FA (P = 0.03 for both). By contrast, SBP at study entry, SBP or pulse pressure at time of neuroimaging, coefficient of variation, SBP load, and SBP peak measured from study entry to time of neuroimaging were not associated with WMH or FA (Supplementary Table 1). The association of antihypertensive medication intake with higher prevalence of WMH was not significant, but the association with lower FA was significant (P = 0.03).The relationships of WMH and FA with antihypertensive medication intake, mean SBP, and load SBP measured from study entry to time of neuroimaging are illustrated in Figure 2. Associations between each type of antihypertensive medication at time of neuroimaging and WMH were also not significant (Supplementary Table 2).
Figure 2.
Proportion of participants taking antihypertensive medication, mean systolic blood pressure (SBP), and SBP load from study entry to time of magnetic resonance imaging stratified by presence (red) or absence (black) of white matter hyperintensity (WMH) greater than or equal to the median (a–c) or fractional anisotropy (FA) less than or equal to the median (d–f). Note that measurements on antihypertensive medications were not obtained in 2000–2001, 2003–2004, and 2005–2006 and that SBP measurements were not obtained in 2003–2004 and 2005–2006.
None of the SBP characteristics were associated with gray matter atrophy (Supplementary Table 1), and this neuroimaging measure did not enter further analyses of spatial distribution.
Higher SBP mean measured from study entry to time of neuroimaging was significantly associated with presence of WMH greater than or equal to the median and with lower FA in most tracts of interests (Table 2), but not with anterior thalamic or corticospinal tracts (data not shown). Associations were stronger for the uncinate and the superior longitudinal fasciculi bilaterally than for the cingulum (Table 2).The associations of overall mean SBP with presence of WMH greater than or equal to the median or with FA in the uncinate and the superior longitudinal fasciculi combined were independent of age, presence of stroke, and antihypertensive use (Table 3). In these multivariable models, 10mm Hg of overall mean SBP corresponded to a nearly 10 times greater probability of having WMH greater than or equal to the median and to smaller FA. Further adjustment for race, diabetes, myocardial infarction, body mass index, physical activity, smoking, and high-density lipoprotein levels also yielded similar results (data not shown). Adding hypertension or SBP or pulse pressure at study entry or SBP at time of neuroimaging did not modify these results.
Table 2.
Associations of overall mean systolic blood pressure (average from study entry to time of neuroimaging) with prevalence of white matter hyperintensity greater than or equal to the median and with mean fractional anisotropy in each tract of interest
| White matter hyperintensity greater than or equal to the median | ||||||
|---|---|---|---|---|---|---|
| Uncinate tract, left | Uncinate tract, right | Superior longitudinal fasciculus, left | Superior longitudinal fasciculus, right | Cingulum, left | Cingulum, right | |
| Mean difference, mm Hg (95% CI) | 5.2 (2.2 to 8.2) | 3.5 (0.5 to 6.6) | 5.1 (2.1 to 8.1) | 5.8 (2.8 to 8.8) | 3.2 (−0.5 to 6.8) | −0.7 (−5.1 to 3.7) |
| Adjusted P value | 0.001 | 0.04 | 0.001 | 0.001 | 0.07 | 0.99 |
| Fractional anisotropy | ||||||
| Uncinate tract, left | Uncinate tract, right | Superior longitudinal fasciculus, left | Superior longitudinal fasciculus, right | Cingulum, left | Cingulum, right | |
| Correlation coefficient | −0.142 | −0.157 | −0.172 | −0.142 | −0.182 | −0.166 |
| Adjusted P value | 0.03 | 0.01 | 0.007 | 0.04 | 0.05 | 0.05 |
P values are from logistic regressions with presence of white matter hyperintensity as the dependent variable and from linear regression with fractional anisotrophy as dependent variable, adjusted for age, stroke, and antihypertensive medication use.
Abbreviation: CI, confidence interval.
Table 3.
Multivariable regression models of overall mean systolic blood pressure with white matter hyperintensity and fractional anisotrophy of uncinate and superior longitudinal fasciculi, adjusted for age, stroke, and antihypertensive medication use
| WMH greater than or equal to the median, odds ratio (95% CI)a | FA, standardized beta (SE)b | |
|---|---|---|
| Independent variable, overall mean SBP of 10mm Hg | 10.4 (10.2–10.6) P < 0.0001 |
−13.54 (4.58) P = 0.003 |
Odds ratio and standardized beta are for 10mm Hg of overall mean systolic blood pressure (SBP). Mean (SD) of fractional anisotrophy (FA) for these tracts in this population is 2.29 (0.12). Therefore, 13.54 in standardized units of FA corresponds to a difference of 1.62 in FA.
Abbreviations: CI, confidence interval; WMH, white matter hyperintensity
aFrom logistic regression model with presence of WMH as the dependent variable.
bFrom linear regression model with FA as dependent variable.
Associations between the 5 SBP trajectory groups and WMH or FA were significant or nearly significant for tracts in the left but not the right hemisphere (Supplementary Table 3) and were stronger for WMH in the left uncinate fasciculus (P = 0.02) and for FA in the left superior longitudinal fasciculus (P = 0.008).Compared with the group with normal SBP and previously stable trajectories, the other 4 trajectory groups had a greater probability of having WMH greater than or equal to the median in the left uncinate fasciculus (Table 4) and had greater FA in the left superior longitudinal fasciculus, independent of age, antihypertensive medication use, and stroke (Table 4). Results were similar after adjusting for SBP at study entry or SBP at time of neuroimaging, race, sex, and other covariables (data not shown). Results were also similar for those without stroke (data not shown). Associations with WMH or with FA appeared stronger for those with previously varying trajectories, even if they had similar levels of SBP at time of neuroimaging. For example, among those with moderate SBP at time of neuroimaging, those with previously higher and varying levels (group 3) had larger regression coefficients as compared with those with previously stable values (group 2); similarly, among those with high SBP at time of neuroimaging, those with previously higher and varying levels (group 5) had larger regression coefficients as compared with those with previously stable values (group 4).
Table 4.
Multivariable regression models of group-based trajectories of systolic blood pressure with white matter hyperintensity of uncinate fasciculus and fractional anisotrophy of superior longitudinal fasciculus, adjusted for age, stroke, and antihypertensive medication use
| Independent variable: group trajectories of SBP |
WMH greater than or equal to the median, odds ratio, (95% CI)a |
FA, standardized beta (SE)b |
|---|---|---|
| 1: Normal SBP at time of neuroimaging (<120mm Hg), previously stable |
Referent | Referent |
| 2: Moderate SBP at time of neuroimaging (120–140mm Hg), previously stable | 2.2 (1.1–4.2) |
0.035 (0.162) |
| 3. Moderate SBP at time of neuroimaging (120–140mm Hg), previously higher and varying | 3.0 (1.4–6.4) |
−0.585 (0.305) |
| 4: High SBP at time of neuroimaging (>140 and ≤120mm Hg), previously stable |
2.7 (0.8–8.9) |
−0.383 (0.192) |
| 5. High SBP at time of neuroimaging (>140 and ≤120mm Hg), previously higher and varying |
8.6 (2.0–36.7) |
−0.431 (0.321) |
| P value | 0.02 | 0.01 |
Abbreviations: CI, confidence interval; FA, fractional anisotropy; SBP, systolic blood pressure; WMH, White matter hyperintensity.
aLogistic regressions with presence of WMH as the dependent variable.
bGeneralized linear models with FA as dependent variable.
Univariable associations of higher overall SBP mean and of SBP trajectory groups with lower DSST were significant (beta = −1.49, SE = 0.58, P = 0.01 for 10mm Hg of SBP; beta = −1.94, SE = 0.77, P = 0.01 for each level of trajectory group. Adjustment for WMH greater than or equal to the median in the uncinate and the superior longitudinal fasciculi attenuated these associations by >10% (beta for DSST = −1.33, SE = 0.59, P = 0.03 for 10mm Hg of SBP; beta = −1.72, SE = 0.78, P = 0.03 for each level of trajectory group). Adjustment for FA in these tracts also attenuated these associations by >10% (beta for DSST = −1.34, SE = 0.58, P = 0.02 for 10mm Hg of SBP; beta = −1.75, SE = 0.77, P = 0.02 for each level of trajectory group). Associations between higher overall SBP and SBP trajectory groups with lower MMSE were also significant (beta for MMSE = −9.5, SE = 0.03, P = 0.001 for 10mm Hg of SBP; beta = −1.38, SE = 0.41, P = 0.001 for each level of trajectory group). However, adjustment for either neuroimaging marker changed these associations by <4%. Of note, WMH greater than or equal to the median and lower FA in the uncinate and the superior longitudinal fasciculi were each associated with lower DSST (F from ANOVA = 4.062, P from ANOVA = 0.045; rho from Spearman = 0.133, P from Spearman = 0.03) but not with lower MMSE (F from ANOVA = 2.898, P from ANOVA = 0.09; rho from Spearman = 0.034, P from Spearman = 0.60).
DISCUSSION
In this group of white and black older adults living in the community, higher and variable SBP levels collected over 10 years were associated with lower white matter integrity of fronto-parietal and medial temporal tracts, independent of markers of arterial stiffness or cardiometabolic conditions. Moreover, these neuroimaging markers substantially attenuated the associations between higher and variable SBP with cognitive function.
These results carry implications for individual practice and at the public health level for both white and black community-dwelling older adults.1,2 Higher WMH is a sign of underlying cerebrovascular disease has been consistently associated with higher risk of mortality, disability, and dementia.22,29,30 Moreover, higher WMH is very common among older adults, with approximately 30% of older adults living in the community without overt neurological or vascular disease presenting with WMH. Patterns of rapid rise and fall in SBP, in addition to overall higher SBP, need to be tested as potential biomarkers of higher WMH and white matter integrity in areas specialized for executive function, even among older adults free from other neurological conditions. Such patterns could help identify individuals who are at higher risk of dementia and disability and prompt individualized approaches to improve cognitive function.
Several prior studies in younger old (e.g., 65–75 years old) have examined the detrimental effects of higher BP on cognition, with variable results.9,10 To date, it is not clear whether lowering BP would have a beneficial effect on cognitive function.7 This study extends our knowledge of the detrimental effects of high SBP on brain health in very old adults because it combines extensive, longitudinal measurements of BP and cognition with advanced neuroimaging measures of brain micro- and macrostructure in a well-characterized cohort of very old white and black adults. We are aware of 1 study relating varying SBP values over 6 years with higher WMH burden in community-dwelling older adults.16 However, this study did not analyze the spatial distribution of WMH or brain microstructure. Two other neuroimaging studies used repeated measures of SBP over long periods of time14,15 to compute overall mean SBP changes but did not examine SBP trajectories.
The associations between SBP and the neuroimaging markers of white matter integrity can provide insights into the potential mechanisms underlying the detrimental effects of higher and variable SBP on dementia and disability. First, the association of overall SBP mean and trajectory groups with WM measures but not with gray matter atrophy supports the notion that white matter is particularly sensitive to vascular-related insults.31 Longitudinal studies of high or variable SBP in older adults to date have not found a significant association with total brain gray matter atrophy, and associations with gray matter of individual regions are contradictory across studies.17,32–35 Related to this, the application of neuroimaging with high spatial resolution and diffusion tensor parameters shows a selected pattern of white matter tract abnormalities associated with higher SBP, particularly the uncinate and the superior longitudinal fasciculi. The uncinate tract connects the orbitofrontal regions with parts of the medial temporal lobe, and the superior longitudinal fasciculus is a long tract connecting the prefrontal regions with the posterior parietal areas. These tracts may be more susceptible to the insults related to higher SBP because of their localization within the watershed areas, which receive blood supply from small, penetrating, and deep lenticulostriate arteries that are highly prone to hypertensive arteriosclerotic changes.21,36 A recent study also observed greater WMH volume in the frontal lobe in hypertensive subjects as compared with nonhypertensive older adults.36 Second, associations remained independent of race or presence of stroke and other cardiovascular conditions, thus suggesting that these relationships reflect subclinical severity of cerebrovascular damage for both white and blacks. This is particularly important in the population of older blacks because they are at high risk of dementia due to a higher risk of vascular diseases. Lastly, we found that the association between higher SBP mean and SBP trajectories with lower digit symbol substitution score was attenuated after adjusting for WMH and FA in the tracts of interest, whereas results were weaker for the MMSE. An interpretation of our results as a whole is that exposure to higher and variable SBP can negatively impact cognitive function, possibly executive cognitive domains to a greater extent than other domains, by damaging selected fronto-parietal and medial temporal tracts.
Our study of trajectories of SBP uncovered a relatively high proportion of participants who had prior varying and higher SBP levels (groups 3 and 5). These groups together accounted for approximately 10% of the entire sample. Compared with those with similar SBP levels at time of neuroimaging but prior stable trajectories (e.g., groups 2 and 4), those in groups 3 and 5 had higher WMH and lower FA, as well as lower cognitive test scores. The potential neuroprotective effects of SBP management for these adults deserve further study in larger samples.
In addition to examining repeated measures of SBP, our study also tested associations with SBP taken at study entry and at time of neuroimaging. Studies indicate that individual absolute SBP measures at 1 point in time during prior years are not associated with severity of white matter lesions15 or risk of stroke.37 Our results comport with this prior evidence and underscore the importance of simultaneously reviewing information on levels and trajectories of SBP collected over time to account for the probability of having WMH and/or microstructural abnormalities in normal-appearing white matter. It is possible that SBP measurements taken more frequently than yearly might improve the precision of estimating presence of WMH or FA. The few studies applying complex measures of rapidly variable and fluctuating SBP over 24 hours have shown a positive association with WMH in community-dwelling older adults.17,18 However, the focus of this study was to examine long-term values of SBP by applying procedures that are simpler and more similar to those used in a physician’s office.38 Further studies are warranted to clarify whether SBP measured at regular intervals over longer periods of time may provide similar information on brain health outcomes compared with SBP measurements obtained over 24 hours.
Vascular dysfunction, and in particular arterial stiffness, has been proposed as a candidate mechanism linking higher BP and higher WMH burden. In this cohort, pulse pressure at time of neuroimaging was related to WMH and FA, and this is consistent with our prior study of arterial pulse wave velocity and WMH.23 However, neither pulse pressure nor pulse wave velocity at time of neuroimaging explained the associations between the longitudinal measures of SBP and the neuroimaging markers. Moreover, the cross-sectional associations between pulse pressure and neuroimaging markers were not significant. Longer incubation times than those hereby examined may underlie the association between higher and variable SBP, arterial stiffness, and brain abnormalities. In the absence of longer follow-up studies, overall discretion in the mechanistic interpretation of these data is suggested.
We recognize several limitations to our study. Because of the lack of MRI data at study entry, this study can not address whether there is a true causal relationship between SBP characteristics and white matter lesion. For example, it is possible that the onset of brain disease might lead to SBP decline. Additionally, it is possible that because of the study design and the voluntary-based participation, those included in the study were healthier than the general population of adults of similar age, and these results may be underestimating the real effect size. Finally, we made multiple comparisons with several tracts, BP measures, and white and gray matter examined separately, so there is a chance our results could be influenced by these issues of multiplicity. However, our hypotheses were made a priori, and thus our analyses were not exploratory in nature.
If these findings are confirmed with future studies, knowledge of prior values of SBP that are high and variable in very old adults may alert clinicians to the potential for underlying focal white matter abnormalities, both at the macro- and microstructural levels. Future studies should also investigate whether these results support individualized BP control approaches to reduce the risk of dementia and disability.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge Lewis Lipsitz for his expert advice, Zachary Marcum with assistance with the medication inventory, and Amy J. Markowitz, JD, for her assistance in editing of the manuscript. This work was supported by National Institute on Aging (NIA) contracts N01 AG62101, N01 AG62103, and N01 AG62106; NIA grants R01 AG29232, R01 AG037451, and R01 AG028050; and National Institute of Nursing Research grant NR012459; and in part by the Intramural Research Program of the NIA, National Institutes of Health.
REFERENCES
- 1. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA 1995; 274:1846–1851. [PubMed] [Google Scholar]
- 2. Rosano C, Newman AB. Cardiovascular disease and risk of Alzheimer’s disease. Neurol Res 2006; 28:612–620. [DOI] [PubMed] [Google Scholar]
- 3. Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005; 4:487–499. [DOI] [PubMed] [Google Scholar]
- 4. Beckett N, Peters R, Tuomilehto J, Swift C, Sever P, Potter J, McCormack T, Forette F, Gil-Extremera B, Dumitrascu D, Staessen JA, Thijs L, Fletcher A, Bulpitt C. Immediate and late benefits of treating very elderly people with hypertension: results from active treatment extension to hypertension in the very elderly randomised controlled trial. BMJ 2012;344:d7541. [DOI] [PubMed] [Google Scholar]
- 5. Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, Greenlick MR, Hadley E, Moye L, Perry HM., Jr Impact of the treatment of isolated systolic hypertension on behavioral variables. Results from the systolic hypertension in the elderly program. Arch Intern Med 1994; 154:2154–2160. [PubMed] [Google Scholar]
- 6. Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI cohort. Neurology 2001; 56:921–926. [DOI] [PubMed] [Google Scholar]
- 7. McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 7(4):CD004034. doi:10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters R, Beckett N, Fagard R, Thijs L, Wang JG, Forette F, Pereira L, Fletcher A, Bulpitt C. Increased pulse pressure linked to dementia: further results from the hypertension in the very elderly trial—HYVET. J Hypertens 2013; 31:1868–1875. [DOI] [PubMed] [Google Scholar]
- 9. Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol 2010; 7:686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. JAMA 1999; 281:438–445. [DOI] [PubMed] [Google Scholar]
- 11. Goodwin JS. Embracing complexity: a consideration of hypertension in the very old. J Gerontol A Biol Sci Med Sci 2003; 58:653–658. [DOI] [PubMed] [Google Scholar]
- 12. Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging Study. Stroke 2002; 33:26–30. [DOI] [PubMed] [Google Scholar]
- 13. Heijer T, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, Breteler MM. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging 2003; 24:307–313. [DOI] [PubMed] [Google Scholar]
- 14. Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, Shibata DK, Knopman DS, Jack CR, Mosley TH., Jr Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (aric) study. Stroke 2010; 41:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo X, Pantoni L, Simoni M, Bengtsson C, Bjorkelund C, Lissner L, Gustafson D, Skoog I. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension 2009; 54:57–62. [DOI] [PubMed] [Google Scholar]
- 16. Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010; 67:564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and the brain: a 5-year follow-up. Neurology 2005; 64:1846–1852. [DOI] [PubMed] [Google Scholar]
- 18. Sander D, Winbeck K, Klingelhofer J, Conrad B. Extent of cerebral white matter lesions is related to changes of circadian blood pressure rhythmicity. Arch Neurol 2000; 57:1302–1307. [DOI] [PubMed] [Google Scholar]
- 19. Joas E, Backman K, Gustafson D, Ostling S, Waern M, Guo X, Skoog I. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension 2012; 59:796–801. [DOI] [PubMed] [Google Scholar]
- 20. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011; 57:160–166. [DOI] [PubMed] [Google Scholar]
- 21. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9:689–701. [DOI] [PubMed] [Google Scholar]
- 22. Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 2005; 53:649–654. [DOI] [PubMed] [Google Scholar]
- 23. Rosano C, Watson N, Chang Y, Newman AB, Aizenstein HJ, Du Y, Venkatraman V, Harris TB, Barinas-Mitchell E, Sutton-Tyrrell K. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension 2013; 61:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the Joint National Committee on Prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 25. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010; 6:109–138. [DOI] [PubMed] [Google Scholar]
- 26. Jones BL ND. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Meth Res 2007; 35:542. [Google Scholar]
- 27. Marcum ZA, Zheng Y, Perera S, Strotmeyer E, Newman AB, Simonsick EM, Shorr RI, Bauer DC, Donohue JM, Hanlon JT. Prevalence and correlates of self-reported medication non-adherence among older adults with coronary heart disease, diabetes mellitus, and/or hypertension. Res Social Adm Pharm 2013;9:817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, 3rd, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006; 148:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuller LH, Arnold AM, Longstreth WT, Jr, Manolio TA, O’Leary DH, Burke GL, Fried LP, Newman AB. White matter grade and ventricular volume on brain mri as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging 2007; 28:1307–1315. [DOI] [PubMed] [Google Scholar]
- 30. Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci 2004; 59:818–826. [DOI] [PubMed] [Google Scholar]
- 31. Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 2002; 31:581–593. [DOI] [PubMed] [Google Scholar]
- 32. den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MM. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology 2005; 64:263–267. [DOI] [PubMed] [Google Scholar]
- 33. Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, Schmidt R. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 2005; 64:1704–1711. [DOI] [PubMed] [Google Scholar]
- 34. Gattringer T, Enzinger C, Ropele S, Gorani F, Petrovic KE, Schmidt R, Fazekas F. Vascular risk factors, white matter hyperintensities and hippocampal volume in normal elderly individuals. Dement Geriatr Cogn Disord 2012; 33:29–34. [DOI] [PubMed] [Google Scholar]
- 35. Wiseman RM, Saxby BK, Burton EJ, Barber R, Ford GA, O’Brien JT. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology 2004; 63:1892–1897. [DOI] [PubMed] [Google Scholar]
- 36. Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging 2012; 33:429 e421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shimizu Y, Kato H, Lin CH, Kodama K, Peterson AV, Prentice RL. Relationship between longitudinal changes in blood pressure and stroke incidence. Stroke 1984; 15:839–846. [DOI] [PubMed] [Google Scholar]
- 38. Mancia G. Prognostic value of long-term blood pressure variability: the evidence is growing. Hypertension 2011; 57:141–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.