Abstract
Rates of amyotrophic lateral sclerosis (ALS) have been reported to be higher among US military veterans, who currently number more than 21 million, but the causal factor(s) has not been identified. We conducted a review to examine the weight of evidence for associations between military service, deployments, and exposures and ALS etiology and survival. Thirty articles or abstracts published through 2013 were reviewed. Although the current evidence suggests a positive association with ALS etiology, it is too limited to draw firm conclusions regarding associations between military service and ALS etiology or survival. Some evidence suggests that deployment to the 1990–1991 Persian Gulf War may be associated with ALS etiology, but there is currently no strong evidence that any particular military exposure is associated with ALS etiology. Future studies should address the limitations of previous ones, such as reliance on mortality as a surrogate for incidence, a dearth of survival analyses, lack of clinical data, low statistical power, and limited exposure assessment. The Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis (GENEVA) Study is one such study, but additional research is needed to determine whether military-related factors are associated with ALS and to assess potential prevention strategies.
Keywords: amyotrophic lateral sclerosis, Gulf War, incidence, military personnel, mortality, motor neuron disease, occupational exposure, veterans
INTRODUCTION
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS), often called Lou Gehrig's disease, is a debilitating neurodegenerative disease that involves loss of motor neurons in the central nervous system (1–3). Specifically, motor neurons die in the brain stem and spinal cord (lower motor neurons) and in the cerebral cortex (upper motor neurons) (1–3). Persons with ALS comprise more than 90% of individuals with motor neuron disease (MND); in the United Kingdom, the term MND is used for ALS (1–3). A diagnosis of ALS requires both upper and lower motor neuron involvement (1–3). Approximately two-thirds of persons with ALS have a spinal form, symptoms of which include muscle wasting and spasticity, progressive weakness, motor dysfunction, and paralysis (1–3). Most remaining persons with ALS have a bulbar form, symptoms of which include difficulty speaking or swallowing, excessive drooling, and facial weakness, although limb symptoms usually appear within 1–2 years of bulbar symptoms in these individuals (1–3). Some forms of ALS also involve cognitive changes that resemble frontotemporal dementia (1–3).
ALS is rare in the general population; incidence is approximately 2 per 100,000 population per year, and prevalence is approximately 6 per 100,000 population (1–3). Nevertheless, 2.7 of every 1,000 deaths in the United States in 2011 were due to ALS (4). In addition, the effect of motor neuron loss in the daily lives of persons with ALS is dramatic and may include an inability to move, eat, talk, or complete other activities of daily living (1). ALS patients are unable to work, have high medical bills, require intensive health care, and are often depressed (2, 3).
Half of persons with ALS die within 3 years of diagnosis, but 4% survive at least 10 years after diagnosis (1–3). The most common cause of death among ALS patients is respiratory failure (2). Riluzole is the only ALS therapy approved by the US Food and Drug Administration, but a recent meta-analysis found it extends survival by only 2–3 months (5). Treatments including physical therapy can relieve ALS symptoms (2, 3), but explanations for the extremely variable survival of ALS cases, particularly explanations that could lead to interventions or therapies, are sorely needed.
Approximately 90%–95% of ALS is classified as sporadic, and 5%–10% is classified as familial (1–3), based on family history. Most familial forms are due to polymorphisms in genes for copper/zinc superoxide dismutase 1 (SOD1), TAR DNA binding protein 43 (TARDP), fused in sarcoma (FUS), or ubiquilin 2 (UBQLN2) or an expansion of the intronic hexanucleotide repeat sequence in the chromosome 9 open reading frame 72 gene (C9ORF72) (1, 6–11). Established risk factors for sporadic ALS include increasing age, male sex, and cigarette smoking (1–3, 12). Other possible risk factors have been investigated (1–3, 13–15), but none have been consistently associated with sporadic ALS.
Factors consistently associated with shorter survival after ALS diagnosis include older age at diagnosis (16–24), bulbar onset (17–22, 24–26), clinical features (e.g., disease progression/disability status, respiratory function) (17–19, 21, 23, 27, 28), time between symptom onset and diagnosis (17–19, 24–26, 29), and diagnosis category (definite, probable, etc.) (17).
Military-related factors and ALS
More than 21 million Americans, or about 1 in 11 of those aged 18 years or more, have served in the military (30). Among American men, who comprise 92% of US military veterans (31), this number jumps to about 1 in 6 (30). US military personnel are exposed to a number of factors encountered in the unique circumstances of their jobs that may lead to health problems later in life (32). For example, US military personnel are exposed to intensive physical activity, detrimental lifestyle factors (cigarette smoking, alcohol consumption), physical trauma (combat-related trauma, fractures), emotional/psychological trauma, transmissible agents (viruses), toxic substances (lead, pesticides), and other environmental toxicants and experiences (33, 34). Concern regarding potential health effects of US military service is demonstrated by the fact that typing “military OR veterans OR soldiers” into the search engine on the Institute of Medicine (IOM) website returns 103 reports published after 1998 (35). Unfortunately, one of the health consequences suspected to be associated with US military service is ALS (34).
An association between military-related factors and ALS was first proposed in connection with the 1990–1991 Persian Gulf War (hereafter called the Gulf War). Six cohort studies on this topic were published between 2000 and 2005 (36–41). In 4 of the studies, investigators reported an increased rate of ALS incidence, hospitalization, or mortality among US military veterans deployed to the Gulf War as compared with veterans deployed to other areas (36–38, 40), but in 1 they did not (39). Another study found that the ALS mortality rate was higher among US men who reported military service compared with men who did not (41). In response to these studies, the US Department of Veterans Affairs (VA) requested that the IOM establish a committee to “review, evaluate, and summarize the scientific literature on ALS in veterans. If an association exists between military service and the development of ALS, then the committee might make recommendations that will help to identify risk factors” (34, p. 9).
The IOM report, published in 2006, concluded that there was “limited and suggestive evidence of an association between military service and later development of ALS” (34, p. 3). The IOM report also recommended that further studies be conducted to “elucidate ALS risk factors relevant to military service” (34, p. 4). Several studies of the association between military-related factors and ALS incidence, prevalence, mortality, or survival were not included in the IOM report, and several new studies on this topic have been conducted since its publication. Therefore, we reviewed the literature to examine the weight of evidence for associations between military service, deployments, and exposures and ALS/MND etiology and survival (we combined ALS and MND together because studies often have not distinguished between them). We also aimed to provide recommendations for future studies of these relationships.
METHODS
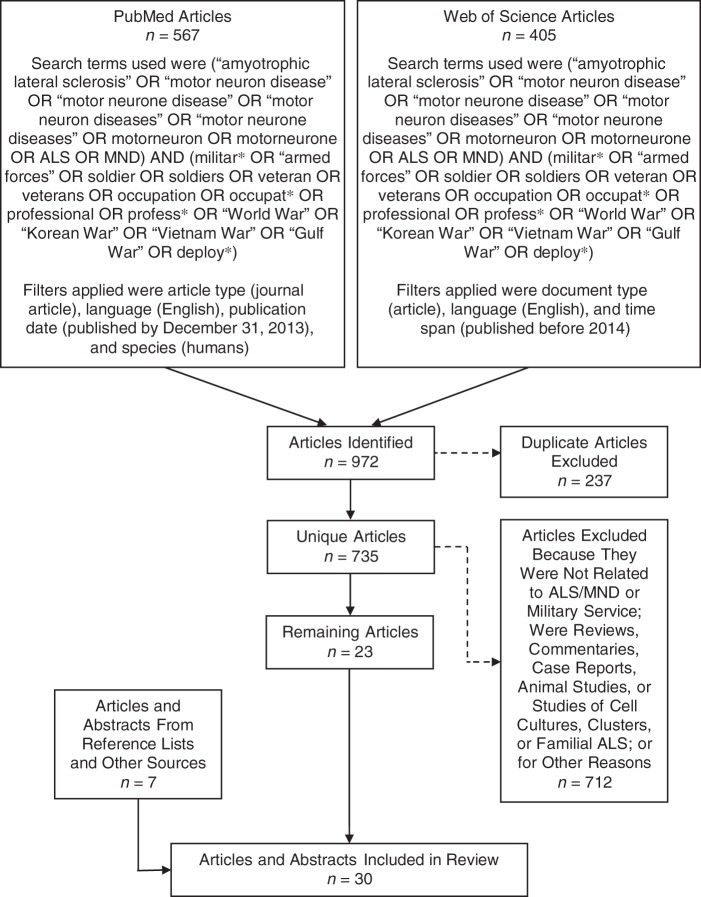
We conducted a search of journal articles that used human subjects and were published in English through the end of 2013 using the PubMed (US National Library of Medicine, Bethesda, Maryland) and Web of Science (Thomson Reuters Corporation, New York, New York) databases. Specifically, we used the following search terms in both databases: (“amyotrophic lateral sclerosis” OR “motor neuron disease” OR “motor neurone disease” OR “motor neuron diseases” OR “motor neurone diseases” OR motorneuron OR motorneurone OR ALS OR MND) AND (militar* OR “armed forces” OR soldier OR soldiers OR veteran OR veterans OR occupation OR occupat* OR professional OR profess* OR “World War” OR “Korean War” OR “Vietnam War” OR “Gulf War” OR deploy*) (Figure 1). After removing duplicates, we excluded articles that did not study ALS/MND etiology or survival as the outcome or military service, deployments, or exposures as the exposure variable. We also excluded articles for other reasons (reviews, commentaries, case reports (no comparison group), animal studies, cell cultures, ALS clusters or possible clusters, familial ALS, etc.) (Figure 1). Finally, we identified relevant articles and abstracts from the reference lists of articles found by our searches of the aforementioned databases or from other sources.
Figure 1.
Article search and selection strategy employed in a review of studies on associations between military service, deployments, and exposures and amyotrophic lateral sclerosis (ALS) etiology and survival. The solid lines represent articles which remained in the review after each step shown, whereas the dashed lines represent articles which were excluded after each step shown. Only articles published through the end of 2013 were considered. MND, motor neuron disease.
After identifying articles that met our selection and inclusion criteria, we extracted the following information: study design, study population, sample (including size), outcome, exposure, matching or adjustment factors, statistical analysis, and results. We then categorized articles into 1) cohort studies of ALS/MND etiology, 2) case-control studies of ALS/MND etiology, 3) cross-sectional studies of ALS/MND etiology, and 4) cohort studies of ALS/MND survival.
RESULTS
We identified 567 and 405 articles by searching PubMed and Web of Science, respectively, for a total of 972 articles (Figure 1). After removing 237 duplicates, we excluded 712 additional articles for the reasons shown in Figure 1. After adding 7 articles and abstracts identified from other sources, we included a total of 30 articles and abstracts in our review (Figure 1). Two cohort studies (42, 43), 4 case-control studies (44–47), and 1 cross-sectional study (48) did not have at least 5 exposed cases or controls or did not report an estimate or P value for the association(s) of interest. We did not consider these studies informative, so we focused the remainder of our review on the other 23 articles and abstracts. For completeness, however, we present extracted information from these 7 studies in Web Table 1 (available at http://epirev.oxfordjournals.org/).
Cohort studies of ALS/MND etiology
There have been 11 cohort studies of ALS/MND etiology and military-related factors (Table 1). The association between military service overall and ALS/MND etiology has been evaluated in 2 cohort studies (41, 49). The first was a prospective study of men in the American Cancer Society's Cancer Prevention Study II cohort (41). Using information on 217 cases among 281,874 US military veterans and 63 cases among 126,414 nonveterans followed for up to 10 years, Weisskopf et al. (41) found that the ALS mortality rate among men who reported military service was 1.53 (95% confidence interval (CI): 1.12, 2.09) times that of men who did not. They also found that, compared with men who did not serve in the military, the ALS mortality rate was elevated for veterans who had served in every branch except the Marine Corps (41). The lower rate among Marines, however, was based on only 4 ALS deaths. The ALS mortality rate was increased in every quintile of years of service, but the increase was not monotonic and the linear trend test was not significant (P = 0.26). In addition, the ALS mortality rate was elevated for men who served during every time period of service evaluated, although service during the time of World War II was the only time period in which the elevated rate was statistically significant (41). Finally, the ALS mortality rate increased with increasing numbers of wars during the time of military service (Ptrend = 0.004 when the reference group, men who did not report military service, was included in the test, but Ptrend = 0.29 when it was not) (41). These results should be interpreted cautiously, however, because Weisskopf et al. did not have information on whether or not men who reported military service were actually deployed to the wars of interest (World War I, World War II, the Korean War, and the Vietnam War), so they inferred it based on time periods of service.
Table 1.
Characteristics of Cohort Studies of the Association Between Military Service, Deployments, or Exposures and Amyotrophic Lateral Sclerosis/Motor Neuron Disease Etiology Published Through the End of 2013
| First Author, Year (Reference No.) | Study Population | Sample | Outcome | Exposure | Matching or Adjustment Factors | Statistical Analysis | Results |
|---|---|---|---|---|---|---|---|
| Gale, 1999 (52) | British male former prisoners of war in Japan followed from 1952 to death or 1997 | 11,134 prisoners of war, 11 deceased MND cases | MND death rate | NA | Age | SMR and PMR (using the entire English and Welsh male populations as the standard for both statistics) | 1952–1965: SMR = 1.33 (95% CI: 0.16, 4.82); n = 2 deceased cases |
| 1966–1980: SMR = 0.91 (95% CI: 0.30, 2.12); n = 5 deceased cases | |||||||
| 1981–1997: SMR = 0.38 (95% CI: 0.10, 1.00); n = 4 deceased cases | |||||||
| 1952–1997: SMR = 0.62 (95% CI: 0.31, 1.11); PMR = 0.73 (95% CI: 0.36, 1.30) | |||||||
| Smith, 2000 (40) | Active-duty Gulf Wara-era US military service personnel followed from October 1988 to July 1997 | 551,841 active-duty service personnel (6 ALS hospitalizations) deployed to the Gulf War; 1,478,704 active-duty service personnel (12 ALS hospitalizations) not deployed to the Gulf War | ALS hospitalization rate | Deployment to the Gulf War (DMDC-provided data, self-reported) | Age | Cox proportional hazards regression | Deployment: HR = 1.66 (95% CI: 0.62, 4.44) |
| Kang, 2001 (39) | Gulf War-era US military veterans followed from May 1991 to death or December 1997 | 621,902 veterans (no. of cases was not reported) deployed to the Gulf War; 746,248 veterans (no. of cases was not reported) from a stratified random sample of all US troops not deployed to the Gulf War | ALS mortality rate | Deployment to the Gulf War (DMDC-provided data, self-reported) | Age, race, marital status, branch of service, and type of service unit | Cox proportional hazards regression | Deployment: HR = 0.59 (95% CI: 0.21, 1.66) |
| Haley, 2003 (37) | Gulf War-era US military veterans followed from 1991 to 1998 | Approximately 695,000 veterans (17 cases) deployed to the Gulf War | ALS incidence rate | NA | Age | SMR (using the entire US male population as the standard) | 1991–1994: SMR = 0.94 (95% CI: 0.26, 2.41); n = 4 cases |
| 1995–1998: SMR = 2.27 (95% CI: 1.27, 3.88); n = 13 cases | |||||||
| Horner, 2003 (38) | Gulf War-era US military veterans (active-duty or activated Reserve and National Guard personnel) followed for 10 years beginning in August 1990 | 696,118 veterans (40 cases) deployed to the Gulf War; 1,786,215 veterans (67 cases) not deployed to the Gulf War | ALS incidence rate | Deployment to the Gulf War (DMDC-provided data, self-reported) | Age | RR and 95% CI | Deployment: RR = 1.92 (95% CI: 1.29, 2.84) |
| Coffman, 2005 (36) | Gulf War-era US military veterans (active-duty or activated Reserve and National Guard personnel) followed for 10 years beginning in August 1990 | 696,118 veterans (40 cases) deployed to the Gulf War; 1,786,215 veterans (67 cases) not deployed to the Gulf War | ALS incidence rate | Deployment to the Gulf War (DMDC-provided data, self-reported) | Case under-ascertainment and age | Capture-recapture methods: log-linear models, sample coverage approach, ecological models; RR and 95% CI | Deployment: RR = 1.77 (lower 95% confidence limit: 1.21); n = 42 deployed cases, n = 76 nondeployed cases |
| Weisskopf, 2005 (41) | ACS CPS II males followed from 1989 to death or 1998 | 281,874 US military veterans (217 cases); 126,414 nonveterans (63 cases) | ALS mortality rate | Military service (veteran status); branch, no. of years, and time period of service; no. of wars during period of service (inferred) | Age, smoking, education, alcohol intake, self-reported exposure to pesticides and herbicides, and main lifetime occupation as a farmer, in a job involving electrical work or welding, or in food preparation | Cox proportional hazards regression | Military service: HR = 1.53 (95% CI: 1.12, 2.09); result was largely independent of branch and no. of years and time period of service; increasing ALS mortality rates with increasing numbers of wars during period of service (inferred) |
| Horner, 2008 (51) | Gulf War-era US military veterans (active-duty or activated Reserve and National Guard personnel) followed from 1991 to death or 2001 | 696,118 veterans (48 cases) deployed to the Gulf War; 1,786,215 veterans (76 cases) not deployed to the Gulf War | ALS incidence rate | Deployment to the Gulf War (DMDC-provided data, self-reported) | Age | SIR (using the nondeployed group as the standard for the deployed group and a group of men in western Washington State (78) as the standard for the nondeployed group), χ2 for homogeneity, χ2 for trend | Deployed: SIR peaked in 1996 at approximately 1.5 and then dropped to nearly 0 in 2001 (P for homogeneity = 0.0003, P for trend = 0.74) |
| Nondeployed: SIR was fairly constant between 0.25 and 0.50 from 1991 to 2001 (P for homogeneity = 0.0002, P for trend = 0.48) | |||||||
| Miranda, 2008 (53) | US military veterans deployed to the Gulf War who were followed from 1991 to death or 2001 | 41 cases | ALS incidence rate | Spatial risk, branch of service, potential exposure to chemical warfare agents in and around Khamisiyah, Iraq | Spatial risk, branch of service, and potential exposure to chemical warfare agents in and around Khamisiyah | Bayesian Poisson regression | Spatial risk was highest for a location about 500 km southeast of Khamisiyah (posterior probability of ALS = 0.91, IRR = 2.01) |
| Branch of service—Army: IRR = 1.0 (referent); Air Force: IRR = 1.7 (95% CrI: 0.6, 4.1); Marine Corps: IRR = 0.2 (95% CrI: 0.03, 0.9); Navy: IRR = 0.3 (95% CrI: 0.06, 1.1) | |||||||
| Khamisiyah—IRR = 1.7, 95% CrI: 0.7, 3.7 | |||||||
| Barth, 2009 (50) | Gulf War-era US military veterans followed from May 1991 to death or 2004 | 621,902 veterans (23 cases) deployed to the Gulf War; 746,248 veterans (38 cases) from a stratified random sample of all US troops not deployed to the Gulf War | ALS mortality rate | DMDC-provided data on deployment to the Gulf War; potential exposure to nerve agents at Khamisiyah, Iraq; potential exposure to oil-well fire smoke | Age, sex, race, marital status, branch of service, and type of service unit; analyses were stratified by sex | Cox proportional hazards regression | Deployment—all veterans: HR = 0.96 (95% CI: 0.56, 1.62); males: HR = 0.91 (95% CI: 0.53, 1.56); females: NA (n = 1 deceased case) |
| Khamisiyah—no association (data not shown) | |||||||
| Oil-well fire smoke—no association (data not shown) | |||||||
| Drouet, 2010 [abstract] (49) | French military personnel identified from the French National Military Health Care Fund, 1991–2007 | 69 male ALS cases, 4 female ALS cases | ALS incidence rate | Branch of service, duration of active duty | Stratified by sex; stratified by age and sex | Incidence rate | Incidence rate among male active-duty military personnel (1.7/100,000 population per year in 2007) was lower than that in the general population but higher in the age groups 40–44 years and 50–54 years (1.9/100,000 population per year and 5.1/100,000 population per year, respectively, in 2007) |
Abbreviations: ACS, American Cancer Society; ALS, amyotrophic lateral sclerosis; CI, confidence interval; CPS II, Cancer Prevention Study II; CrI, credible interval; DMDC, Defense Manpower Data Center; HR, hazard ratio; IRR, incidence rate ratio; MND, motor neuron disease; NA, not applicable/available; PMR, proportional mortality ratio; RR, risk ratio; SIR, standardized incidence ratio; SMR, standardized morbidity/mortality ratio.
a The 1990–1991 Persian Gulf War.
The second study that considered military service overall in relation to ALS/MND etiology was a retrospective cohort study of French military personnel, including 69 male ALS cases and 4 female ALS cases, identified from the French National Military Health Care Fund (1991–2007) (49). The overall ALS incidence rate among male active-duty military personnel, 1.7/100,000 population per year in 2007, was lower than that in the general population, but age-specific rates were higher in the age groups 40–44 years and 50–54 years (1.9/100,000 population per year and 5.1/100,000 population per year, respectively, in 2007) (49).
Military deployment to the Gulf War has been evaluated in relation to ALS/MND etiology in 7 cohort studies (36–40, 50, 51) (Table 1). The first of these used US Department of Defense Defense Manpower Data Center (DMDC) deployment information for US active-duty service personnel and found that 551,841 personnel deployed to the Gulf War were 1.66 (95% CI: 0.62, 4.44) times as likely to experience an ALS hospitalization during 9 years of follow-up as 1,478,704 nondeployed personnel who served in the military during the same era (40). This result, however, was based on only 6 ALS hospitalizations among deployed personnel and 12 ALS hospitalizations among nondeployed personnel (40). In the second study, which also used DMDC deployment information, Kang and Bullman (39) reported that, during 7 years of follow-up, 621,902 US military veterans deployed to the Gulf War had an ALS mortality rate 0.59 (95% CI: 0.21, 1.66) times that of a stratified random sample of 746,248 veterans who were serving in the military at that time but were not deployed to the Gulf War. The analysis was repeated after an additional 6 years of follow-up, and the updated hazard ratio, based on 23 deployed cases and 38 nondeployed cases, was 0.96 (95% CI: 0.56, 1.62) (50). Although information on military rank, branch of service, deployment date, and type of service unit was available, associations between these factors and ALS mortality rates were not reported in either analysis (39, 50).
Observed and expected incidence rates of ALS by age 45 years were compared in another study of 695,000 US veterans of the Gulf War followed for 8 years (37). Military service and deployment to the Gulf War were confirmed via a nonmilitary online database. Age-adjusted standardized morbidity ratios, using the entire US male population as the standard, were calculated for 2 separate time intervals to allow a lag from exposure to onset of ALS symptoms. For 1991–1994, based on 4 confirmed cases, the age-adjusted standardized morbidity ratio was 0.94 (95% CI: 0.26, 2.41) (37). The age-adjusted standardized morbidity ratio for 1995–1998, based on 13 confirmed cases, increased to 2.27 (95% CI: 1.27, 3.88). In a study of cumulative incidence rates of ALS over a 10-year period which used DMDC deployment information, Horner et al. (38) found that 696,118 veterans deployed to the Gulf War were 1.92 (95% CI: 1.29, 2.84) times as likely to be diagnosed with ALS as 1,786,215 veterans who were not deployed but served in the military during the same era. Although information was available, associations between ALS rates and branch of service or type of service unit were not reported in that study. In a follow-up study of the same cohort, Coffman et al. (36) aimed to evaluate the effect on the estimated risk ratio of probable underascertainment of ALS cases among veterans who were not deployed to the Gulf War. The underascertainment-corrected risk ratio decreased slightly to 1.77 (lower 95% confidence limit: 1.21) (36). In a second follow-up study of the same cohort, Horner et al. (51) reported that deployment to the Gulf War was associated with increased ALS incidence only during the first 10 years after the war and that the incidence rate was highest in 1996.
Associations between specific military exposures and ALS/MND etiology have been evaluated in 3 cohort studies (50, 52, 53) (Table 1), one of which analyzed past experience as a prisoner of war. In that study, Gale et al. (52) used records from the United Kingdom War Pensions Agency to form a cohort of 11,134 British men who were prisoners of war in Japan and then followed them for 46 years. Using the entire English and Welsh male populations as the standard, they found that the standardized mortality ratio for MND death was 0.62 (95% CI: 0.31, 1.11) and the proportional mortality ratio was 0.73 (95% CI: 0.36, 1.30) (52). In a spatial analysis of the deployment locations (obtained from the DMDC) of 41 ALS cases who were Gulf War veterans, Miranda et al. (53) reported that a location about 500 km southeast of Khamisiyah, Iraq, where US troops destroyed munitions warehouses, bunkers, and rockets that contained nerve agents, had the highest spatial risk of ALS (posterior probability = 0.91, incidence rate ratio = 2.01) of any location evaluated. They also reported significantly lower ALS incidence for members of the Marine Corps compared with members of the Army (incidence rate ratio = 0.2, 95% credible interval: 0.03, 0.9), but no overall test of the association between service branch and ALS incidence was reported. Veterans potentially exposed to nerve/chemical warfare agents in and around Khamisiyah, determined using a US Department of Defense dispersion model (54), were 1.7 (95% credible interval: 0.7, 3.7) times as likely as unexposed veterans to later develop ALS (53). In another study of 621,902 US military veterans deployed to the Gulf War, Barth et al. (50) used the same Department of Defense dispersion model (54) and reported that potential exposure to chemical warfare agents in and around Khamisiyah was not associated with ALS mortality after 13 years of follow-up. Barth et al. also reported no association between potential exposure to oil-well fire smoke during Gulf War deployment, determined via Department of Defense data (55), and ALS mortality (50).
Case-control studies of ALS/MND etiology
Relationships between military service and ALS/MND etiology have been evaluated in 4 case-control studies (14, 26, 56, 57) (Table 2). In a study based on the death certificates of 4,745 cases and 14,235 matched controls in Texas, White et al. (57) reported an odds ratio of 2.28 (95% CI: 1.34, 3.87) for the association between veteran status and ALS/MND death among men but an odds ratio of 1.13 (95% CI: 0.77, 1.67) for the same association among women. In a study of 95 cases and 106 controls in Boston, Massachusetts, Qureshi et al. (26) found a significant inverse association between being a self-reported male veteran and ALS (odds ratio (OR) = 0.22, 95% CI: 0.09, 0.54). They also reported a significant inverse association between being a male veteran who had performed active duty and ALS (OR = 0.23, 95% CI: 0.08, 0.62) (26). No association was found between self-reported branch of service and ALS in a study of 77 cases and 185 controls in Rome, Italy (56). In a study of 109 cases and 253 frequency-matched controls conducted in New England, Fang et al. (14) did not find an association between self-reported military service and ALS (OR = 1.2, 95% CI: 0.7, 2.4). Subgrouping study participants by branch or duration of service or wartime service did not reveal notable variation in the association between military service and ALS (14).
Table 2.
Characteristics of Case-Control Studies of the Association Between Military Service, Deployments, or Exposures and Amyotrophic Lateral Sclerosis/Motor Neuron Disease Etiology Published Through the End of 2013
| First Author, Year (Reference No.) | Study Population | Sample | Outcome | Exposure | Matching or Adjustment Factors | Statistical Analysis | Results |
|---|---|---|---|---|---|---|---|
| Kurtzke, 1980 (59) | Male US World War II veterans, 1963–1967 | 504 deceased cases; 504 randomly selected, living controls | ALS death | Branch of service, arm of service within the Army, decorations and awards, highest rank attained, duration of service, location of any service overseas, campaign credits, whether service extended to Korean conflict, immunizations during military service, military occupational specialties, whether a prisoner of war | Year of birth, date of entry into military service, and branch of service | Direct comparison of counts; continuity-corrected χ2, matched-pair analysis, 2 × r χ2 | All P's > 0.05 |
| Kondo, 1981A (58) | Japan, 1965–1966 | 458 deceased male cases, 254 deceased female cases; 216 living male controls (husbands of female cases), 421 living female controls (wives of male cases) | MND death | War prisoner outside of Japan | Sex | RR | Males: RR = 0.94, P > 0.05; females: RR = 1.00, P > 0.05 |
| Kondo, 1981B (58) | Japan, 1973 | 104 male cases, 54 female cases; 104 male controls (unrelated healthy persons or hospital patients), 54 female controls (unrelated healthy persons or hospital patients) | MND | War prisoner outside of Japan | Individually matched on age, sex, and residence | RR | Males: RR = 0.96, P > 0.05; females: NA |
| Nicolson, 2002 (61) | United States, Great Britain, Australia, Netherlands | 8 Gulf Wara veteran cases; 28 civilian cases, 70 civilian controls | ALS | Systemic mycoplasmal infections in blood (Mycoplasma penetrans, Mycoplasma fermentans, Mycoplasma hominis, Mycoplasma pneumoniae) | Age and sex | Percentages, P values (test not specified) | Any infection—Gulf War veteran cases: 100%; civilian cases: 79%; civilian controls: 9% (P < 0.001 for all cases vs. controls) |
| Mycoplasma fermentans—Gulf War veteran cases: 88%; civilian cases: 46%; civilian controls: 3% (P < 0.001 for all cases vs. controls) | |||||||
| White, 2002 [abstract] (57) | Texas deaths, 1979–2000 | 4,745 deceased cases; 14,235 deceased controls | ALS/MND death | Veteran status | Age group, year of death, and sex | Conditional logistic regression | Males: OR = 2.28 (95% CI: 1.34, 3.87); females: OR = 1.13 (95% CI: 0.77, 1.67) |
| Qureshi, 2006 (26) | Neuromuscular clinic at Massachusetts General Hospital in Boston, Massachusetts, April 1998–August 2002 | 95 cases; 106 controls (51 non- blood-related relatives of cases, 55 friends or unrelated persons) |
ALS | Male veteran, male veteran who performed active duty | Unrelated controls matched on age; adjusted for age and sex | Multiple logistic regression | Male veterans: OR = 0.22 (95% CI: 0.09, 0.54); male veterans who performed active duty: OR = 0.23 (95% CI: 0.08, 0.62) |
| Binazzi, 2009 (56) | Neurology clinics in Rome, Italy, January 2005–July 2006 | 77 cases; 185 controls (healthy relatives or persons accompanying non-ALS outpatients at the clinics) | ALS | Military service (Army, Aviation [Air Force], Navy) | Age and sex | Mantel-Haenszel OR | No association (data not shown) |
| Fang, 2009 (14) | Cases from 2 referral centers (Tufts-New England Medical Center and Brigham and Women's Hospital) in Boston, Massachusetts; controls from random telephone screening, 1993–1996 | 109 ALS cases, 253 controls | ALS | Ever serving in military, branch of service, duration of service, wartime service | Frequency-matched on age, sex, and region of residence within New England; adjusted for age, sex, region of residence within New England, smoking history, and educational level; stratified by smoking history | Multiple logistic regression | Ever serving in military: OR = 1.2 (95% CI: 0.7, 2.4); no clear variation in ORs when persons who had ever served in the military were subgrouped by branch or duration of service or wartime service |
| Kwee, 2009 [abstract] (60) | US military veterans; cases enrolled beginning in April 2003, controls enrolled beginning in January 2006 | 1,165 veteran cases; 530 randomly selected veteran controls | ALS | Interactions between Vietnam deployment (as a crude surrogate for organophosphate exposure) and 12 single-nucleotide polymorphisms in the PON gene cluster | Controls frequency- matched to some cases on age and use of the VA system for health care; no adjustment factors mentioned |
Logistic regression | Interaction terms not significant (data not shown) |
Abbreviations: ALS, amyotrophic lateral sclerosis; CI, confidence interval; MND, motor neuron disease; NA, not available; OR, odds ratio; PON, paraoxonase gene; RR, risk ratio; VA, Department of Veterans Affairs.
a The 1990–1991 Persian Gulf War.
Other military-related factors have been evaluated in relation to ALS/MND etiology in 5 case-control studies (58–61) (Table 2). In a study of 504 deceased US World War II veteran cases and 504 matched living controls conducted in 1963–1967, no significant associations (P > 0.05) were found between ALS death and branch of service, arm of service within the Army, decorations and awards, highest rank attained, duration of service, location of any service overseas, campaign credits, whether service extended to the Korean conflict, immunizations during military service, military occupational specialties, or whether the veteran had been a prisoner of war (data on all of which were obtained from military records) (59). In 2 studies of Japanese veterans, Kondo and Tsubaki (58) reported that being a self-reported war prisoner outside of Japan was not associated with MND (for males, risk ratio = 0.96; P > 0.05) or MND death (for males, risk ratio = 0.94 (P > 0.05); for females, risk ratio = 1.00 (P > 0.05)); 1 study was conducted during 1965–1966 and included 712 MND deaths and 637 matched living controls, and the other was conducted in 1973 and included 158 MND cases and 158 individually matched controls. In a study of 8 Gulf War-veteran ALS cases, 28 civilian cases, and 70 matched civilian controls from the United States, Great Britain, Australia, and the Netherlands, Nicolson et al. (61) reported that ALS cases (veterans and civilians together) were more likely to have evidence of systemic mycoplasmal infections (especially Mycoplasma fermentans) in their blood than civilian controls (83% vs. 9%; P < 0.001) and that the occurrence of infections was higher among the 8 ALS cases who were Gulf War veterans than in the 28 civilian cases (100% vs. 79%). Finally, in a study of 1,165 US military veteran cases and 530 veteran controls, 12 single-nucleotide polymorphisms in the paraoxonase (PON) gene cluster did not modify the association between deployment to the Vietnam War (used as a surrogate for organophosphate exposure) and ALS (60).
Cross-sectional studies of ALS/MND etiology
The association between military service overall and ALS/MND etiology has been evaluated in 1 cross-sectional study (62) (Table 3). This study used information from death certificates for 1982–1991 from 27 US states and found a positive association (proportionate mortality ratio = 1.88; P < 0.05) between occupation as military personnel and MND death among persons aged 15–55 years (62).
Table 3.
Characteristics of a Cross-Sectional Study of the Association Between Military Service and Amyotrophic Lateral Sclerosis/Motor Neuron Disease Etiology Published Through the End of 2013
| First Author, Year (Reference No.) | Study Population | Sample | Outcome | Exposure | Matching or Adjustment Factors | Statistical Analysis | Results |
|---|---|---|---|---|---|---|---|
| Schulte, 1996 (62) | 27 US states, 1982–1991 | 9,435 deceased MND cases; 130,420 total neurodegenerative disease deaths | MND death | Occupation as military personnel | Age-standardized; stratified by sex and race | PMR | Ages 15–55 years: PMR = 188, P < 0.05; n = 27 deceased cases |
Abbreviations: ALS, amyotrophic lateral sclerosis; MND, motor neuron disease; PMR, proportionate mortality ratio.
Cohort studies of ALS/MND survival
Relationships between military service or deployment and ALS/MND survival or progression have been evaluated in 4 cohort studies (24, 26, 63, 64) (Table 4). In a study of 961 veteran ALS cases and 2,571 nonveteran cases in the Clinical Assessment, Research and Education Program database, a nonmilitary database of ALS patients from North America (65), Brooks et al. (63) reported no relationship between veteran status and ALS survival. In a study of 95 cases in Boston, Massachusetts, Qureshi et al. (26) reported that after 1 year of follow-up, both being a male veteran and being a male veteran who had performed active duty were associated with faster disease progression, measured by the ALS Functional Rating Scale (27), and with declining respiratory function, measured by predicted percentage of forced vital capacity. Another study found that ALS survival, defined as the time between onset of symptoms and death, the beginning of ventilation, or the end of follow-up (October 2002), was significantly shorter (hazard ratio = 0.62, 95% CI: 0.40, 0.96) among 43 US veterans deployed to the Gulf War (median survival time, 40.2 months), defined using DMDC data, than among 66 veterans who were not deployed but served in the military during the same era (median survival time, 57.0 months) (64). Finally, in a study of 1,063 US military-veteran ALS cases, 603 of whom had died or begun using ventilation by October 2006, Pastula et al. (24) reported that DMDC-defined deployment to the Vietnam War was associated with shorter survival after ALS diagnosis (hazard ratio = 1.73, 95% CI: 1.36, 2.19), but ALS survival was not associated with cumulative years or branch of military service or with deployment to the Korean War or the Gulf War.
Table 4.
Characteristics of Cohort Studies of the Association Between Military Service or Deployments and Amyotrophic Lateral Sclerosis/Motor Neuron Disease Survival Published Through the End of 2013
| First Author, Year (Reference No.) | Study Population | Sample | Outcome | Exposure | Matching or Adjustment Factors | Statistical Analysis | Results |
|---|---|---|---|---|---|---|---|
| Brooks, 2003 [abstract] (63) | ALS CARE database, beginning in 1996 | 946 male veteran cases, 15 female veteran cases; 1,154 male nonveteran cases, 1,417 female nonveteran cases (no. of deaths not specified) | ALS survival | Veteran status | Not specified, but investigated age, sex, region affected and disease course, comorbid conditions, modes of treatment, site of treatment, and caregiver status | Stepwise multiple logistic regression | Decreased survival among veterans, but decrease disappeared after adjustment for age (data not shown) |
| Qureshi, 2006 (26) | Neuromuscular clinic at Massachusetts General Hospital in Boston, Massachusetts, followed for 1 year after enrollment (April 1998–August 2002) | 95 cases | ALSFRS, predicted %FVC | Male veteran, male veteran who performed active duty | Sex | Random slope regression | Male veterans—change in ALSFRS: β = −0.02 (SE, 0.01), P = 0.13; change in predicted %FVC: β = −0.07 (SE, 0.03), P = 0.04 |
| Male veterans who performed active duty—change in ALSFRS: β = −0.02 (SE, 0.01), P = 0.03; change in predicted %FVC: β = −0.08 (SE, 0.04), P = 0.04 | |||||||
| Kasarskis, 2009 (64) | Gulf Wara-era US military veterans (active-duty or activated Reserve and National Guard personnel) with ALS followed from 1991 to death or October 2002 | 43 cases deployed to the Gulf War; 66 cases not deployed to the Gulf War (no. of deaths not specified) | ALS survival (from onset of symptoms to death or ventilation) | Deployment to the Gulf War (DMDC- provided data, self-reported) | Age, site of onset | Cox proportional hazards regression | Median survival time—deployed: 40.2 months; nondeployed: 57.0 months; HR = 0.62 (95% CI: 0.40, 0.96) |
| Pastula, 2009 (24) | US military veterans with ALS followed from April 2003 to death or October 2006 | 1,063 veteran cases (603 dead or on ventilation) | ALS survival (from diagnosis to death or ventilation) | Cumulative military service (years); branch of service; deployment to Korean War, Vietnam War, or Gulf War | Age; race; site of onset; ALS type; time from onset of symptoms to diagnosis; history of myocardial infarction, stroke/cerebrovascular disease, diabetes, cancer, or a major traumatic injury; cigarette smoking; cumulative military service; branch of service; and deployment to Korean War, Vietnam War, or Gulf War | Cox proportional hazards regression | Cumulative military service (per 10-year increase): HR = 1.03 (95% CI: 0.91, 1.17) |
| Branch of service—Air Force: HR = 1.00 (referent); Army: HR = 1.08 (95% CI: 0.84, 1.41); Coast Guard: HR = 2.14 (95% CI: 0.92, 4.98); Marines: HR = 1.26 (95% CI: 0.91, 2.03); Navy: HR = 1.24 (95% CI: 0.93, 1.66) | |||||||
| Deployment location—Korean War: HR = 0.98 (95% CI: 0.74, 1.30); Vietnam War: HR = 1.73 (95% CI: 1.36, 2.19); Gulf War: HR = 0.92 (95% CI: 0.57, 1.50) |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS, ALS Functional Rating Scale; CARE, Clinical Assessment, Research and Education Program; CI, confidence interval; DMDC, Defense Manpower Data Center; %FVC, percentage of forced vital capacity; HR, hazard ratio; MND, motor neuron disease; SE, standard error.
a The 1990–1991 Persian Gulf War.
DISCUSSION
The IOM report (34) concluded that there were suggestive, though not conclusive, findings that ALS rates may be higher among US military veterans, currently numbering more than 21 million, compared with nonveterans (41), particularly among those deployed to the Gulf War (36–38, 40). We conducted a review of the relevant literature, incorporating new research published since the IOM report. Our review focused on studies of associations between military service, deployments, and exposures and ALS/MND etiology and survival.
Associations between military service-related factors and ALS/MND etiology were evaluated in 3 cohort studies (41, 49, 53), 5 case-control studies (14, 26, 56, 57, 59), and 1 cross-sectional study (62). Six of these studies (2 cohort, 3 case-control, and 1 cross-sectional) evaluated military service overall and ALS/MND etiology; 3 reported positive associations (41, 57, 62), 1 reported a null association (14), and 2 reported inverse associations (26, 49). These differences may be explained in part by limitations of study design, such as control selection, statistical power, and sources of information on exposure and outcome. For example, one of the case-control studies that found an inverse association, conducted from April 1998 to August 2002 in Boston, used non-blood-related relatives of the cases (e.g., spouses), friends who accompanied the cases to the clinics, or unrelated persons as controls (26). These controls were probably not representative of the exposure distribution in the source population giving rise to the cases, potentially biasing the results. For example, the proportions of veterans among cases (mean age = 54 years) and controls (mean age = 53 years) were 8% and 24%, respectively (26). However, only 6% of Boston residents aged 18 years or more were US military veterans in 2000 (4% of residents aged 18–64 years and 20% of residents aged 65 years or more were veterans) (66).
The cohort study that observed an inverse association overall found positive associations with ALS incidence in the age groups 40–44 years and 50–54 years (49). That study identified military personnel from the French National Military Health Care Fund, so military personnel who retired, left the military for other reasons, or obtained health care elsewhere were not included in the database (49). The study that found a null association (OR = 1.2, 95% CI: 0.7, 2.4) was based on 109 ALS cases, only 24 of whom had ever served in the military (14), so perhaps the weak positive association was not statistically significant because of low power. For comparison, 2 of the 3 studies that found positive associations had 217 (41) and 1,476 (57) exposed cases, respectively, although the third had only 27 exposed cases (62). Two of the studies with positive associations ascertained information on occupation from death certificates (57, 62), which is likely to have resulted in nondifferential misclassification and bias toward the null (67, 68). Furthermore, one of those studies found a positive association with MND mortality only after restricting the analysis to persons aged 15–55 years (62). Results of the other study (57) were published only in abstract form, which makes it difficult to evaluate the robustness of the reported findings.
Use of ALS/MND mortality as a surrogate for incidence was a problem common to all 3 studies reporting positive associations with military service (41, 57, 62). This could have resulted in nondifferential misclassification and bias toward the null if military service was not related to survival, or in differential misclassification and bias either toward the null or away from the null if it was. In a recent review of ALS mortality studies, Marin et al. (69) stated that ALS/MND mortality can accurately reflect trends in incidence. This statement was based on 2 high-quality studies (70, 71), but neither study investigated the possible occurrence of bias when ALS/MND mortality is used to estimate measures of association between exposures and incidence, such as rate differences or rate ratios. Therefore, while it is suggestive of a positive association, the current literature is too limited to draw conclusions regarding a possible association between military service and ALS/MND etiology. If such an association exists, it may be age-specific (49, 62), but it does not appear to vary by branch, duration, or time period of service (14, 41, 53, 56, 59).
Relationships between military deployment-related factors and ALS/MND etiology were evaluated in 8 cohort studies (36–41, 50, 51) and 2 case-control studies (14, 59). Deployment to the Gulf War and ALS/MND etiology was evaluated in 7 of the cohort studies. Five of these studies reported positive associations (36–38, 40, 51) and 2 did not (39, 50). Two studies (37, 40) that found positive associations, however, were based on small numbers of exposed cases. One of these studies (37) had additional limitations (e.g., military service was confirmed via a nonmilitary online database, expected incidence rates were calculated in an unorthodox manner). The remaining 5 studies arose from 2 different VA research groups. The first of these groups published 3 studies that found a positive association between Gulf War deployment and ALS incidence (36, 38, 51). The other group published 2 studies that used ALS mortality data and found no association (39, 50). Other features of the studies conducted by the 2 groups, such as design, population, and exposure assessment, were similar (36, 38, 39, 50, 51). A limitation of the first study by the group using incidence data (38) was potential underascertainment of cases among veterans not deployed to the Gulf War (36). The second study by this group used capture-recapture methods to estimate the number of missing cases among the nondeployed veterans and to correct the original analysis for this potential underascertainment (36). The new results were slightly attenuated relative to the first, but the positive association remained (36). Studies by the group using mortality data (39, 50) were limited by low statistical power; these studies had 43% fewer cases than the incidence studies. In addition, using mortality instead of incidence data could have resulted in differential or nondifferential misclassification and bias toward or away from the null (see above). Therefore, the truth probably lies somewhere in between the results of the 2 VA research groups: There may be a moderate association between Gulf War deployment and ALS/MND etiology, but it is probably not as strong as that reported in the 3 positive studies (36, 38, 51). No association with any other military deployment or deployment-related factor is apparent from published literature (14, 41, 59).
Associations between several military exposures and ALS/MND etiology were considered in 3 cohort studies (50, 52, 53) and 5 case-control studies (58–61). Nevertheless, there is currently not strong evidence that any particular military exposure is associated with ALS/MND etiology. The higher prevalence of systemic mycoplasmal infections among ALS cases (veterans and civilians together) relative to civilian controls and the higher prevalence of these infections among Gulf War veteran ALS cases compared with civilian cases (61) needs to be confirmed in other nonmilitary populations with larger sample sizes.
Two cohort studies of associations between military service and ALS/MND survival or progression found disparate results: One reported no association with survival (63) and the other reported a significant association with faster progression (26). The results of the first study (63), however, were published in abstract form only, which makes it difficult to evaluate the robustness of the reported findings. The study that used progression as the outcome did not adjust for age at diagnosis (26), which is one of the most consistently reported prognostic factors for ALS survival (16–24). Two other cohort studies of the association between Gulf War deployment and ALS survival found conflicting results: One found an elevated hazard ratio (64) and the other found no association (24). Both of these studies were plagued by immortal person-time bias, which arises when follow-up time is counted as person-time at risk for an outcome even though that time occurs before the last event required to enter the risk set (72, 73). A likely explanation for the discrepancy between the results of the 2 studies is that the first study—which found a positive association—was restricted to Gulf War-era veterans (64), whereas the other—with no association—included non-Gulf War-era veterans in the comparison group (24). The latter also reported a positive association between Vietnam War deployment and ALS survival (24). Clearly, associations between military service or deployment and ALS survival need to be evaluated in other populations before conclusions can be drawn.
Some overall conclusions can be made regarding limitations of previous studies of military service, deployments, and exposures and ALS etiology and survival. First, ALS mortality was used as the outcome in 8 of 21 (38%) etiology studies (39, 41, 50, 52, 57–59, 62), which makes it difficult to determine whether reported associations were associations with ALS incidence, survival after ALS diagnosis, or a mixture of the two (see above). Second, only 4 of 25 (16%) studies were formal survival analyses (24, 26, 63, 64), but immortal person-time bias was probably present in at least 2 of them (24, 64), and 1 other study (63) did not provide enough information to make this determination. Third, important clinical information, such as date of diagnosis, site of onset, respiratory function, etc., was used to subgroup ALS cases in only 2 of 21 (10%) etiology studies (26, 56). Fourth, 18 of 25 (72%) studies had 124 or fewer ALS cases (14, 26, 36–40, 49–53, 56, 58, 61, 64), and the number of exposed cases was 48 or less in all but 1 of these studies (it had 73) (49), so power to detect associations between military-related factors and ALS etiology or survival was often limited. Fifth, associations between ALS etiology or survival and specific aspects of military service or specific deployments or exposures were usually not evaluated (because the relevant information was not collected) or were evaluated in a limited way. For example, only 9 of 25 (36%) studies evaluated associations between more than 1 military-related factor and ALS etiology or survival (14, 24, 26, 41, 49, 50, 53, 59), and only 1 of these studies examined more than 5 military-related factors (it examined 11) (59). In addition, exposure metrics were often vague or defined differently among studies. Furthermore, information on exposure gradient was used to evaluate exposure-response relationships for at least 1 exposure in only 5 of 25 (20%) studies (14, 24, 41, 49, 59). Clearly, studies that address the limitations of previous studies of military service, deployments, and exposures and ALS etiology and survival are needed. Studies that evaluate associations between extensive, hypothesis-driven lists of factors related to military service, deployments, and exposures and ALS etiology or survival would be particularly valuable.
As pointed out in the IOM report (34), studies conducted in nonmilitary populations may provide clues regarding factors that may be more generally associated with ALS etiology (or survival) and are also likely to be related to military service. For example, the IOM report lists intensive physical activity, lifestyle factors, trauma, transmissible agents, occupational toxicants, and environmental toxicants as potential risk factors for ALS etiology that are likely to be experienced by military populations (34).
The request for the IOM to review the 6 cohort studies published between 2000 and 2005 (36–41) was initiated by the VA (34). In addition, the VA established a National Registry of Veterans with ALS (hereafter called the Registry) (74, 75), which enrolled approximately 2,000 living veteran cases from April 2003 to September 2007. The Registry (74, 75) was established with the goal of identifying and enrolling all living US military veterans with ALS, following their health and disease progression over time, collecting information that could be used for epidemiologic studies of ALS, and providing a way to inform veterans with ALS about clinical trials for which they might be eligible (74, 76).
Soon after veterans with ALS began to enroll in the Registry, researchers at Duke University (Durham, North Carolina), in collaboration with the VA, began enrolling cases from the Registry into the Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis (GENEVA) Study, a case-control study of 630 cases from the Registry and 975 controls identified via VA databases and frequency-matched to cases on age and use of the VA health-care system (76). The goal of the GENEVA Study is to evaluate associations between ALS and environmental factors, genetic susceptibility, and their interactions (76).
Several of the limitations of previous studies of military service, deployments, and exposures and ALS etiology and survival outlined above can be addressed using data from the GENEVA Study. Specifically, the GENEVA Study can be used for both etiological and survival research because of the following features. Living cases were identified via the Registry and ALS diagnoses were confirmed via medical record review (76). Important and high-quality clinical information, such as date of diagnosis, site of onset, respiratory function, etc., is also available. In addition, controls were identified via VA databases and frequency-matched to cases on age and use of the VA health-care system (76). Information on the vital status of ALS cases is current through July 2013. Formal survival analyses, with the appropriate mitigation of immortal person-time bias, can be conducted. Thus, associations between military-related factors and ALS etiology or survival can be evaluated more carefully than in previous studies. In addition, having 630 ALS cases makes the GENEVA Study one of the largest studies of military-related factors and ALS etiology or survival ever conducted and will provide better statistical power for evaluating associations of interest. Finally, the GENEVA Study investigators collected extensive information on various aspects of military service and deployments and on more than 30 specific exposures related to military service (76), including pesticides, chemicals, paint, gas, diesel fuel, solvents, ionizing and nonionizing radiation, vaccines, and riot control substances, which was not available in any previous study of military exposures and ALS etiology or survival. Other studies that address the limitations of previous studies of military service, deployments, and exposures and ALS etiology and survival are also needed.
The GENEVA Study may also have some limitations. Recall bias may be possible and may be differential by war of service, because the GENEVA Study interview occurred up to 65 years after participants served in the military (74, 76). There seems to be little evidence, however, that cases with various diseases and controls recall past exposures differentially (77). Another possible limitation of the GENEVA Study and possibly other case-control studies of ALS, especially register- or population-based ones, is potential selection bias arising from nonparticipation of potential controls and the fact that the patients with the most quickly progressing disease probably died before they could enroll. Therefore, investigators analyzing the GENEVA Study data will need to evaluate and address these and other potential sources of bias.
In conclusion, although the current literature suggests a positive association between military service and ALS/MND etiology, it is too limited to make definitive statements regarding the presence of possible associations between military service and ALS/MND etiology or survival. An association between Gulf War deployment and ALS/MND etiology is possible. There is not currently strong evidence that any particular military exposure is a risk factor for ALS/MND. Future studies of military service, deployments, and exposures and ALS etiology and survival should address limitations of previous ones, such as reliance on mortality as a surrogate for incidence, a dearth of survival analyses, lack of clinical data, low statistical power, and limited exposure assessment. The GENEVA Study provides one such attempt, but other, additional studies are clearly needed to determine whether military-related factors are associated with ALS and to assess potential prevention strategies.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (John D. Beard); and Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (John D. Beard, Freya Kamel).
This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences (grant Z01 ES049005). J.D.B. was also supported by training grants from the National Institute of Environmental Health Sciences (grant T32ES007018) and the National Institute for Occupational Safety and Health (grant T42OH00867302).
Conflict of interest: none declared
REFERENCES
- 1.Armon C. Amyotrophic lateral sclerosis. In: Nelson LM, Tanner CM, Van Den Eeden SK, et al., editors. Neuroepidemiology: From Principles to Practice. New York, NY: Oxford University Press; 2004. pp. 162–187. [Google Scholar]
- 2.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 3.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics, Centers for Disease Control and Prevention. About underlying cause of death, 1999–2011. http://wonder.cdc.gov/ucd-icd10.html. Accessed July 22, 2014.
- 5.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;3:CD001447. doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng HX, Chen W, Hong ST, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477(7363):211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 9.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 10.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vance C, Rogelj B, Hortobágyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009;73(20):1693–1698. doi: 10.1212/WNL.0b013e3181c1df48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cronin S, Hardiman O, Traynor BJ. Ethnic variation in the incidence of ALS: a systematic review. Neurology. 2007;68(13):1002–1007. doi: 10.1212/01.wnl.0000258551.96893.6f. [DOI] [PubMed] [Google Scholar]
- 14.Fang F, Quinlan P, Ye W, et al. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117(9):1387–1392. doi: 10.1289/ehp.0900580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire V, Longstreth WT, Jr, Nelson LM, et al. Occupational exposures and amyotrophic lateral sclerosis: a population-based case-control study. Am J Epidemiol. 1997;145(12):1076–1088. doi: 10.1093/oxfordjournals.aje.a009070. [DOI] [PubMed] [Google Scholar]
- 16.Alonso A, Logroscino G, Jick SS, et al. Association of smoking with amyotrophic lateral sclerosis risk and survival in men and women: a prospective study. BMC Neurol. 2010;10:6. doi: 10.1186/1471-2377-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiò A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10(5-6):310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czaplinski A, Yen AA, Appel SH. Amyotrophic lateral sclerosis: early predictors of prolonged survival. J Neurol. 2006;253(11):1428–1436. doi: 10.1007/s00415-006-0226-8. [DOI] [PubMed] [Google Scholar]
- 19.Czaplinski A, Yen AA, Simpson EP, et al. Predictability of disease progression in amyotrophic lateral sclerosis. Muscle Nerve. 2006;34(6):702–708. doi: 10.1002/mus.20658. [DOI] [PubMed] [Google Scholar]
- 20.Gil J, Preux PM, Alioum A, et al. Disease progression and survival in ALS: first multi-state model approach. Amyotroph Lateral Scler. 2007;8(4):224–229. doi: 10.1080/17482960701278562. [DOI] [PubMed] [Google Scholar]
- 21.Gordon PH, Cheng B, Salachas F, et al. Progression in ALS is not linear but is curvilinear. J Neurol. 2010;257(10):1713–1717. doi: 10.1007/s00415-010-5609-1. [DOI] [PubMed] [Google Scholar]
- 22.Mandrioli J, Faglioni P, Nichelli P, et al. Amyotrophic lateral sclerosis: prognostic indicators of survival. Amyotroph Lateral Scler. 2006;7(4):211–220. doi: 10.1080/17482960600947648. [DOI] [PubMed] [Google Scholar]
- 23.Paillisse C, Lacomblez L, Dib M, et al. Prognostic factors for survival in amyotrophic lateral sclerosis patients treated with riluzole. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(1):37–44. doi: 10.1080/14660820510027035. [DOI] [PubMed] [Google Scholar]
- 24.Pastula DM, Coffman CJ, Allen KD, et al. Factors associated with survival in the National Registry of Veterans with ALS. Amyotroph Lateral Scler. 2009;10(5-6):332–338. doi: 10.3109/17482960802320545. [DOI] [PubMed] [Google Scholar]
- 25.Ganesalingam J, Stahl D, Wijesekera L, et al. Latent cluster analysis of ALS phenotypes identifies prognostically differing groups. PLoS One. 2009;4(9):e7107. doi: 10.1371/journal.pone.0007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi MM, Hayden D, Urbinelli L, et al. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS) Amyotroph Lateral Scler. 2006;7(3):173–182. doi: 10.1080/14660820600640596. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann P, Levy G, Thompson JL, et al. The ALSFRSr predicts survival time in an ALS clinic population. Neurology. 2005;64(1):38–43. doi: 10.1212/01.WNL.0000148648.38313.64. [DOI] [PubMed] [Google Scholar]
- 28.Süssmuth SD, Sperfeld AD, Hinz A, et al. CSF glial markers correlate with survival in amyotrophic lateral sclerosis. Neurology. 2010;74(12):982–987. doi: 10.1212/WNL.0b013e3181d5dc3b. [DOI] [PubMed] [Google Scholar]
- 29.Rauchway AC, Kaiboriboon K, Bansal SK, et al. A role for functional classification in the early identification of prognostic factors in ALS. Amyotroph Lateral Scler. 2007;8(4):214–216. doi: 10.1080/08037060701318234. [DOI] [PubMed] [Google Scholar]
- 30.Bureau of the Census, US Department of Commerce. Washington, DC: Bureau of the Census 2012; 2012. American Community Survey 1-year estimates. Veteran status http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_12_1YR_S2101&prodType=table. Accessed July 22, 2014. [Google Scholar]
- 31.National Center for Veterans Analysis and Statistics, US Department of Veterans Affairs. National Survey of Veterans, Active Duty Service Members, Demobilized National Guard and Reserve Members, Family Members, and Surviving Spouses. Rockville, MD: Westat, Inc.; 2010. [Google Scholar]
- 32.Hernandez LM, Durch JS, Blazer DG 2nd, et al. Institute of Medicine. Gulf War Veterans: Measuring Health. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 33.Hoerster KD, Lehavot K, Simpson T, et al. Health and health behavior differences: U.S. military, veteran, and civilian men. Am J Prev Med. 2012;43(5):483–489. doi: 10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 34.Institute of Medicine of the National Academies. Amyotrophic Lateral Sclerosis in Veterans: Review of the Scientific Literature. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 35.Institute of Medicine of the National Academies. Washington, DC: Institute of Medicine of the National Academies; 2014. About reports. http://www.iom.edu/Reports.aspx. Updated July 18, 2014. Accessed March 6, 2014. [Google Scholar]
- 36.Coffman CJ, Horner RD, Grambow SC, et al. Estimating the occurrence of amyotrophic lateral sclerosis among Gulf War (1990–1991) veterans using capture-recapture methods. Neuroepidemiology. 2005;24(3):141–150. doi: 10.1159/000083297. [DOI] [PubMed] [Google Scholar]
- 37.Haley RW. Excess incidence of ALS in young Gulf War veterans. Neurology. 2003;61(6):750–756. doi: 10.1212/wnl.61.6.750. [DOI] [PubMed] [Google Scholar]
- 38.Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742–749. doi: 10.1212/01.wnl.0000069922.32557.ca. [DOI] [PubMed] [Google Scholar]
- 39.Kang HK, Bullman TA. Mortality among US veterans of the Persian Gulf War: 7-year follow-up. Am J Epidemiol. 2001;154(5):399–405. doi: 10.1093/aje/154.5.399. [DOI] [PubMed] [Google Scholar]
- 40.Smith TC, Gray GC, Knoke JD. Is systemic lupus erythematosus, amyotrophic lateral sclerosis, or fibromyalgia associated with Persian Gulf War service? An examination of Department of Defense hospitalization data. Am J Epidemiol. 2000;151(11):1053–1059. doi: 10.1093/oxfordjournals.aje.a010147. [DOI] [PubMed] [Google Scholar]
- 41.Weisskopf MG, O'Reilly EJ, McCullough ML, et al. Prospective study of military service and mortality from ALS. Neurology. 2005;64(1):32–37. doi: 10.1212/01.WNL.0000148649.17706.D9. [DOI] [PubMed] [Google Scholar]
- 42.Gibberd FB, Simmonds JP. Neurological disease in ex-Far-East prisoners of war. Lancet. 1980;2(8186):135–137. doi: 10.1016/s0140-6736(80)90015-x. [DOI] [PubMed] [Google Scholar]
- 43.Thomas TL, Kang HK. Mortality and morbidity among Army Chemical Corps Vietnam veterans: a preliminary report. Am J Ind Med. 1990;18(6):665–673. doi: 10.1002/ajim.4700180605. [DOI] [PubMed] [Google Scholar]
- 44.Chió A, Meineri P, Tribolo A, et al. Risk factors in motor neuron disease: a case-control study. Neuroepidemiology. 1991;10(4):174–184. doi: 10.1159/000110267. [DOI] [PubMed] [Google Scholar]
- 45.Gresham LS, Molgaard CA, Golbeck AL, et al. Amyotrophic lateral sclerosis and occupational heavy metal exposure: a case-control study. Neuroepidemiology. 1986;5(1):29–38. doi: 10.1159/000110810. [DOI] [PubMed] [Google Scholar]
- 46.Gunnarsson LG, Lindberg G, Söderfeldt B, et al. Amyotrophic lateral sclerosis in Sweden in relation to occupation. Acta Neurol Scand. 1991;83(6):394–398. doi: 10.1111/j.1600-0404.1991.tb03970.x. [DOI] [PubMed] [Google Scholar]
- 47.Sutedja NA, Veldink JH, Fischer K, et al. Lifetime occupation, education, smoking, and risk of ALS. Neurology. 2007;69(15):1508–1514. doi: 10.1212/01.wnl.0000277463.87361.8c. [DOI] [PubMed] [Google Scholar]
- 48.Holloway SM, Mitchell JD. Motor neurone disease in the Lothian Region of Scotland 1961–81. J Epidemiol Community Health. 1986;40(4):344–350. doi: 10.1136/jech.40.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drouet A, Desjeux G, Balaire C, et al. Retrospective study of ALS in French military personnel. Rev Neurol (Paris) 2010;166(6-7):621–629. doi: 10.1016/j.neurol.2010.01.004. [abstract] [DOI] [PubMed] [Google Scholar]
- 50.Barth SK, Kang HK, Bullman TA, et al. Neurological mortality among U.S. veterans of the Persian Gulf War: 13-year follow-up. Am J Ind Med. 2009;52(9):663–670. doi: 10.1002/ajim.20718. [DOI] [PubMed] [Google Scholar]
- 51.Horner RD, Grambow SC, Coffman CJ, et al. Amyotrophic lateral sclerosis among 1991 Gulf War veterans: evidence for a time-limited outbreak. Neuroepidemiology. 2008;31(1):28–32. doi: 10.1159/000136648. [DOI] [PubMed] [Google Scholar]
- 52.Gale CR, Braidwood EA, Winter PD, et al. Mortality from Parkinson's disease and other causes in men who were prisoners of war in the Far East. Lancet. 1999;354(9196):2116–2118. doi: 10.1016/S0140-6736(99)06264-9. [DOI] [PubMed] [Google Scholar]
- 53.Miranda ML, Overstreet Galeano MA, Tassone E, et al. Spatial analysis of the etiology of amyotrophic lateral sclerosis among 1991 Gulf War veterans. Neurotoxicology. 2008;29(6):964–970. doi: 10.1016/j.neuro.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Winkenwerder W., Jr . Washington, DC: Office of the Assistant Secretary of Defense (Health Affairs) and Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illnesses, Medical Readiness, and Military Deployments, US Department of Defense; 2002. US demolition operations at Khamisiyah: final report. [Google Scholar]
- 55.Rostker B. Washington, DC: Office of the Special Assistant to the Deputy Secretary of Defense for Gulf War Illnesses, US Department of Defense; 2000. Environmental exposure report: oil well fires. [Google Scholar]
- 56.Binazzi A, Belli S, Uccelli R, et al. An exploratory case-control study on spinal and bulbar forms of amyotrophic lateral sclerosis in the province of Rome. Amyotroph Lateral Scler. 2009;10(5-6):361–369. doi: 10.3109/17482960802382313. [DOI] [PubMed] [Google Scholar]
- 57.White DL, Harati Y, del Junco D, et al. ALS/MND and veterans: are vets at increased risk? J Neurol Sci. 2002;199(suppl 1):S46. [abstract] [Google Scholar]
- 58.Kondo K, Tsubaki T. Case-control studies of motor neuron disease: association with mechanical injuries. Arch Neurol. 1981;38(4):220–226. doi: 10.1001/archneur.1981.00510040046007. [DOI] [PubMed] [Google Scholar]
- 59.Kurtzke JF, Beebe GW. Epidemiology of amyotrophic lateral sclerosis: 1. A case-control comparison based on ALS deaths. Neurology. 1980;30(5):453–462. doi: 10.1212/wnl.30.5.453. [DOI] [PubMed] [Google Scholar]
- 60.Kwee LC, Allen KD, Oddone EZ, et al. Examination of paraoxonase gene cluster and VietNam deployment in amyotrophic lateral sclerosis. Neuroepidemiology. 2009;33(1):71–72. [abstract] [Google Scholar]
- 61.Nicolson GL, Nasralla MY, Haier J, et al. High frequency of systemic mycoplasmal infections in Gulf War veterans and civilians with amyotrophic lateral sclerosis (ALS) J Clin Neurosci. 2002;9(5):525–529. doi: 10.1054/jocn.2001.1075. [DOI] [PubMed] [Google Scholar]
- 62.Schulte PA, Burnett CA, Boeniger MF, et al. Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through 1991. Am J Public Health. 1996;86(9):1281–1288. doi: 10.2105/ajph.86.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brooks BR, Anderson FA, Gowda N, et al. Amyotrophic lateral sclerosis (ALS) outcomes in US military veterans compared with non-veterans: neuroepidemiological insights from the ALS CARE Database. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(suppl 1):114. [abstract] [Google Scholar]
- 64.Kasarskis EJ, Lindquist JH, Coffman CJ, et al. Clinical aspects of ALS in Gulf War veterans. Amyotroph Lateral Scler. 2009;10(1):35–41. doi: 10.1080/17482960802351029. [DOI] [PubMed] [Google Scholar]
- 65.Miller RG, Anderson FA, Jr, Bradley WG, et al. The ALS Patient Care Database: goals, design, and early results. ALS C.A.R.E. Study Group. Neurology. 2000;54(1):53–57. doi: 10.1212/wnl.54.1.53. [DOI] [PubMed] [Google Scholar]
- 66.US Census Bureau. Washington, DC: Bureau of the Census, US Department of Commerce; 2014. Census 2000 summary file 3. http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_00_SF3_P039&prodType=table. Accessed July 22, 2014. [Google Scholar]
- 67.Dick S, Semple S, Dick F, et al. Occupational titles as risk factors for Parkinson's disease. Occup Med (Lond) 2007;57(1):50–56. doi: 10.1093/occmed/kql109. [DOI] [PubMed] [Google Scholar]
- 68.McLaughlin JK, Mehl ES. A comparison of occupational data from death certificates and interviews. Am J Ind Med. 1991;20(3):335–342. doi: 10.1002/ajim.4700200306. [DOI] [PubMed] [Google Scholar]
- 69.Marin B, Couratier P, Preux PM, et al. Can mortality data be used to estimate amyotrophic lateral sclerosis incidence? Neuroepidemiology. 2011;36(1):29–38. doi: 10.1159/000321930. [DOI] [PubMed] [Google Scholar]
- 70.Dean G, Quigley M, Goldacre M. Motor neuron disease in a defined English population: estimates of incidence and mortality. J Neurol Neurosurg Psychiatry. 1994;57(4):450–454. doi: 10.1136/jnnp.57.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fong KY, Yu YL, Chan YW, et al. Motor neuron disease in Hong Kong Chinese: epidemiology and clinical picture. Neuroepidemiology. 1996;15(5):239–245. doi: 10.1159/000109913. [DOI] [PubMed] [Google Scholar]
- 72.Rothman KJ, Greenland S, Lash TL. Cohort studies. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 100–110. [Google Scholar]
- 73.Lash TL, Cole SR. Immortal person-time in studies of cancer outcomes. J Clin Oncol. 2009;27(23):e55–e56. doi: 10.1200/JCO.2009.24.1877. [DOI] [PubMed] [Google Scholar]
- 74.Allen KD, Kasarskis EJ, Bedlack RS, et al. The National Registry of Veterans with Amyotrophic Lateral Sclerosis. Neuroepidemiology. 2008;30(3):180–190. doi: 10.1159/000126910. [DOI] [PubMed] [Google Scholar]
- 75.Kasarkis EJ, Dominic K, Oddone EZ. The National Registry of Veterans with Amyotrophic Lateral Sclerosis: Department of Veterans Affairs Cooperative Studies Program (CSP) #500a. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(suppl 1):129–132. doi: 10.1080/17434470410019915. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt S, Allen KD, Loiacono VT, et al. Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis: the GENEVA Study. Rationale, study design and demographic characteristics. Neuroepidemiology. 2008;30(3):191–204. doi: 10.1159/000126911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teschke K, Olshan AF, Daniels JL, et al. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med. 2002;59(9):575–593. doi: 10.1136/oem.59.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGuire V, Longstreth WT, Jr, Koepsell TD, et al. Incidence of amyotrophic lateral sclerosis in three counties in western Washington State. Neurology. 1996;47(2):571–573. doi: 10.1212/wnl.47.2.571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.