Abstract
STUDY QUESTION
What is the effect of maternal exposure to perfluorooctane sulfonate (PFOS), perflurooctanoic acid (PFOA) and perfluorohexane sulfonate (PFHxS) on female fecundity?
SUMMARY ANSWER
Increasing concentrations of PFOA or PFHxS in maternal plasma were associated with reduced fecundability and infertility.
WHAT IS KNOWN ALREADY
Perfluorinated chemicals (PFCs) are a group of synthetic compounds used in industrial production. There is a concern about the effect of PFCs on fecundity, as measured by time-to-pregnancy (TTP). Although some recent studies suggest that increasing concentrations of PFCs may decrease fecundity, divergence in the methodological approaches used to evaluate this association have prevented firm conclusions being reached.
STUDY DESIGN, SIZE, DURATION
The Maternal-Infant Research on Environmental Chemicals (MIREC) Study is a cohort study of 2,001 women recruited before 14 weeks of gestation in 10 cities across Canada between 2008 and 2011.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A questionnaire was administered and medical chart data and biospecimens were collected from participants. After excluding women who withdrew, those for whom data were incomplete, those whose pregnancies followed birth control failure, and accounting for male fertility, 1743 participants remained. TTP was defined as the number of months of unprotected intercourse needed to become pregnant in the current pregnancy, as self-reported in the first trimester of pregnancy. Plasma concentrations of PFOA, PFOS and PFHxS measured in the first trimester were considered as a surrogate of preconception exposure. Fecundability odds ratios (FORs) were estimated using Cox proportional hazard models for discrete time. FOR < 1 denote a longer TTP and FORs >1 denote a shorter TTP. The odds of infertility (TTP > 12 months or infertility treatment in the index pregnancy) were estimated using logistic regression. Each chemical concentration (ng/ml) was log-transformed and divided by its SD.
MAIN RESULTS AND THE ROLE OF CHANCE
The cumulative probabilities of pregnancy at 1, 6 and 12 months were 0.42 (95% confidence interval (CI) 0.40–0.45), 0.81 (95% CI 0.79–0.83) and 0.90 (95% CI 0.89–0.92), respectively. The mean maternal age was 32.8 (SD 5.0) years. The geometric means (ng/ml) of PFOA, PFOS and PFHxS were 1.66 (95% CI 1.61–1.71), 4.59 (95% CI 4.46–4.72) and 1.01 (95% CI 0.97–1.05), respectively. After adjustment for potential confounders, PFOA and PFHxS were associated with a 11 and 9% reduction in fecundability per one SD increase (FOR = 0.89; 95% CI 0.83–0.94; P < 0.001 for PFOA and FOR = 0.91; 95% CI 0.86–0.97; P = 0.002 for PFHxS), while no significant association was observed for PFOS (FOR = 0.96; 95% CI 0.91–1.02; P = 0.17). In addition, the odds of infertility increased by 31% per one SD increase of PFOA (odds ratio (OR) = 1.31; 95% CI 1.11–1.53; P = 0.001) and by 27% per one SD increase of PFHxS (OR = 1.27; 95% CI 1.09–1.48; P = 0.003), while no significant association was observed for PFOS (OR = 1.14; 95% CI 0.98–1.34; P = 0.09).
LIMITATIONS, REASONS FOR CAUTION
Women with the highest concentrations of PFCs might have been excluded from the study if there is a causal association with infertility. The MIREC study did not assess concentrations of PFCs in males, semen quality, menstrual cycle characteristics or intercourse frequency.
WIDER IMPLICATIONS OF THE FINDINGS
Our results add to the evidence that exposure to PFOA and PFHxS, even at lower levels than previously reported, may reduce fecundability.
STUDY FUNDING/COMPETING INTEREST(S)
The MIREC study is supported by the Chemicals Management Plan of Health Canada, the Canadian Institutes for Health Research (CIHR, grant no. MOP – 81285) and the Ontario Ministry of the Environment. M.P.V. was supported by a CIHR Fellowship Award, and a CIHR-Quebec Training Network in Perinatal Research (QTNPR) Ph.D. scholarship. W.D.F. is supported by a CIHR Canada Research Chair. There are no conflicts of interest to declare.
Keywords: time to pregnancy, fecundity, perfluorooctane sulfonate, perflurooctanoic acid, perfluorohexane sulfonate
Introduction
Perfluorinated chemicals (PFCs) have recently received attention because of their high-volume production, ubiquitous environmental presence and possible association with adverse health effects. PFCs were introduced in 1950 and have since been widely used in the manufacture of both domestic and industrial products having applications as grease-or-water repellents and protective coatings for clothes, furniture and other products, and also as constituents of floor polish, adhesives, firefighting foam and insulation of electrical wire (Centers for Disease Control and Prevention, 2009).
The two PFCs made in the largest quantities in the USA were perflurooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) (ATSDR, 2009). A replacement for PFOA was introduced recently which resulted in PFOA no longer being used in manufacturing processes and the key producer of PFOS phased out worldwide production in 2002 (DuPont, 2013; 3M, 2014). Direct uses in Canada have largely been phased out (Health Canada, 2008)—voluntarily, by most of the manufacturers of PFOA, and by federal regulation through the Canadian Environmental Protection Act (CEPA) for PFOS (Environment Canada, 2012). Nonetheless, the production of PFOS has continued in China, and therefore, products containing its precursors may be still entering the Canadian market (Lim et al., 2011).
Although the production of several PFCs has declined during the last decade, recent national biomonitoring surveys in the USA (Centers for Disease Control and Prevention, 2009) and Canada (Health Canada, 2010; 2013) have shown that nearly all participants were found to have low levels of PFOA, PFOS and perfluorohexane sulfonate (PFHxS) in their blood. These PFCs have also been detected in cord blood (Arbuckle et al., 2013a,b) and breast milk (Fromme et al., 2010). While the primary source of exposure in the general population is food (Tittlemier et al., 2007), water is an important source in contaminated areas, for example, in communities near production facilities (Hoffman et al., 2011). In addition, PFCs have been detected in dust (Bjorklund et al., 2009) and in indoor and outdoor air (Shoeib et al., 2004; Shoeib et al., 2011).
There is a concern about the potential adverse developmental and reproductive effects of PFCs due to their persistence in the environment and long serum elimination half-lives (i.e. 3.8, 5.4 and 8.5 years for PFOA, PFOS and PFHxS, respectively) (Olsen et al., 2007). Evidence is emerging about the potential role of PFCs as endocrine-disrupting chemicals (EDCs). In vitro, some PFCs have the potential to affect estrogen-receptor and androgen-receptor transactivity (Kjeldsen and Bonefeld-Jorgensen, 2013). In rats, exposure to PFOS has shown estrous cyclicity disruption and neurotransmitter imbalance in adult females (Austin et al., 2003), decreased serum concentrations of thyroxine (T4) and triiodothyronine (T3) during pregnancy (Thibodeaux et al., 2003; Luebker et al., 2005; Chang et al., 2008), and reduced total T4 levels in pups (Lau et al., 2003; Luebker et al., 2005). In humans, PFOS concentrations in Inuit adults were negatively associated with thyroid-stimulating-hormone (TSH), total T3 and thyroxine-binding globulin (TBG) and positively with free T4 concentrations (Dallaire et al., 2009). In pregnant women, exposure to high concentrations of perfluorononanoic acid (PFNA), perfluoroundecanoic acid (PFUnDA) and perfluorododecanoic acid (PFDoDA) were associated with lower free T4 and total T4 levels (Wang et al., 2014).
Several epidemiological studies have explored the potential association between PFCs and fecundity, as measured by time-to-pregnancy (TTP). In a retrospective pregnancy-based TTP study within the Danish National Birth Cohort, Fei et al. (2009) observed a strong association between higher concentrations of PFOA and PFOS and longer TTP. In a case–control study within the Norwegian Mother and Child Cohort (MoBa), Whitworth et al. (2012) reported higher odds of subfecundity (TTP > 12 months) at increasing concentrations of PFOA and PFOS. A recent analysis of the same MoBa cohort testing different statistical approaches replicated the association between PFOA and longer TTP (Ding et al., 2014). Nonetheless, Whitworth et al. (2012), under the assumption of reverse causation suggested by Olsen et al. (2009) (i.e. parous women with longer time-to-pregnancy have higher PFCs levels because they have long interpregnancy intervals allowing re-accumulation of PFCs), found no association among nulliparous women after stratification by parity. When such stratification was used by Fei et al. (2012), they found stronger associations for PFOS and PFOA in nulliparous women and for PFOA in multiparous women. In prospective couple-based cohort designs, Vestergaard et al. (2012) observed no consistent pattern between eight PFCs and TTP in a Danish cohort, and Buck Louis et al. (2013) reported just one (perfluorooctane sulfonamide, PFOSA) of the seven PFCs assessed in the LIFE Study (Michigan and Texas) associated with decreased fecundability. Why these studies have reported conflicting results on the association between PFCs and Time-to-Pregnancy remains unclear.
The aim of the present study was to evaluate the association between selected PFCs (PFOA, PFOS and PFHxS) and TTP in the Maternal-Infant Research on Environmental Chemicals (MIREC) Study, a Canadian pregnancy and birth cohort.
Methods
Population and study design
The MIREC Study was established to examine potential adverse health effects of exposure to priority environmental chemicals on pregnancy and infant health in Canada. The cohort profile of the MIREC Study was recently published (Arbuckle et al., 2013a,b). Briefly, pregnant women from the general population who were attending early prenatal clinics from 10 cities across Canada between 2008 and 2011 were invited to participate, reaching a participation rate of 39%. Ethical considerations did not permit the collection of information on those who refused to participate. Among those who were ineligible to participate in MIREC, 52% were not planning on delivering in the participating hospitals, 20% were outside the required gestational age at recruitment and 14% were not willing to provide a sample of cord blood for the study. Some 2000 women were followed during each trimester of pregnancy, at delivery and in the early post-natal period. MIREC participants tended to smoke less (5.9 versus 10.5%), be older (mean 32.2 versus 29.4 years) and have a higher education (62.3 versus 35.1% with a university degree) than women giving birth in Canada.
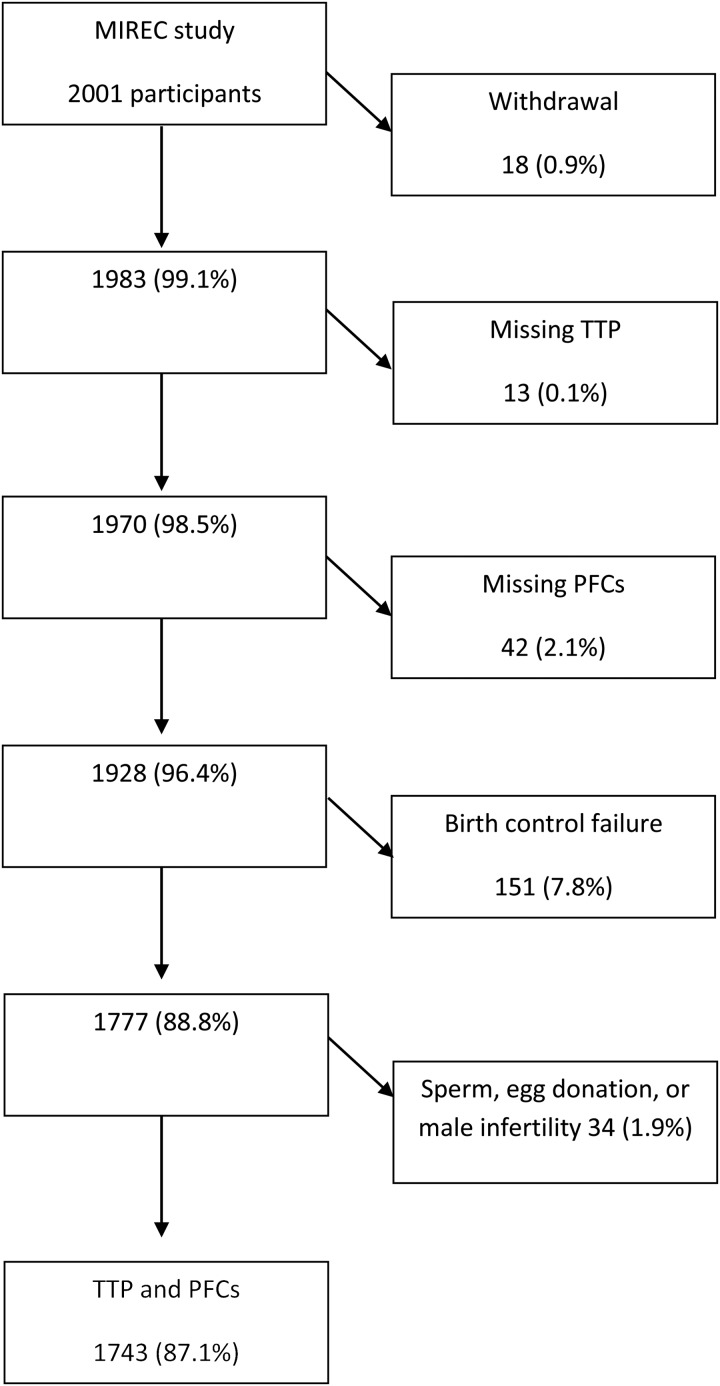
In this specific pregnancy-based retrospective TTP study, socio-demographic and lifestyle data, as well as biospecimens collected during the first trimester of pregnancy (6 to <14 weeks), from 1743 participants were analyzed. In MIREC, women were asked about the type of birth control they used before this pregnancy. If a method was indicated, women were then asked if they had stopped it before the pregnancy started or if there was a birth control failure. Women who answered that there was a birth control failure were excluded from the present analysis (Fig. 1). Sixteen patients who became pregnant with sperm donation, 3 with egg donation and 15 whose male partners required some infertility treatment were also excluded.
Figure 1.
Participant's selection flow-chart.
The MIREC study was approved by the Research Ethics Board of Health Canada, the research ethics committee of the coordinating center of Ste-Justine's Hospital in Montreal, and the academic and hospital ethics committees of the 10 study sites across Canada. All the participants signed informed consent forms.
Study variables
Time-to-pregnancy
TTP was collected as a discrete variable (i.e. number of months of unprotected intercourse before conception) by this question: ‘How long did it take you to get pregnant with this pregnancy?’ (Number of months.)
Infertility was defined as having a TTP of more than 12 months or requiring infertility treatment for this pregnancy.
PFOA, PFOS and PFHxS exposure
Maternal blood samples collected during the first trimester of pregnancy were considered a surrogate of the preconception exposure to PFOS, PFOA and PFHxS. Maternal blood was collected in 10-ml sterile vacutainer tubes. Within 2 h of the blood draw, the samples were centrifuged and the plasma aliquoted into smaller cryovials to be stored at −80°C until analysis.
Chemical analyses were carried out by the toxicology laboratory of the Institut national de santé publique du Québec (INSPQ), https://www.inspq.qc.ca/ctq/, which is accredited by the Standards Council of Canada under ISO 17025 and CAN-P-43. The analytes were extracted at alkaline pH with methyl tertbutyl ether, and ion-pairing done with tetrabutylammonium hydrogensulfate, evaporated to dryness and dissolved in the mobile phase. Samples were analyzed with a Waters Acquity ultra performance liquid chromatography—tandem mass spectrometer (Milford, MA, USA) operated in the multiple reaction monitoring mode with an electrospray ion source in negative mode. The between-assay coefficients of variation for the assays were PFOS, PFOA and PFHxS were 3.6, 5.8 and 10%, respectively.
Statistical analysis
Descriptive statistics, including the percentage detected, medians and geometric means, were computed for all chemicals, and arithmetic means were found for important demographic variables. Concentrations below the limit of detection (LOD) were set to the LOD divided by 2. Each chemical concentration (ng/ml) was log-transformed to achieve normality and then divided by its SD so that the measure of effect could be interpreted per 1 − SD change in the log-transformed chemical concentration (Buck Louis et al., 2013).
Fecundity odds ratios (FOR) were estimated using the Cox model, modified for discrete-time data (Allison, 2010). FORs estimate the odds of becoming pregnant each cycle, given exposure to the specific PFC, conditional on not being pregnant in the previous cycle. FORs <1 denote reduction in fecundity or longer TTP, and FORs >1 denote a shorter TTP. TTP was censored at the 13th month. The proportional hazard assumptions were verified for the discrete-time models (Allison, 2010). In addition, we used logistic regression to estimate the odds ratios (ORs) for infertility (TTP > 12 months or infertility treatment). Statistical significance was assessed using an alpha level of 0.05.
Potential confounders included gestational age at blood draw (as an indicator of length of recall), maternal age, country of birth, education, household income, maternal and paternal smoking, and pre-pregnancy BMI. Maternal and paternal age were highly correlated (r = 0.73), which precluded inclusion of paternal age in the model. However, we conducted a sensitivity analysis by including the difference between paternal and maternal age as a covariate. Variables with a P-value of <0.20 in the univariate analysis were potentially eligible for the multivariate model.
There is controversy on how to consider parity in the assessment of the toxicological effect of PFCs on TTP. Biologically, parity is influenced by a woman's fecundability. TTP is used as an epidemiological metric for the assessment of women's fecundability. Thus, we consider that conditioning (i.e. adjusting, stratifying, or restricting) on parity is redundant and would cause overadjustment, as parity is the result, among other factors, of proven fecundability. Based on the modern theory of diagrams for causal inference using Directed Acyclic Graphs (DAGs), as described in Howards et al. (2012), we assume that E (exposure) is PFC, A is fecundability, S1 is the previous TTP, and that S2 is the current TTP (Fig. 1A in Howards et al. (2012)). In a sense, S1 is a proxy for parity and for fecundability. Adjusting for it is like imperfectly adjusting for an intermediate, and could cause overadjustment bias (Schisterman et al., 2009). Furthermore, assuming that Us is an unmeasured risk factor for the previous TTP (S1) and the current TTP (S2) (Fig. 1B in Howards et al. (2012)), then S1 is a collider on a path from E to S2. Conditioning on a collider is susceptible to collider-stratification bias (Greenland, 2003; Schisterman et al., 2009). It would open the blocked backdoor path between exposure and outcome (Howards et al., 2012).
In addition to DAGs, there is also toxicokinetic data to support the exclusion of parity in models for fecundity-related outcomes (Buck Louis et al., 2012). PFCs are not lipophilic and while transfer during pregnancy and lactation has been reported in animal models (Loccisano et al., 2013) and humans (Fromme et al., 2010; Arbuckle et al., 2013a,b), the magnitude of change is minimal compared with lipophilic compounds. Moreover, daily exposure to PFCs via diet has been documented (Fromme et al., 2009), supporting the continual exposure for women including pregnant women.
Statistical analysis was performed using STATA 10.0 (Stata Corporation, College Station, TX, USA) and SAS 9.3 (SAS Institute Inc., Cary, NC, USA), specifically for the discrete-time Cox proportional models.
Results
The cumulative probabilities of pregnancy for the cohort at months 1, 6 and 12 were 0.42 (95% confidence interval (CI) 0.40–0.45), 0.81 (95% CI 0.79–0.83) and 0.90 (95% CI 0.89–0.92), respectively. Socio-demographic characteristics of the population and their association with TTP are presented in Table I. The mean maternal age was 32.8 (SD 5.0) years. About two-thirds of the women had a university degree, most were born in Canada, more than one-third reported a household income higher than $100 000 CAD, half had at least one prior pregnancy with a live birth and about 15% were obese or active smokers during the preconception period. Women in the higher income categories were older and reported a higher proportion of infertility treatment for the index pregnancy. Maternal and paternal age, pre-pregnancy BMI and parity were associated with TTP. Maternal or paternal active smoking, gestational age at which the sample was collected, country of birth, household income and education were not associated with TTP.
Table I.
Characteristics of the study population and association with TTP: the Maternal-Infant Research on Environmental Chemicals (MIREC) study.
| Continuous variables | n | Mean (SD)a | Median | Minimum | Maximum | P value** |
|---|---|---|---|---|---|---|
| Maternal age (years) | 1743 | 32.83 (4.96) | 32.74 | 18.34 | 46.35 | <0.001 |
| Paternal age (years) | 1510 | 34.78 (5.68) | 34.51 | 18.66 | 58.74 | <0.001 |
| Age difference (years) | 1510 | 1.80 (3.98) | 1.00 | −15 | 26 | 0.18 |
| Gestational age (weeks) | 1741 | 12 (1.52) | 12.43 | 6 | 14 | 0.19 |
| Categorical variables | n | % |
TTP (months) |
|||
| Mean (SD)a | Median (p25–p75) | |||||
| Education | ||||||
| Some college or less | 236 | 13.5 | 5.8 (12.8) | 1 (1–5) | 0.46 | |
| College diploma | 399 | 22.9 | 5.2 (8.9) | 2 (1–5) | ||
| Undergraduate | 644 | 37.0 | 5.7 (12.2) | 2 (1–5) | ||
| Graduate (MSc PhD) | 464 | 26.6 | 4.3 (6.4) | 2 (1–4) | ||
| Country of birth | ||||||
| Canada | 1412 | 81.0 | 5.3 (10.8) | 2 (1–5) | 0.93 | |
| USA | 27 | 1.5 | 4.7 (6.4) | 2 (1–6) | ||
| Mexico | 8 | 0.5 | 5.1 (4.8) | 4.5 (1–9) | ||
| China | 17 | 1.0 | 3.8 (4.8) | 1 (1–5) | ||
| Other | 279 | 16.0 | 5.1 (8.4) | 2 (1–6) | ||
| Household income | ||||||
| <$60 000 | 361 | 20.7 | 4.5 (10.1) | 2 (1–4) | 0.06 | |
| $60 001–100 000 | 609 | 35.0 | 5.2 (9.9) | 2 (1–5) | ||
| >$100 000 | 691 | 39.6 | 5.7 (10.7) | 2 (1–6) | ||
| No response | 82 | 4.7 | 5.2 (10.9) | 2 (1–4) | ||
| Parity conditional on gravidity | ||||||
| No prior pregnancy | 501 | 28.8 | 6.5 (13.7) | 2 (1–6) | <0.001 | |
| Prior pregnancy without live birth(s) | 270 | 15.5 | 6.8 (12.1) | 2 (1–6) | ||
| Prior pregnancy with live birth(s) | 971 | 55.7 | 4.2 (7.2) | 2 (1–4) | ||
| Maternal smoking | ||||||
| Never | 1077 | 62.9 | 5.1 (8.9) | 2 (1–5) | 0.85 | |
| Former | 392 | 22.5 | 5.3 (11.8) | 2 (1–5) | ||
| Currentb | 272 | 15.6 | 5.7 (13.1) | 2 (1–4) | ||
| Pre-pregnancy BMI (kg/m2) | ||||||
| <24.9 | 1034 | 63.6 | 4.9 (9.6) | 2 (1–5) | 0.003 | |
| 25–29.9 | 355 | 21.9 | 5.2 (11.4) | 2 (1–4) | ||
| >30 | 236 | 14.5 | 6.7 (11.3) | 3 (1–7) | ||
| Current paternal smoking | ||||||
| No | 1217 | 83.0 | 5.2 (9.9) | 2 (1–5) | 0.83 | |
| Yes | 249 | 17.0 | 5.6 (12.0) | 2 (1–5) | ||
BMI, body mass index.
aArithmetic mean and SD.
bWomen who stopped during pregnancy or 1 year before.
**P-values for the association with TTP: likelihood ratio for continuous variables, log-rank test for categorical variables.
Table II presents the distribution of PFC concentrations in maternal plasma. PFOA, PFOS and PFHxS were detected in at least 95% of the samples. The geometric means (ng/ml) of PFOA, PFOS and PFHxS were 1.66 (95% CI 1.61–1.71), 4.59 (95% CI 4.46–4.72) and 1.01 (95% CI 0.97–1.05), respectively.
Table II.
Perfluoroalkyl acid levels (ng/ml) in maternal plasma (n = 1743).
| LOD | n (%) <LOD | Median | Minimum | Maximum | GM (95% CI) | |
|---|---|---|---|---|---|---|
| PFOA | 0.1 | 2 (0.12) | 1.7 | <LOD | 16 | 1.66 (1.61–1.71) |
| PFOS | 0.3 | 2 (0.12) | 4.7 | <LOD | 36 | 4.59 (4.46–4.72) |
| PFHxS | 0.2 | 69 (4.28) | 1 | <LOD | 25 | 1.01 (0.97–1.05) |
LOD, limit of detection; GM, geometric mean; CI, confidence interval; PFOA, perflurooctanoic acid; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonate.
Crude FORs per one SD increase in log-transformed serum concentrations were significantly lower for PFOA 0.91 (95% CI 0.86–0.96) and PFHxS 0.94 (95% CI 0.89–1.00), while there was no statistically significant association with PFOS 0.97 (95% CI 0.92–1.03) (Table III). Fecundability decreased by 4% per 1 year increase in maternal age (FOR = 0.96; 95% CI 0.95–0.97). Obesity (BMI >30 kg/m2) was associated with a 25% reduction in fecundability (FOR = 0.75; CI 95% 0.63–0.90), and annual household income >$100 000 CAD with a 21% decrease (FOR = 0.79; CI 95% 0.67–0.93). Education and smoking status were not associated with fecundability reduction.
Table III.
Crude fecundity odds ratios (FOR) for TTP.
| n | Crude FORs | 95% CI | P valueb | |
|---|---|---|---|---|
| PFOAa, ng/ml | 1743 | 0.91 | 0.86–0.96 | 0.001 |
| PFOSa, ng/ml | 1743 | 0.97 | 0.92–1.03 | 0.33 |
| PFHxSa, ng/ml | 1743 | 0.94 | 0.89–1.00c | 0.04 |
| Age | 1743 | 0.96 | 0.95–0.97 | <0.001 |
| Gestational age | 1743 | 0.98 | 0.94–1.01 | 0.18 |
| Pre-pregnancy BMI (kg/m2) | 0.04 | |||
| <24.9 | 1034 | 1 | ||
| 25–29.9 | 355 | 1.03 | 0.89–1.20 | |
| >30 | 236 | 0.75 | 0.63–0.90 | |
| Education | 0.68 | |||
| Graduate (MSc PhD) | 464 | 1 | ||
| Undergraduate | 644 | 0.95 | 0.82–1.10 | |
| College diploma | 399 | 0.91 | 0.77–1.08 | |
| Some college or less | 236 | 0.97 | 0.82–1.21 | |
| Household income | ||||
| <60 000 | 361 | 1 | 0.04 | |
| 60 000–100 000 | 609 | 0.86 | 0.73–1.01 | |
| >100 000 | 691 | 0.79 | 0.67–0.93 | |
| No response | 82 | 0.89 | 0.65–1.20 | |
| Maternal smoking | 0.63 | |||
| Never | 1077 | 1 | ||
| Former | 392 | 1.05 | 0.91–1.21 | |
| Currentd | 272 | 1.08 | 0.91–1.27 | |
| Current paternal smoking | 0.68 | |||
| No | 1217 | 1 | ||
| Yes | 249 | 1.05 | 0.91–1.21 |
PFOA, perflurooctanoic acid; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonate.
aChemical plasma concentrations were log-transformed and divided by their SDs.
bWald PHREG procedure.
cAfter rounding.
dThose who quit during pregnancy or <1 year prior to the study visit.
In models adjusted for maternal age and BMI, PFOA and PFHxS were associated with an 11% (FOR = 0.89; 95% CI 0.83–0.94) and 9% (FOR = 0.91; 95% CI 0.86–0.97) reduction in fecundability per one SD increase in log-transformed serum concentrations, respectively; no significant association was observed for PFOS (FOR = 0.96; 95% CI 0.91–1.02) (Table IV). When added to the model with the other variables, household income was no longer significant.
Table IV.
Adjusted FORs for TTP (n = 1625)a.
| Adjusted FORs | 95% CI | P valueb | |
|---|---|---|---|
| PFOA (ng/ml) | 0.89 | 0.83–0.94 | <0.001 |
| Age | 0.96 | 0.94–0.97 | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 0.001 | ||
| <24.9 | 1 | ||
| 25–29.9 | 1.03 | 0.89–1.20 | |
| >30 | 0.71 | 0.60–0.86 | |
| PFOS (ng/ml) | 0.96 | 0.91–1.02 | 0.17 |
| Age | 0.96 | 0.95–0.97 | <0.001 |
| Pre-pregnancy BMI (kg/m2) | <0.001 | ||
| <24.9 | 1 | ||
| 25–29.9 | 1.03 | 0.89–1.20 | |
| >30 | 0.72 | 0.60–0.86 | |
| PFHxS (ng/ml) | 0.91 | 0.86–0.97 | 0.002 |
| Age | 0.96 | 0.94–0.97 | <0.001 |
| Pre-pregnancy BMI (kg/m2) | <0.001 | ||
| <24.9 | 1 | ||
| 25–29.9 | 1.05 | 0.90–1.22 | |
| >30 | 0.73 | 0.61–0.87 |
Chemical plasma concentrations were log-transformed and divided by their SDs.
PFOA, perflurooctanoic acid; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonate.
a1625 women (118 missing values for pre-pregnancy weight).
bWald χ2 test PHREG procedure.
The adjusted odds of infertility (TTP > 12 months or infertility treatment to become pregnant) increased by 31% per one SD increase of PFOA (OR = 1.31; 95% CI 1.11–1.53) and by 27% per one SD increase of PFHxS (OR = 1.27; 95% CI 1.09–1.48). No significant association was observed between concentrations of PFOS and the odds of infertility (odds ratio (OR) = 1.14; 95% CI 0.98–1.34) (Table V).
Table V.
Adjusted odd ratios for infertility (n = 1625)a.
| Odds ratios | 95% CI | P value | |
|---|---|---|---|
| PFOA (ng/ml) | 1.31 | 1.11–1.53 | 0.001 |
| PFOS (ng/ml) | 1.14 | 0.98–1.34 | 0.09 |
| PFHxS (ng/ml) | 1.27 | 1.09–1.48 | 0.003 |
Chemical plasma concentrations were log-transformed and divided by their SDs.
PFOA, perflurooctanoic acid; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonate.
a1625 women (118 missing values for pre-pregnancy weight).
Including the difference between paternal and maternal age resulted in very similar results for all the models presented. However, due to missing information on paternal age, the sample is reduced from 1625 to 1419 women. For this reason, our final models did not include the age difference, a variable that was also not significant in the univariate analysis (Table I).
Discussion
The MIREC study is the largest cohort of pregnant women to date measuring the concentrations of PFOA, PFOS and PFHxS in plasma samples collected during the first trimester of pregnancy. The participation rate of 39% in the MIREC study is consistent with participation rates of several large prospective cohort studies. There is evidence from similar pregnancy cohorts that this level of participation does not increase the risk of bias (Nohr et al., 2006). The time-frame of the study, from 2008 to 2011, reflects current levels of exposure to these chemicals, especially after the move to reduce and phase out several PFCs over the last decade. We observed that increased concentrations of PFOA and PFHxS were associated with decreased fecundability as measured by a longer TTP, and increased odds of infertility, even at lower levels of exposure than previously documented. The median concentrations in our participants (1.7 ng/ml for PFOA, and 4.7 ng/ml for PFOS) were lower than those reported for women in the Danish National Birth Cohort conducted between 1996 and 2002 (5.3 ng/ml for PFOA and 33.7 ng/ml for PFOS) (Fei et al., 2009), the Norwegian Mother and Child Cohort Study conducted between 2003 and 2004 (2.2 ng/ml for PFOA and 13.0 ng/ml for PFOS) (Whitworth et al., 2012), and a Danish study conducted among trade union workers between 1992 and 1995 (5.6 ng/ml for PFOA and 36.3 ng/ml for PFOS) (Vestergaard et al., 2012). Also, our geometric mean concentrations (1.66 ng/ml; 95% CI 1.61–1.71 for PFOA and 4.59 ng/ml; 95% CI 4.46–4.72 for PFOS) were lower than those reported in the LIFE study conducted in Texas and Michigan between 2005 and 2007 (3.11 ng/ml; 95% CI 2.91–3.33 for PFOA and 11.76 ng/ml; 95% CI 11.01–12.57 for PFOS) (Buck Louis et al., 2013).
Two previous studies have evaluated the effect of PFOA and PFOS on TTP using a retrospective pregnancy TTP design. Our results are in agreement with those on PFOA reported by Fei et al. (2009) in a subset of 1400 women randomly selected from the Danish National Birth Cohort; however, we did not find an association between TTP and PFOS, which might be explained by the differences in exposure. Indeed, while our median PFOA level was 1/3 of those reported by Fei et al. (2009), our median PFOS was 1/14 their level. The covariates included in our models were similar, except for the consideration of parity as a confounder by Fei et al. (2009).
A second study, a case–control analysis within the Norwegian Mother and Child Cohort, reported an increased odds of subfecundity (TTP > 12 months) with elevated PFOA and PFOS levels in a model (not adjusted for parity) (Whitworth et al., 2012). A recent analysis of the MoBa cohort, using the discrete-time Cox proportional hazard model, also reported diminished fecundability at increasing concentrations of PFOA (FOR 0.83; 95% CI 0.75–0.91) (Ding et al., 2014). In the study by Whitworth et al. (2012), however, the authors considered that reverse causation could account for their reported association in parous women (Olsen et al., 2009). Thus, women with longer TTP would have higher PFCs levels due to only a longer time since their previous pregnancy, allowing re-accumulation of the PFCs that had decreased during pregnancy and post-partum through placenta transfer and breastfeeding. Based on this assumption, they reported their final conclusion according to a second model, stratifying by parity. In this model, the ORs for subfecundity were elevated only among parous women, which, according to the authors, support the reverse causation hypothesis (Whitworth et al., 2012). However, these results were not replicated by Fei et al. (2012) after stratification by parity, where stronger associations were reported for PFOS and PFOA in nulliparous women, and for PFOA in the case of multiparous women, thus refuting reverse causation.
In agreement with other authors, we considered that neither adjustment nor stratification for parity should be conducted when studying the reproductive adverse effects of PFCs, as this will introduce overadjustment through collider-stratification bias (Greenland, 2003; Schisterman et al., 2009; Sallmen et al., 2015). To describe this type of bias, Greenland (2003) used the example of the effect of exposure to an unopposed estrogen therapy (E) and endometrial cancer (D), stratifying (conditioning) on uterine bleeding (C). It was common practice to assess the effect of estrogen therapy on endometrial cancer only among women presenting with uterine bleeding, as a marker of estrogen use and cancer. However, it was demonstrated that the measure of effect among these women would be much smaller than the true causal association because of the strong effects of both the therapy and the cancer on bleeding risk, and therefore, the bias induced by stratification on bleeding was toward the null (Greenland, 2003). The same bias would be introduced if the association of PFCs (E) and TTP (D) were conditioned on parity (C).
Besides collider-stratification bias, there are also toxicokinetic data to support the exclusion of parity in our models (Buck Louis et al., 2012). Compared with lipophilic compounds, the magnitude of changes for PFCs during pregnancy and lactation appear minimal, as indicated by the relatively small changes in maternal serum concentrations during pregnancy or through 6 months post-partum reported in a pregnancy cohort study (Fromme et al., 2010). In addition, PFCs have the capacity to bind to serum albumin (Han et al., 2003), which may account for breast milk concentrations being ∼1000 times lower than blood concentrations (Fromme et al., 2010; Kato et al., 2011). Furthermore, independently of parity, women are continuously exposed to PFCs, not only due to the long half-lives of these chemicals, but also through an estimated daily uptake of 2–3 ng/kg of PFOS and PFOA, with 90% coming from dietary sources (Fromme et al., 2009).
In addition, we included women in our study who had had fertility treatment for the index pregnancy, i.e. 7.3% of the study population, as was also done by Fei et al. (2009). In our descriptive phase of analysis, we found that the geometric mean levels of PFOA and PFHxS were significantly higher in participants receiving fertility treatment for the index pregnancy than those of untreated participants, and this was maintained after age-adjustment (data not shown). We also noted that half of these patients reported a TTP < 12 months, even if these treatments (i.e. ovulation induction, intrauterine insemination and IVF) are rarely prescribed before 12 months of attempting pregnancy. Our hypothesis is that some of the women reported their TTP as the number of months since their first cycle of fertility treatment until the successful cycle, some considered the number of months of trying before starting fertility treatment, and others counted both. Moreover, if they had used fertility treatment for a previous pregnancy and therefore were able to access it for this pregnancy without having to wait during 12 months of trying, the reported TTP would probably be <12 months. In Whitworth et al. (2012) cases were randomly selected from women who reported a TTP > 12 months but not according to fertility treatment, likely leading to the exclusion of some potential cases that received fertility treatment but reported a TTP < 12 months.
Two additional studies have addressed the association between PFCs and TTP using a prospective couple-based cohort design. In a survey conducted among trade union workers in Denmark from 1992 to 1995, no consistent pattern was observed between TTP and eight PFCs, including PFOA, PFOS, PFHxS and PFOSA, measured in the serum of 222 women attempting pregnancy for the first time, followed for up to six cycles of trying (Vestergaard et al., 2012). More recently, Buck Louis et al. (2013) reported the results from the LIFE study, including biomonitoring data from 501 couples followed for upto 12 months of attempting during 2005 and 2007. Decreased fecundability was observed at higher concentrations of PFOSA in females in the adjusted model; however, no association was found for the other 6 PFCs assessed, including PFOA and PFOS in their unadjusted analysis. PFOSA was not measured in the MIREC study. Detection of this chemical is currently very low in the USA (Centers for Disease Control and Prevention, 2009) due to its phasing-out since 2002 (3M, 2014). In fact, PFOSA was not detected in 90% of the samples from the LIFE study (Buck Louis et al., 2013).
Some methodological aspects of our study need to be considered. First, in pregnancy-based TTP, infertile couples who do not opt for fertility treatment or have no access to it and those whose fertility treatment is unsuccessful are excluded from the study, resulting in the systematic underrepresentation of infertile women and the selection of a healthier population (Joffe et al., 2005). Nonetheless, pregnancy-based TTP studies have been successful in identifying environmental exposures that may adversely affect fertility, as it is expected that at current levels of exposure, the toxic effect of these contaminants do not lead to complete infertility (Weinberg and Wilcox, 2008). Secondly, TTP was assessed at a mean gestational age of 12 weeks (SD 1.52) in MIREC, similar to the gestational age at which TTP was assessed by Fei et al. (2009), but earlier than in Whitworth et al. (2012), which was at ∼17 weeks of gestation. Studies that have assessed the validity of this recall have reported reasonable validity if collected in the short-term (Zielhuis et al., 1992; Cooney et al., 2009). Thirdly, we used the first trimester concentration of PFCs as a surrogate of the preconception exposure. Since the half-lives of these chemicals are considerably long (Olsen et al., 2007) and they are persistent in the environment, we considered that these concentrations reflected those at the time of the pregnancy attempts. In addition, one recent study reported robust correlations between two subsequent measures of PFCs in blood samples of 53 men collected 6 years apart, in 2001 and 2007 (Spearman's ρ = 0.75 for PFOA and 0.81 for PFOS, and PFHxS) (Nost et al., 2014).
One limitation of our study is that we do not have information on male partner exposure to PFCs. The LIFE study is the only study that has measured concentrations of PFCs in both partners (Buck Louis et al., 2013), reporting high correlations between partner concentrations. Since TTP is an epidemiological metric of couple fecundity, we are unable to determine whether the effects are female, male or both. Furthermore, we did not assess specific end-points of fecundability such as menstrual cycle characteristics, markers of ovulation or semen quality (Buck Louis et al., 2014).
With regard to the biological mechanism of action, there are limited toxicological studies assessing the effect of PFCs on reproductive outcomes. In vitro, some PFCs have the potential to affect estrogen-receptor and androgen-receptor transactivity (Kjeldsen and Bonefeld-Jorgensen, 2013). In rats, exposure to PFOS has shown estrous cyclicity disruption and neurotransmitter imbalance (Austin et al., 2003). Thyroid dysfunction could also be an endocrine target for PFCs, with some evidence from animal (Lau et al., 2003; Thibodeaux et al., 2003; Luebker et al., 2005; Chang et al., 2008) and human studies (Dallaire et al., 2009; Wang et al., 2014) showing decreased levels of some thyroid hormones at higher concentrations of exposure. Although these studies suggest a possible endocrine-disrupting effect, additional studies need to be assessed to support an effect and its mechanistic pathway. Recently, PFOA and perfluorononanoic acid (PFNA) were reported to be associated with endometriosis in a cohort of 495 women undergoing laparoscopy/laparotomy in the ENDO study (Endometriosis—Natural History, Diagnosis and Outcomes Study) (Buck Louis et al., 2012). Endometriosis is a condition associated with impaired fecundability. Therefore, endometriosis or its determinants could be on the etiologic or causal pathways of the association between PFCs and TTP.
In conclusion, our results add to the evidence that exposure to PFOA and PFHxS, even at lower levels than previously reported, may reduce fecundability, as measured by a longer TTP and increased odds of infertility (TTP > 12 months). Future research should focus on the mechanisms involved in this potential endocrine-disrupting effect. Methodological differences in the causal models of previous studies have impaired possible conclusions about the potential adverse effect of PFCs and TTP.
Authors' roles
M.P.V., T.E.A. and W.D.F. were all involved in the conception and design of the study. M.P.V. carried out analysis and interpretation of data, in addition to drafting the manuscript. T.E.A. and W.D.F. were the co-principal investigators of the MIREC Study and contributed to data interpretation and review of the manuscript.
Funding
The MIREC study is supported by the Chemicals Management Plan of Health Canada, the Canadian Institutes for Health Research (CIHR, grant no. MOP–81285), and the Ontario Ministry of the Environment. M.P.V. is supported by a CIHR Fellowship Award, and a CIHR-Quebec Training Network in Perinatal Research (QTNPR) Ph.D. scholarship. W.D. is supported by a CIHR Canada Research Chair. Funding to pay the Open Access publication charges for this article was provided by Health Canada.
Conflict of interest
None declared.
Acknowledgements
We thank MIREC participants for their valuable commitment to the study, as well as the MIREC study group and the staff at each recruitment site and at the coordinating center at CHU Sainte-Justine. Special thanks to an anonymous reviewer for the insightful comments on a previous version of this manuscript.
References
- 3M. 2014. 3M's Phase Out and New Technologies http://solutions.3m.com/wps/portal/3M/en_US/PFOS/PFOA/Information/phase-out-technologies/ 16 June 2014, date last accessed.
- Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd edn. Cary, NC: SAS Institute Inc.; 2010. [Google Scholar]
- Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, von Dadelszen P, Hemmings DG, et al. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinat Epidemiol. 2013a;27:415–425. doi: 10.1111/ppe.12061. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Kubwabo C, Walker M, Davis K, Lalonde K, Kosarac I, Wen SW, Arnold DL. Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int J Hyg Environ Health. 2013b;216:184–194. doi: 10.1016/j.ijheh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- ATSDR. Draft Toxicological Profile for Perfluoroalkyls. Agency for Toxic Substances and Disease Registry; 2009. http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=1116&tid=237. (date last accessed, 16 June 2014). [PubMed] [Google Scholar]
- Austin ME, Kasturi BS, Barber M, Kannan K, MohanKumar PS, MohanKumar SM. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect. 2003;111:1485–1489. doi: 10.1289/ehp.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund JA, Thuresson K, De Wit CA. Perfluoroalkyl compounds (PFCs) in indoor dust: concentrations, human exposure estimates, and sources. Environ Sci Technol. 2009;43:2276–2281. doi: 10.1021/es803201a. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Hediger ML, Croughan MS, Sundaram R, Stanford JB, Fujimoto VY, Varner MW, Giudice LC, et al. Perfluorochemicals and endometriosis: the ENDO study. Epidemiology. 2012;23:799–805. doi: 10.1097/EDE.0b013e31826cc0cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Maisog J, Kim S, Chen Z, Barr DB. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121:231–236. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. 2014;101:1359–1366. doi: 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. 2009.
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, Butenhoff JL. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS) Toxicology. 2008;243:330–339. doi: 10.1016/j.tox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20:56–59. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect. 2009;117:1380–1386. doi: 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Zhou H, Liu Y, Cai J, Longnecker MP. Estimating effect of environmental contaminants on women's subfecundity for the MoBa study data with an outcome-dependent sampling scheme. Biostatistics. 2014;15:636–650. doi: 10.1093/biostatistics/kxu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont. 2013. About PFOA http://www2.dupont.com/PFOA2/en_US/index.html. 8 December 2014, date last accessed.
- Environment Canada. 2012. Perfluorooctane Sulfonate and its Salts and Certain Other Compounds Regulations (PFOS Regulations) http://www.ec.gc.ca/toxiques-toxics/4284EC2C-8BA0-4049-AF03-994FD409D523/907_PFOS%20factsheet_04_e.pdf. (date last accessed, 16 June 2014)
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod. 2009;24:1200–1205. doi: 10.1093/humrep/den490. [DOI] [PubMed] [Google Scholar]
- Fei C, Weinberg CR, Olsen J. Commentary: perfluorinated chemicals and time to pregnancy: a link based on reverse causation? Epidemiology. 2012;23:264–266. doi: 10.1097/EDE.0b013e3182467608. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds—exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44:7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- Greenland S. Quantifying biases in causal models: classical confounding 1 collider-stratification bias. Epidemiology. 2003;14:300–306. [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem Res Toxicol. 2003;16:775–781. doi: 10.1021/tx034005w. [DOI] [PubMed] [Google Scholar]
- Health Canada. 2008. Perfluorooctane Sulfonate (PFOS) and Health http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/perflurooctane_sulfonate-eng.php. 16 June 2014, date last accessed.
- Health Canada. Report on Human Biomonitoring of Environmental Chemicals in Canada. 2010. Results of the Canadian Health Measures Survey Cycle 1 (2007–2009) http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/chms-ecms/report-rapport-eng.pdf. (date last accessed, 16 June 2014)
- Health Canada. Second Report on Human Biomonitoring of Environmental Chemicals in Canada. 2013. Results of the Canadian Health Measures Survey Cycle 2 (2009–2011) http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/chms-ecms-cycle2/index-eng.php. (date last accessed, 16 June 2014)
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ Health Perspect. 2011;119:92–97. doi: 10.1289/ehp.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. ‘Toward a clearer definition of confounding’ revisited with directed acyclic graphs. Am J Epidemiol. 2012;176:506–511. doi: 10.1093/aje/kws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162:115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int. 2013;20:8031–8044. doi: 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci. 2003;74:382–392. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Lim TC, Wang B, Huang J, Deng S, Yu G. Emission inventory for PFOS in China: review of past methodologies and suggestions. ScientificWorldJournal. 2011;11:1963–1980. doi: 10.1100/2011/868156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loccisano AE, Longnecker MP, Campbell JL, Jr, Andersen ME, Clewell HJ., III Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J Toxicol Environ Health A. 2013;76:25–57. doi: 10.1080/15287394.2012.722523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebker DJ, York RG, Hansen KJ, Moore JA, Butenhoff JL. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: dose-response, and biochemical and pharamacokinetic parameters. Toxicology. 2005;215:149–169. doi: 10.1016/j.tox.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17:413–418. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- Nost TH, Vestergren R, Berg V, Nieboer E, Odland JO, Sandanger TM. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environ Int. 2014;67:43–53. doi: 10.1016/j.envint.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol. 2009;27:212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Sallmen M, Bonde JP, Lindbohm ML, Kristensen P. Selection bias due to parity-conditioning in studies of time trends in fertility. Epidemiology. 2015;26:85–90. doi: 10.1097/EDE.0000000000000190. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Ikonomou M, Kannan K. Indoor and outdoor air concentrations and phase partitioning of perfluoroalkyl sulfonamides and polybrominated diphenyl ethers. Environ Sci Technol. 2004;38:1313–1320. doi: 10.1021/es0305555. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, MWebster G, Lee SC. Indoor sources of poly- and perfluorinated compounds (PFCS) in Vancouver, Canada: implications for human exposure. Environ Sci Technol. 2011;45:7999–8005. doi: 10.1021/es103562v. [DOI] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci. 2003;74:369–381. doi: 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao XL, Dabeka RW. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J Agric Food Chem. 2007;55:3203–3210. doi: 10.1021/jf0634045. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, Nielsen F, Andersson AM, Hjollund NH, Grandjean P, Andersen HR, Jensen TK. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum Reprod. 2012;27:873–880. doi: 10.1093/humrep/der450. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, Longnecker MP, Wang SL. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect. 2014;122:529–534. doi: 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ. Methodological issues in reproductive epidemiology. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 620–640. [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, et al. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology. 2012;23:257–263. doi: 10.1097/EDE.0b013e31823b5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielhuis GA, Hulscher ME, Florack EI. Validity and reliability of a questionnaire on fecundability. Int J Epidemiol. 1992;21:1151–1156. doi: 10.1093/ije/21.6.1151. [DOI] [PubMed] [Google Scholar]