Abstract
STUDY QUESTION
Are the genes that gained novel expression in the endometria of Eutherian (placental) mammals more likely to be dysregulated in patients with endometrial-associated recurrent early pregnancy loss (REPL)?
SUMMARY ANSWER
There was a significant enrichment of genes dysregulated in REPL patients among the Eutherian-specific endometrial genes.
WHAT IS KNOWN ALREADY
Pregnancy loss is the most common complication of human pregnancy. REPL has multiple etiologies, including dysregulation of endometrial function, leading to ‘suboptimal’ implantation. Although the implantation process is tightly regulated in Eutherian (placental) mammals, the molecular factors contributing to dysregulated endometrial gene expression patterns in women with REPL are largely unknown.
STUDY DESIGN, SIZE, DURATION
Endometrial biopsies were obtained from 32 REPL patients during the mid-luteal phase, and evaluated for glandular development arrest based on elevated nuclear cyclin E levels in gland cells, and for out-of-phase endometrial development based on histology. Gene expression levels were measured using Illumina Human HT-12v4 BeadChip arrays.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Differentially expressed genes were identified between patients with (i) out-of-phase (n = 10) versus normal (n = 22) histological dating and (ii) abnormally elevated (n = 9) versus normal (n = 23) cyclin E levels in the nuclei of endometrial glands, using a likelihood ratio test. Enrichment of dysregulated genes in REPL endometria among Eutherian-specific genes was tested by permutation. Gene ontology and pathway enrichment analyses were carried out for the dysregulated genes.
MAIN RESULTS AND THE ROLE OF CHANCE
Fifty-eight and eighty-one genes were identified as differentially expressed at P < 0.001 in women with out-of-phase histological dating and abnormally elevated glandular cyclin E levels, respectively. Genes that were recruited into endometrial expression during the evolution of pregnancy in Eutherian mammals were significantly enriched for dysregulated genes (P = 0.002 for histology, P = 0.021 for cyclin E), as well as for genes involved in immune response and signaling pathways with essential roles in implantation and endometrial biology.
LIMITATIONS, REASONS FOR CAUTION
Small sample size limits the statistical power to detect dysregulated genes, and the lack of non-REPL control women does not allow us to test for the contribution of these genes to overall risk of REPL.
WIDER IMPLICATIONS OF THE FINDINGS
Enrichment of functional gene categories, as well as genes gained expression in the Eutherian endometria, help to identify molecular etiologies that contribute to normal functioning of the endometrium. These pathways are also strong candidates for successful pregnancy outcomes. Using the evolutionary history of mammalian gene expression in the endometrial tissue may be a promising approach to discover genes involved in female reproductive disorders.
STUDY FUNDING/COMPETING INTEREST(S)
This work is supported by National Institutes of Health (NIH) grant R01 HD21244 to C.O. Authors declare no competing interests.
Keywords: recurrent early pregnancy loss, endometrium, reproductive disorders, eutherian mammals, forward genomics
Introduction
Pregnancy loss during the first trimester is the most common complication in human pregnancy, occurring in nearly 15% of clinically recognized pregnancies (Stephenson and Kutteh, 2007). Recurrent early pregnancy loss (REPL), defined here as two or more miscarriages under 10 weeks of gestation, affects up to 5% of couples trying to conceive (Stephenson and Kutteh, 2007). The state of endometrial receptivity during the mid-luteal phase (endometrial cycle day 20–24) is critical for successful implantation (Ruiz-Alonso et al., 2012), and therefore, has been focus of many genome-wide transcriptomic studies that aim to identify predictive markers of early pregnancy in healthy, fertile women (for example, Borthwick et al., 2003; Riesewijk et al., 2003; Mirkin et al., 2005; Talbi et al., 2006; Haouzi et al., 2009). In contrast, there have been many fewer studies that characterized gene expression in patients with reproductive disorders (Altmae et al., 2010; Ledee et al., 2011; Othman et al., 2012). As a result, the specific genes and pathways that are involved in preparation of the endometrium for successful implantation and their roles in the etiology of REPL are poorly characterized (Ruiz-Alonso et al., 2012).
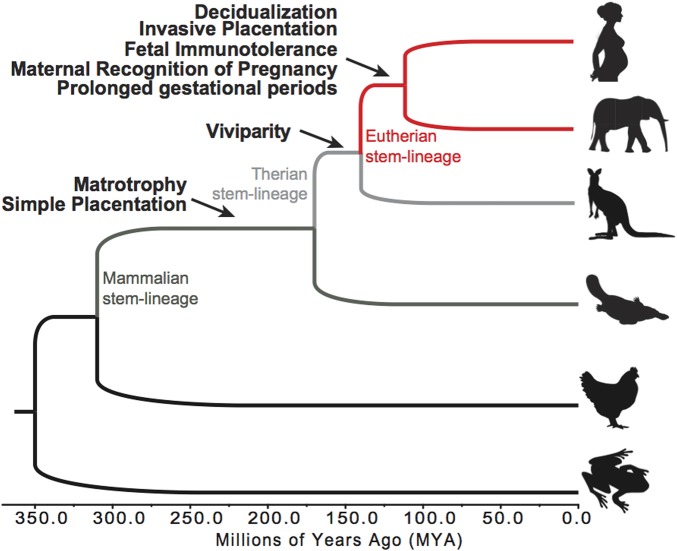
These traditional approaches, however, have not considered that complex biological systems like pregnancy have an evolutionary history that can be leveraged to reconstruct their origin and provide unique insights into critical genes and pathways whose evolution was critical for the origin of Eutherian pregnancy. For example, extant mammals span several major evolutionary transitions during the origins of pregnancy (Fig. 1). Although monotremes are oviparous and lay leathery shelled eggs, the developing embryo is retained in the uterus for ∼21 days during which time it is nourished from uterine secretions delivered through a simple placenta derived from the yolk sac. Live birth (viviparity) evolved in the Therian stem-lineage (common ancestor), with the loss of the eggshell and yolk, and elaboration of the placenta. Different Therian mammals, however, have dramatically different reproductive life histories. Marsupials lack implantation and strong maternal recognition of pregnancy, have noninvasive placentas, and very short gestations (∼14 days). In contrast, Eutherian (‘placental’) mammals evolved decidualization of endometrial stromal cells, direct implantation of fetal tissue into the richly vascular uterine endometrium, pronounced maternal recognition of pregnancy, maternal immunotolerance of the fetal allograft, and prolonged pregnancies (up to 670 days). Therefore, many of the genes that underlie the successful establishment and maintenance of pregnancy likely evolved endometrial expression in Eutherian mammals. Indeed the evolutionary origin of pregnancy in mammals was accompanied by the recruitment of thousands of genes into endometrial and placental expression, including many that are essential for pregnancy (Hou et al., 2012; Lynch et al., 2012).
Figure 1.
Evolution of pregnancy in animals.
Here we used the evolutionary history of gene expression in the endometrium as a forward genomics approach to identify dysregulated genes in REPL patients with endometrial abnormalities, which may contribute to the establishment and maintenance of pregnancy. We included 32 women with REPL who were evaluated during the mid-luteal phase and classified into three groups based on the results of two independent tests of endometrial function: the endometrium of 10 women showed out-of-phase dating by histology, the endometrium of nine women showed glandular developmental arrest as determined by abnormally elevated cyclin E levels in the nuclei of endometrial glands (Dubowy et al., 2003), and the endometria of the remaining 13 women were both in-phase by histology and had normal levels of cyclin E (Table I). We first show that REPL women with out-of-phase histology can be differentiated from women with abnormal cyclin E levels based on gene expression profiles in mid-luteal phase endometrial biopsies. Moreover genes that evolved endometrial expression in the stem-lineage of Eutheria were significantly enriched among differentially expressed genes in each group of women. These results suggest that an evolutionary-based forward genomics approach can identify groups of genes whose transcriptional regulation in the mid-luteal endometrium is necessary to provide optimal conditions for implantation and successful pregnancy outcome.
Table I.
Summary characteristics of the patients included in the differential gene expression analyses (Groups 1–3) or studies of gene expression in response to progesterone treatment (Group 4).
| Group | Abnormal histology? | Abnormal cyclin E? | N | Mean age (years) (SD) | Mean number of prior miscarriages (range) | Mean % glandular cyclin E (SD) |
|
|---|---|---|---|---|---|---|---|
| Before progesterone | After progesterone | ||||||
| 1 | Y | N* | 10 | 34.8 (4.0) | 3.9 (2–9) | 11.7 (7.6) | NA |
| 2 | N | Y | 9 | 33.4 (2.7) | 3.8 (2–6) | 44.4 (11.3) | NA |
| 3 | N | N | 13 | 33.4 (2.5) | 4.5 (2–7) | 8.1 (4.3) | NA |
| 4 | N | Y | 5** | 36.3 (2.1) | 4.0 (3–5) | 58.0 (13.0) | 19.0 (13.0) |
*7 of 10 women with abnormal histology were not evaluated for cyclin E levels (see Methods).
**Does not overlap with 9 women in group 2.
Methods
Ethics statement
Written informed consent was obtained from each subject prior to the endometrial biopsy; all studies were approved by the Institutional Review Board at the University of Chicago.
Subjects and sample collection
This study included 32 women of European ancestry, who had REPL, defined as two or more documented unexplained miscarriages (excluding those due to numeric unbalanced chromosome errors) of <10 weeks duration. The REPL screening protocol was completed in all couples, and consisted of cytogenetic analyses of both partners, maternal thyroid stimulating hormone, prolactin and antiphospholipid antibodies (APA) (anticardiolipin IgG/IgM and lupus anticoagulant), office hysteroscopy to assess uterine cavity, and an endometrial biopsy to assess histology, nuclear cyclin E levels in gland cells, and whether endometritis was present, as previously described (Stephenson et al., 2010). Couples with genetic, anatomic and infections factors associated with miscarriage were excluded from this study (Stephenson, 1996). All women had endometrial biopsies performed during their mid-luteal phase, 9–11 days following the endogenous LH surge, detected by the patient testing her urine. Endometrial dating and Endometrial Function Test (EFT®) (Dubowy et al., 2003) were performed on each biopsy (see Supplementary Data for details), and based on the results of these tests, patients were classified into three groups. The first study group was composed of patients with out-of-phase endometrial histology dating, defined as ≥3 days of endometrial developmental delay (n = 10). The second group exhibited glandular developmental arrest associated with abnormally high cyclin E levels (>20%) in the nuclei of endometrial glands (Dubowy et al., 2003), but had ‘in-phase’ endometrial histological dating (n = 9). The third group comprised the remaining women with REPL who had normal cyclin E levels and in-phase histology dating (n = 13). Five women in this group had tested positive for APAs. The EFT® was not performed in seven women in the first group and, as a result, their % glandular cyclin E levels were not available. However, based on the EFT® results of ∼100 REPL patients evaluated, only one woman (<1%) who was not included in this study had both abnormal cyclin E levels and out-of-phase histological dating. Therefore, although we cannot rule out that any one of these seven patients with out-of-phase dating did not also have abnormal cyclin E levels, it is highly unlikely. Characteristics of the 32 women are described in Table I; more detailed descriptions of all subjects are given in Supplementary Table SI. In addition to these 32 women, five women with abnormally high nuclear cyclin E levels in endometrial glands (not overlapping with women in the second group) had endometrial biopsies collected both before and after vaginal administration of 100 mg Prometrium® every 12 h starting 3 days after LH surge in order to study the effects of progesterone on gene expression levels.
Differential gene expression analysis
100 μg of each total RNA sample was sent to the Southern California Genotyping Consortium (SCGC) as one batch, where they were processed and hybridized to Illumina HT-12 v4 Expression BeadChips (Illumina, San Diego, CA, USA). Details regarding the low-level analysis of the microarray data, including the quality control steps and covariate selection are described in Supplementary Data; distribution of array intensities before and after normalization for each sample is shown in Supplementary Fig. S1. To identify differentially expressed genes between patients in different diagnostic groups, we used a likelihood ratio test approach within a fixed-effects linear model framework. For each gene, we assessed the linear model:
where YExp is the residual gene expression estimates after inclusion of covariates, μ is the mean gene expression level across all the samples, XExp is the diagnostic classification while β is the fixed effect, and ϵ is an error term assumed to be normally distributed with mean 0 and variance σ2Exp. To test for the effect of the diagnostic classification on gene expression levels, we compared the null model where the variable has no effect on gene expression (i.e. XExp = 0) to an alternative model where the variable has an effect on gene expression (i.e. XExp ≠ 0). Because null and alternative models are nested, we assumed that the differences in log-likelihood between the two models are χ2-distributed with 1 degree of freedom. Significance of each variable on gene expression levels was determined by an F-statistic. P-values were corrected for multiple testing using the false discovery rate (FDR) approach of Benjamini and Hochberg (Benjamini and Hochberg, 1995).
Because we did not have similar data for healthy fertile women, we stratified our sample based on their underlying endometrial diagnoses for comparisons. Therefore, two comparisons were performed to identify differentially expressed genes in REPL subjects with endometrial diagnoses. First, we tested for differential gene expression between women with out-of phase histology dating (first group, n = 10) and those with normal histology dating (second and third groups combined, n = 22). For the second comparison, we tested for differential gene expression between women with abnormally high nuclear cyclin E expression (second group, n = 9) and those with normal nuclear cyclin E levels (first and third groups combined, n = 23).
Analysis of enrichment in the Eutherian-specific genes
We investigated the differential expression of genes that were recruited into endometrial expression in the stem-lineage of Eutherian mammals, a clade that is mainly distinguished from other mammals by the evolution of implantation and placentation. Endometrial expression was detected in Eutherian mammals only for 827 of the 8897 one-to-one orthologous genes across 12 species, including eight Eutherian mammals (human, rhesus monkey, mouse, dog, horse, cow, pig, armadillo), two marsupial mammals (short-tailed opossum, wallaby), an egg-laying monotreme (platypus) and chicken (Lynch et al., 2012). We hypothesized that regulation of these 827 genes should be under tight constraint, and perturbations could lead to pregnancy loss. To test this hypothesis, we performed an enrichment analysis of 827 Eutherian endometrium-specific genes within the differentially expressed genes in the endometria from women with REPL. Distributions of the P-values of these 827 genes in the histology and cyclin E analyses were compared with the P-values of 827 randomly selected genes, after 10 000 iterations. Empirical P-values for enrichment were calculated as the fraction of 10 000 iterations, for which the total number of genes with differential expression P-values less than the chosen threshold was higher than the observed number (e.g. number of iterations that yielded >75 genes with P < 0.05 in histology comparison; Table II). To better understand if the observed enrichment is specific to only the Eutherian lineage or whether it is a general trend for genes expressed in the endometria of all species, the same analysis was repeated for two additional gene sets: 1294 genes expressed in the endometria of all amniote species analyzed and 660 genes expressed in all Therians (Eutherians and marsupials).
Table II.
Enrichment of Eutherian lineage-specific genes among the differentially expressed genes in the histology and cyclin E comparisons at three different P-value thresholds of 0.05, 0.01 and 0.001.
| Gene groups | N | Histology |
Cyclin E |
|||||
|---|---|---|---|---|---|---|---|---|
| P < 0.05 (%) | P < 0.01 (%) | P < 0.001 (%) | P < 0.05 (%) | P < 0.01 (%) | P < 0.001 (%) | |||
| All genes | 14 281 | 1219 (8.5) | 348 (2.4) | 58 (0.4) | 1672 (11.7) | 529 (3.7) | 81 (0.6) | |
| Eutherian-recruits | 596 | Obs | 75 (12.6) | 25 (4.2) | 7 (1.2) | 113 (19.0) | 34 (5.7) | 7 (1.2) |
| Exp | 50.8 (8.5) | 14.5 (2.4) | 2.32 (0.4) | 69.7 (11.7) | 22.1 (3.7) | 3.4 (0.6) | ||
| Enrich | 1.48 | 1.75 | 3.00 | 1.62 | 1.54 | 2 | ||
| P-value | 0.0003 | 0.003 | 0.002 | <0.0001 | 0.005 | 0.021 | ||
| Therian-recruits | 526 | Obs | 55 (10.5) | 17 (3.2) | 3 (0.6) | 57 (10.8) | 16 (3.0) | 3 (0.6) |
| Exp | 44.9 (8.5) | 12.8 (2.4) | 2.0 (0.4) | 61.5 (11.7) | 19.5 (3.7) | 3.0 (0.6) | ||
| Enrich | 1.24 | 1.33 | 1.50 | 0.92 | 0.81 | 1.00 | ||
| P-value | 0.07 | 0.14 | 0.34 | 0.75 | 0.83 | 0.58 | ||
| Ancestrally expressed | 1104 | Obs | 85 (7.7) | 26 (2.4) | 4 (0.4) | 129 (11.7) | 37 (3.4) | 8 (0.7) |
| (Expressed in all species) | Exp | 94.3 (8.5) | 26.9 (2.4) | 4.3 (0.4) | 129.1 (11.7) | 40.9 (3.7) | 6.3 (0.6) | |
| Enrich | 0.91 | 1.00 | 1.00 | 1.00 | 0.92 | 1.17 | ||
| P-value | 0.86 | 0.60 | 0.64 | 0.52 | 0.77 | 0.29 | ||
Significant enrichments are shown in boldface.
N: number of expressed genes in the recurrent early pregnancy loss (REPL) endometrium; Obs: observed number of genes with P-values smaller than the given threshold within the given category; Exp: expected number of genes with P-values smaller than the given threshold, calculated as the mean number of genes with a P-value smaller than the given threshold obtained from 10 000 iterations; Enrich: fold-enrichment in the observed data; P-value: empirical P-value corresponding to fraction of the 10 000 iterations that yielded higher number of smaller differential expression P-values than the observed number.
Statistical analysis
For descriptive statistics, sample sizes, sample means and SDs or minimum and maximum ranges were reported, as appropriate. ‘Residual gene expression’ values refer to the median value for all probes mapping to that gene, after log2 transformation and quantile normalization, and the effects of the covariates (microarray chip and season of the biopsy collection) were regressed out. For all duplicate samples, mean of the residual gene expression is calculated. Comparison of gene expression levels between the groups was performed by likelihood ratio test or Wilcoxon signed rank test, as appropriate. False discovery rates are reported to adjust for multiple testing, as indicated. Enrichments of differentially expressed genes among Eutherian-recruited, Therian-recruited or ancestrally expressed gene sets were determined by permutation, and empirical P-values were reported, corresponding to the fractions of permutations that yielded higher number of genes with differential expression P-values less than each threshold P-value, compared with the observed number. GeneTrail [http://genetrail.bioinf.uni-sb.de (2 January 2015, date last accessed)], and Mouse Genome Informatics [www.informatics.jax.org (2 January 2015, date last accessed)] databases were used to obtain gene ontology (GO) and mammalian phenotype (MP) classifications, respectively. Enrichment of GO categories within the Eutherian-recruited genes among all genes represented in the GO catalog was calculated using Fisher's exact test (2-by-2 table). Enrichment of MP within the Eutherian-recruited genes compared with those among ancestrally expressed genes was calculated using the binomial distribution. All statistical analyses were conducted using R statistical software [http://www.r-project.org/ (2 January 2015, date last accessed)].
Results
Endometrial expression profiles and sample clustering
Gene expression levels were measured using the Illumina Human HT-12v4 Expression BeadChips, and compared between women with (i) out-of-phase histological dating (n = 10) and those with normal histology (n = 22), or (ii) abnormally elevated nuclear cyclin E levels (n = 9) and normal nuclear cyclin E levels (n = 23), using a likelihood ratio test. Of the 47 231 probes present on the arrays, 18 065 were detected as expressed in at least one woman with a detection P-value of 0.01, representing 14 281 unique RefSeq genes. Hierarchical clustering was performed using gene expression levels after adjusting for significant covariates (see Supplementary Data/Methods), for all 14 281 genes. We observed a distinct clustering between women with out-of-phase histology compared with those with elevated nuclear cyclin E levels with high confidence probabilities (Supplementary Fig. S2). Women with REPL who have normal histology and nuclear cyclin E levels did not show a clear pattern of clustering among themselves or either with the out-of-phase histology or the elevated nuclear cyclin E groups. These results indicate that the two types of endometrial diagnoses, specifically, out-of-phase endometrial dating and elevated nuclear cyclin E levels, can be differentiated based on gene expression profiles in mid-secretory phase endometrium. Not surprisingly the remaining REPL women do not share a common endometrial expression profile reflecting the underlying etiologic heterogeneity of this group.
Differential gene expression in women with out-of-phase histology and abnormal cyclin E
Raw and normalized expression values are publicly available in Gene Expression Omnibus (GEO) repository (accession number GSE63901). In the comparison between women with out-of-phase histology versus those with in-phase histology, no genes were differentially expressed at a FDR of 5% but 58 genes were differentially expressed at a nominal significance level of P < 0.001 (Supplementary Table SIIA). In the comparison between women with elevated nuclear cyclin E levels in endometrial glands versus those with normal nuclear cyclin E levels, the differential expression of one gene, zinc finger protein 789 (ZNF789), reached significance at a FDR of 5% (P = 3.77 × 10−6, FDR = 0.05). Overall, 81 genes were differentially expressed at P < 0.001 (Supplementary Table SIIB). Notably, we did not observe differential expression in any of the cyclin E subunits, cyclin-dependent kinases or inhibitor genes between women with elevated nuclear cyclin E levels and women with normal nuclear cyclin E levels in endometrial glands. The lack of differential expression of the cyclin E1 gene, CCNE1, was validated by quantitative real-time PCR (Supplementary Fig. S3). This may be attributed to the fact that the endometrial biopsies comprise glands, stroma and endothelial cells, whereas dynamic changes of cyclin E levels throughout the menstrual cycle are only seen in the cytoplasm and the nuclei of the endometrial glands (although only nuclear expression is detected during the luteal phase; Dubowy et al., 2003). Therefore, even though it is still possible that elevated nuclear cyclin E protein levels are due to increased mRNA levels, we were unable to detect this difference in the mRNA extracted from total biopsy tissues.
Among the genes that were differentially expressed at P < 0.01 in the endometrium of women with abnormal histology, 210 (60.3%) were up-regulated and 138 (39.7%) were down-regulated. Significant GO enrichments were detected within the up-regulated genes only, which showed overrepresentation of biological processes including cell adhesion (2.7-fold, FDR = 7.72 × 10−4), cell motility/migration/movement (3.2-fold, min FDR = 7.72 × 10−4), transforming growth factor (TGF)-β receptor signaling (6.4-fold, FDR = 2.15 × 10−3) and system/organ development (1.7-fold, min FDR = 4.23 × 10−3). Genes with functions in metabolic processes and gene expression were underrepresented in this group (1.6- to 1.7-fold, FDR < 0.01) (Supplementary Table SIIIA). In contrast, among the genes that were differentially expressed at P < 0.01 in the endometrium of women with elevated nuclear cyclin E expression, there were overall similar numbers of up- and down-regulated genes (278 [52.7%] up-regulated and 250 [47.3%] down-regulated). In this group, we detected significant gene annotation enrichments only within the down-regulated genes. GO biological processes that were significantly overrepresented among these down-regulated genes include immune response (3.4-fold, FDR = 1.50 × 10−6), signaling/signal transduction (1.7-fold, min FDR = 1.97 × 10−5), leukocyte/lymphocyte/T-cell activation (3.4-fold, min FDR = 8.78 × 10−4), response to stimulus (5.6-fold, FDR = 1.24 × 10−3), organ/system development (1.8-fold, min FDR = 1.74 × 10−3) and cell adhesion (2.1-fold, FDR = 9.55 × 10−3), while terms that include nucleic acid metabolic process and gene expression were significantly underrepresented (1.3- to 2.0-fold, FDR < 0.05) (Supplementary Table SIIIB).
Significant enrichment of Eutherian-recruited genes among differentially expressed genes
We next asked whether genes that evolved expression in the endometrium of Eutherian mammals, and therefore likely mediate successful pregnancies, were more likely to be dysregulated in the endometria of women with REPL than expected by chance or compared with gene expressed in the endometria of all species. Of 827 genes that evolved endometrial expression in the stem-lineage of the Eutherian mammals (Lynch et al., 2012), 596 were detected as expressed in the mid-secretory phase endometrium in our samples (Supplementary Table SII). The 596 Eutherian-recruited genes were significantly enriched among the genes that were dysregulated in women with out-of-phase histology (1.5- to 3-fold at different P-value thresholds, P = 0.003 − 0.0003) or with elevated nuclear cyclin E expression (1.5- to 2-fold at different P-value thresholds, P = 0.02 − <0.0001), based on 10 000 permutations (Table II). In stark contrast, genes recruited into endometrial expression in Therian mammals or genes that were ancestrally expressed across all amniotes were not enriched among dysregulated genes in REPL women with either out-of-phase histology or elevated nuclear cyclin E levels (Table II).
Our observation that genes that evolved endometrial expression in the stem-lineage of Eutherian mammals are preferentially dysregulated in the endometria with histological and molecular abnormalities, both of which are associated with REPL, brings about the possibility that they may also play important roles in the maintenance of early human pregnancies. To infer what these roles may be, we examined GO terms and phenotypes observed in knockout mouse models of these genes. We found that Eutherian-recruited genes that were dysregulated in REPL endometria were significantly enriched in GO terms related to immune responses such as ‘positive regulation’ of immune cell proliferation, activation and differentiation (12.4-fold, min FDR = 0.002), as well as ‘cell adhesion’ (3.2-fold, FDR = 0.02), ‘response to stimulus' (1.5-fold, FDR = 0.02) and ‘calcium-mediated signaling’ (9.9-fold, FDR = 0.04) (Table IIIA and Supplementary Table SIV), and in knockout mouse phenotypes such as ‘decreased T-cell proliferation’ (8.8-fold, FDR = 3.62 × 10-5), ‘abnormal humoral immune response’ (14.2-fold, FDR = 4.57 × 10-5) and ‘decreased interleukin-2 secretion’ (8.8-fold, FDR = 0.0091) compared with ancestrally expressed genes (Table IIIB and Supplementary Table SV).
Table III.
Enrichment of (A) gene ontology (GO) biological process categories and (B) mammalian phenotypes (MP) among differential expressed Eutherian-specific genes in REPL.
| A. | ||||
|---|---|---|---|---|
| Gene ontology | ||||
| Accession | Term | Data set | Fold-enrich. | P-value (FDR) |
| GO:0042102 | Positive regulation of T-cell proliferation | Cyclin E | 12.4 | 2.37 × 10−3 |
| GO:0050865 | Regulation of cell activation | Cyclin E | 5.4 | 2.42 × 10−3 |
| GO:0007155 | Cell adhesion | Cyclin E | 3.2 | 2.12 × 10−2 |
| GO:0050896 | Response to stimulus | Cyclin E | 1.5 | 2.34 × 10−2 |
| GO:0019722 | Calcium-mediated signaling | Cyclin E | 9.9 | 4.15 × 10−2 |
| GO:0006023 | Aminoglycan biosynthetic process | Histology | 8.1 | 3.95 × 10−2 |
| GO:0006024 | Glycosaminoglycan biosynthetic process | Histology | 8.1 | 1.97 × 10−2 |
| B. | ||||
|
Mouse knockout phenotypes | ||||
| MP ID | MP TERM | Data set | Fold-enrich. | P-value (FDR) |
| MP:0005095 | Decreased T-cell proliferation | Cyclin E | 8.8 | 3.62 × 10−5 |
| MP:0001800 | Abnormal humoral immune response | Cyclin E | 14.2 | 4.57 × 10−5 |
| MP:0005078 | Abnormal cytotoxic T cell physiology | Cyclin E | 35.4 | 1.68 × 10−3 |
| MP:0008075 | Decreased CD4-positive T cell number | Cyclin E | 7.7 | 7.05 × 10−3 |
| MP:0008688 | Decreased interleukin-2 secretion | Cyclin E | 8.8 | 9.05 × 10−3 |
| MP:0002497 | Increased IgE level | Cyclin E | 17.7 | 9.05 × 10−3 |
| MP:0009199 | Abnormal external male genitalia morphology | Histology | 42.7 | 3.44 × 10−2 |
FDR, false discovery rate.
Functional roles of dysregulated recruited genes
A large proportion of the dysregulated Eutherian-recruited genes are down-regulated in women with high cyclin E (79 [69.9%] of 113 genes with P < 0.01), and many of these genes have known roles in immune response based on their GO annotations and knockout phenotypes (24 of 113 [21.2%]). Among the more notable dysregulated recruits with immune functions were integrin alpha L (ITGAL), inositol polyphosphate-5-phosphatase (INPP5D) and hepatitis A virus cellular receptor 2 (HAVCR2). ITGAL encodes the α-chain (CD11a) of the lymphocyte function associated antigen-1 (LFA-1), which acts as a receptor on CD56++ NK cells to mediate cell adhesion and cytotoxic activity. CD56++ NK cells are present in elevated numbers in human endometrium where they play an immunoregulatory role in pregnancy when they initiate remodeling of the decidua basalis and uterine spiral arteries (Fukui et al., 1999). CD11a levels present on CD56++ NK cells are critical for implantation success (Kodama et al., 1998; Fukui et al., 1999). Two other genes, INPP5D and HAVCR2, both have roles in immunomodulation. Mice homozygous null for INPP5D, a gene involved in biological processes such as blood coagulation, T-cell receptor signaling and negative regulation of immune response, fails to reject fully mismatched allogeneic marrow grafts (Wang et al., 2002). In addition, INPP5D is a direct target of p53 (Lion et al., 2013), whose function in implantation is crucial, and interestingly, is also significantly downregulated in women with high cyclin E (P = 2.17 × 10−4). HAVCR2 encodes a Th1-specific cell surface protein. Functional inhibition of HAVCR2 during pregnancy results in increased inflammation and fetal rejection in mice (Chabtini et al., 2013), while abnormal expression of the gene was found in human miscarriages (Zhao et al., 2009).
Six recruited genes are intracellular mediators of the Rho GTPases Rac1 and RhoA, which have opposing roles in regulating the migration and motility and of human endometrial stromal cells and act in concert to regulate implantation (Grewal et al., 2008) (Fig. 2A). Although neither RAC1 nor RHOA were differentially expressed in our data, the intracellular mediators activating these proteins, namely the Rac guanine nucleotide exchange factors DOCK2 (also known as dedicator of cytokinesis 2), DOCK10 (also known as dedicator of cytokinesis 10), and ARHGEF6, and the Rho GTPase activating proteins ARHGAP22, ARHGAP25, and ARHGAP30 were all expressed at lower levels in women with high cyclin E levels (Fig. 2B). Furthermore, the expression levels of these six genes were highly correlated with average pair-wise Pearson's r of 0.67 (SD 0.25; P < 1 × 10−6 based on permutation; Supplementary Fig. S4A), supporting the hypothesis that these Rho GTPase signaling genes are members of a co-regulated biological module that are coordinately down-regulated in women with abnormal levels of cyclin E.
Figure 2.
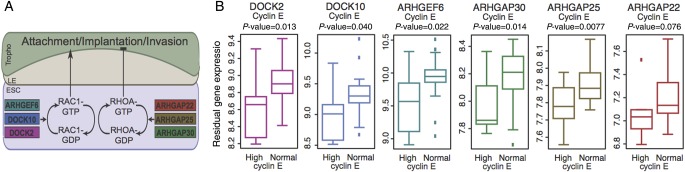
Differential expression of Rho/Rac guanine nucleotide exchange factors and RhoGTPase-activating proteins in women with elevated nuclear cyclin E levels. (A) The roles of six intracellular mediators of RAC1 and RHOA in the endometrium (ESC: endometrial stromal cells; LE: luminal epithelium; Tropho: trophoblast cells). (B) Expression level differences for the six genes in women with elevated versus normal nuclear cyclin E levels (DOCK10: dedicator of cytokinesis 10; DOCK2: dedicator or cytokinesis 2; ARHGEF6: Rac/Cdc42 guanine nucleotide exchange factor (GEF) 6; ARHGAP22: Rho GTPase activating protein 22; ARHGAP25: Rho GTPase activating protein 25; ARHGAP30: Rho GTPase activating protein 30).
Calcium influx into uterine epithelial cells is also required to trigger adhesiveness of the endometrium (Tinel et al., 2000) for the trophoblast. We found three Eutherian-recruited genes with roles in calcium signaling (transient receptor potential cation channel subfamily C member 4 (TRPC4), oxytocin receptor (OXTR) and serotonin receptor 2B (HTR2B)) that were underexpressed in REPL subjects with elevated nuclear cyclin E levels (Supplementary Fig. S5 and Table SII). For example, TRPC4 encodes a transient receptor potential cation channel, whereas OXTR encodes the oxytocin receptor; and both genes are involved in contractile properties of the pregnant myometrium and initiation of labor (Dalrymple et al., 2004; Terzidou et al., 2011). HTR2B mediates cell-cycle progression by activating cyclin E (Nebigil et al., 2000), which may provide a mechanism for the observed cyclin E dysregulation in these women. In addition to these genes, cytoplasmic FMR1 interacting protein 1 (CYFIP2), FYVE RhoGEF and PH domain containing 3 (FGD3), and ITGAL, which were also underexpressed in women with elevated nuclear cyclin E levels, play roles in regulating the actin cytoskeleton, the proper organization of which is required for the adhesion of trophoblasts to the uterine epithelium (Thie and Denker, 2002). Lastly, one of the most differentially expressed Eutherian-recruited genes in women with elevated nuclear cyclin E levels is a complement gene, C1QA, a pathway with known roles in both maintenance of healthy pregnancies and risk of recurrent miscarriage (Denny et al., 2013).
In contrast to Eutherian-recruited genes dysregulated in women with high cyclin E, many of the Eutherian-recruited genes dysregulated in women with out-of-phase endometria are up-regulated (51/75 genes [68%] with P < 0.01). Examples of this trend for the most dysregulated genes in both categories are shown in Supplementary Fig. S5. Although there have been few previous studies on most of the genes that are up-regulated in this group, VCAN, which encodes the extracellular matrix component protein versican, is a notable exception. Altmae et al. (2012) identified VCAN as one of the proteins in a large module of an embryo-endometrium interaction network at the time of implantation, where it clustered with several other cell adhesion molecules such as collagens, integrins and laminins. VCAN may also influence the invasive properties of endometrial cells (Aghajanova and Giudice, 2011). In addition to VCAN, two of the calcium signaling molecules that were down-regulated in women with high cyclin E, OXTR and HTR2B, were significantly up-regulated in women with out-of-phase histology as well (Supplementary Table SII).
Changes in expression of Rho GTPase signaling genes in response to progesterone
Our finding that genes involved in Rho GTPase signaling were down-regulated in women with abnormal cyclin E levels was intriguing due to the critical role of this pathway in modulating remodeling of endometrial stromal cells and invasion of the human embryo through the stroma (Grewal et al., 2008). Because we hypothesized that these genes are co-regulated as a biological module, we were interested in knowing how the expression of these genes changed in response to progesterone, a common treatment that some of the participants in our study received following their first endometrial biopsy.
Five women with elevated nuclear cyclin E levels in endometrial glands, who were not included in the analyses of differential expression described above, had endometrial biopsies collected in the mid-luteal phase both prior to and post-treatment with progesterone (Prometrium® 100 mg per vagina every 12 h starting 3 days after LH surge). Examining the gene expression data for the six dysregulated genes in these five paired samples revealed a nearly uniform up-regulation of all genes following treatment with progesterone: the expression of all six genes increased in four of the five women, whereas the expression of all six genes decreased in just one woman (Supplementary Fig. S4B and C). Although the Wilcoxon signed rank tests for paired samples were not significant for any of the individual genes (likely due to very small sample size), there was a statistically significant overall increase in gene expression levels when all observations were considered in a single test (P = 4.97 × 10−5). It was possible, however, that this highly significant result was inflated due to the fact that the expression levels of these six genes are highly correlated. Therefore, to assess significance while accounting for this correlation, we selected 1000 random sets of six genes that were correlated in the pretreated samples within 1 SD of the observed data (r = 0.42–0.92) to generate a null distribution for the combined test. In this random data, only one set out of 1000 yielded a P < 4.97 × 10−5 in a combined test of increased gene expression following progesterone treatment. This provides an empirical P-value of 0.001 for the observation that the expression of nearly all six Rho GTPase signaling genes increased after progesterone treatment in nearly all women. Moreover, pair-wise correlation between the six Rho GTPase signaling genes was itself quite unusual in that no other set of six expressed genes were as correlated in the untreated data. As a result, the permuted sets have a mean correlation (r) of 0.45 (range 0.42–0.65) compared with a mean pairwise r of 0.67 in the six Rho GTPase signaling genes. In addition, the magnitude of the average pair-wise correlations in the permuted data sets was not correlated with overall patterns of up- or down-regulation following progesterone treatment, or on the significance of the Wilcoxon signed rank test (Supplementary Fig. S4D and E). Therefore, even though the significantly high correlation between the six Rho GTPase genes makes the interpretation of statistical tests less straightforward, the consistent up-regulation of six genes upon progesterone treatment in women with abnormal cyclin E further suggests that they are co-regulated as a part of biological network, and points to biological pathways whose dysregulation may be associated with molecular changes that may result in a proportion of couples with a history of recurrent pregnancy loss.
Other genes significantly dysregulated in the endometria of women with REPL
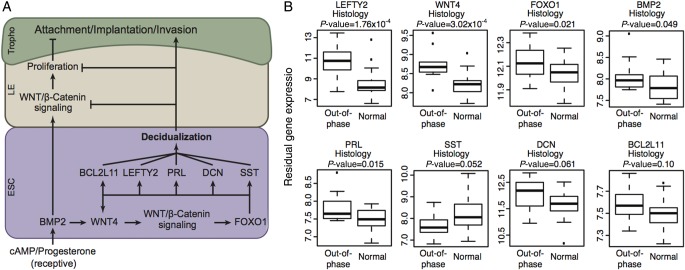
Although most genes were not significantly differentially expressed after adjusting for multiple testing, some genes with the largest magnitude of gene expression difference in each comparison have known important roles in the endometrial cycle. One striking example is left-right determination factor 2 (LEFTY2), which encodes endometrial bleeding associated factor, and had a 4.6-fold up-regulation in women with out-of-phase endometrial histology compared with those with normal histology (P = 1.76 × 10−4). Overabundance of LEFTY2 during the receptive endometrium stage has been associated with increased implantation failure (Tabibzadeh et al., 2000; Tang et al., 2005). In addition, LEFTY2 is co-regulated with the transcriptional factor forkhead box O1 (FOXO1), developmental protein wingless-type MMTV integration site family member 4 (WNT4), a TGF-β family member bone morphogenetic protein 2 (BMP2), pituitary hormone prolactin, extracellular matrix protein decorin (DCN), endocrine regulator somatostatin (SST) and apoptosis facilitator BCL2L11, all of which are required for differentiation of endometrial stromal cells into decidual cells (Grinius et al., 2006; Takano et al., 2007; Li et al., 2013) (Fig. 3A). Consistent with this model, we also observed up-regulation for most of these co-regulated genes in REPL women with out-of-phase histology (Fig. 3B). Collectively, these results suggest that increased expression of genes in this pathway have adverse effects on endometrium development, implantation and maintenance of early pregnancy.
Figure 3.
Differential expression of left-right determination factor 2 (LEFTY2) and Wnt/β-catenin pathway genes in women with out-of-phase endometrium. (A) The roles of Wnt/β-catenin pathway genes in the endometrium. (B) Expression level differences for Wnt/β-catenin pathway genes in women with recurrent early pregnancy loss with out-of-phase versus normal endometrial dating (WNT4: wingless-type MMTV integration site family, member 4; FOXO1: forkhead box O1; BMP2: bone morphogenetic protein 2; PRL: prolactin; SST: somatostatin; DCN: decorin; BCL2L11: BCL2-like 11 (apoptosis facilitator)).
Discussion
While global gene expression profiles in the receptive endometrium have been investigated extensively in healthy, fertile women (Borthwick et al., 2003; Riesewijk et al., 2003; Mirkin et al., 2005; Talbi et al., 2006; Haouzi et al., 2009), few studies have been performed in patients with reproductive disorders to identify specific genes or pathways underlying these conditions (Altmae et al., 2010; Othman et al., 2012). In this study we tested the hypothesis that the genes that gained endometrial expression during the evolution of pregnancy in Eutherian mammals, and therefore likely mediate successful pregnancies, are also more likely to be dysregulated in the endometria of women with endometrial-associated REPL. Indeed, we found that among genes differentially expressed in REPL endometria with abnormal histology or with elevated nuclear cyclin E levels of endometrial glands, there is a highly significant overrepresentation of genes that were recruited into endometrial expression in the stem-lineage of Eutherian mammals (Lynch et al., 2012). In stark contrast, there was no such enrichment for genes that were recruited into endometrial expression in the stem-lineage of Therian mammals or genes that were expressed in all species (Table II). These results indicate that the transcriptional regulation of genes that evolved endometrial expression in Eutherian mammals, coincident with the origin of traits like implantation, maternal–fetal communication, and maternal immunotolerance, is critical for proper endometrial functioning and in turn, successful pregnancy. Thus, these genes are excellent candidates for studies of human pregnancy disorders, potentially revealing novel molecular pathways and regulatory mechanisms with crucial roles in pregnancy.
Studying subgroups of REPL patients also gave us the opportunity to investigate and compare the gene expression profiles in two groups of women with endometrial-associated REPL, specifically, delayed endometrial development as determined by histology and elevated nuclear cyclin E levels. We found little overlap between the dysregulated genes in these two groups (Supplementary Table SIII) and distinct clustering of these two groups of women with endometrial-associated REPL based on their gene expression profiles (Supplementary Fig. S2). For the women with out-of-phase histology, the up-regulated genes were enriched for those involved in cell adhesion or cell migration, processes that characterize early-secretory phase endometrium and help to prepare endometrium for the receptive mid-secretory phase (Ruiz-Alonso et al., 2012). In addition, WNT/β-catenin signaling is also known to be tightly regulated during this phase (Ruiz-Alonso et al., 2012), and we have shown here that several members of this pathway were significantly up-regulated in REPL patients with out-of-phase endometrium (Fig. 3). In contrast, significantly down-regulated genes in women with elevated nuclear cyclin E levels were enriched for biological processes involved in immune response, cell signaling and communication, organ/system development, and cell adhesion. This enrichment suggests that cyclin E may be acting as a master negative regulator of genes involved in processes that characterize early to mid- and mid-secretory phase endometrium (Ruiz-Alonso et al., 2012) and that dysregulation of cyclin E causes widespread deficiencies of essential gene products. The importance of these functions for implantation and successful pregnancy has been highlighted in previous studies of gene expression in the mid-secretory phase endometrium of healthy individuals and of women with unexplained infertility, recurrent miscarriage or implantation failure (Altmae et al., 2010, 2012; Ledee et al., 2011). Aberrant expression of genes in these pathways may also affect the synchrony between different biological processes that collectively determine the temporal and spatial molecular landscape in the endometrium, and in turn, its ability to maintain early pregnancy.
Our findings may also impact clinical practice. The American Society of Reproductive Medicine (ASRM) Practice Committee considers endometrial dating by histology to be unreliable (Practice Committee of the American Society for Reproductive, 2012), although Coutifaris et al. recommended endometrial assessment with molecular markers (Coutifaris et al., 2004). Our study indicates that both histology and molecular markers, such as nuclear cyclin E levels, identify distinct groups of dysregulated genes and pathways. Repeated gene expression studies before and after treatment with vaginal micronized progesterone administration in five women with elevated nuclear cyclin E levels in endometrial glands suggest that this treatment may normalize aberrant gene expression profiles in women with endometrial-associated REPL. Although we did not have post-progesterone treatment biopsies on women with out-of-phase histology, the dysregulated pathways identified in this group are also known to be regulated by progesterone (e.g. Fig. 3A), further suggesting that these women may also benefit from this treatment. Thus, our study provides a molecular rationale for treating women with endometrial-associated REPL with vaginal micronized progesterone. RCTs are needed to clinically validate this observation.
Despite the potential clinical significance of our findings, this study was limited by small sample size and the lack of fertile control women. Although the size of our sample was comparable to many previous studies with similar aims (Carson et al., 2002; Borthwick et al., 2003; Riesewijk et al., 2003; Mirkin et al., 2005; Altmae et al., 2010), our study was underpowered for detecting significant differential expression at the genome-wide level. As a result, we have likely missed important differentially expressed genes, and some of our results could be false positives. However, the observed enrichment of functional gene categories that are crucial during the time of implantation and early pregnancy, as well as an enrichment of genes that evolved a function in the endometrium of Eutherian mammals, supports the validity of our data. The lack of fertile women without a history of REPL limited our ability to test whether expression patterns of the same or other genes in mid-secretory endometria are associated with REPL per se. We therefore suggest that these results form the basis for future studies to investigate this hypothesis further, and possibly shed additional light on the biological mechanisms that influence early maintenance of pregnancy.
In conclusion, the results of this study demonstrate unique expression profiles in the mid-luteal phase endometria collected from women with a history of REPL, with two types of endometrial diagnoses, out-of-phase histology and elevated cyclin E levels in endometrial glands, both of which may influence implantation and the maintenance of early pregnancy, thus impacting the likelihood of successful pregnancy outcome. We identified many candidate genes in each group of women that should lead to future molecular and clinical studies. Finally, our study demonstrates the potential of using an evolutionary-based forward genomics approach as a novel strategy for discovery of genes involved in REPL and possibly for other female reproductive disorders.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
Author contributions are as follows: G.K. designed research, performed research, analyzed data and wrote the manuscript; M.D.S. designed research, recruited patients, contributed data to the project and reviewed the manuscript; V.J.L. contributed data to the project, supervised the project and wrote the manuscript; C.O. designed research, supervised project and wrote the manuscript.
Funding
This work is supported by National Institutes of Health (NIH) grant R01 HD21244 to C.O.
Conflict of interest
The authors declare no competing financial interests.
Supplementary Material
Acknowledgements
We thank Patricia Schultz and Deborah Smith for their assistance in biopsy collection and patient coordination, and Christine Billstrand for her assistance with biopsy collection, sample storage and qRT–PCR analyses.
References
- Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci. 2011;18:229–251. doi: 10.1177/1933719110386241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmae S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16:178–187. doi: 10.1093/molehr/gap102. [DOI] [PubMed] [Google Scholar]
- Altmae S, Reimand J, Hovatta O, Zhang P, Kere J, Laisk T, Saare M, Peters M, Vilo J, Stavreus-Evers A, et al. Research resource: interactome of human embryo implantation: identification of gene expression pathways, regulation, and integrated regulatory networks. Mol Endocrinol. 2012;26:203–217. doi: 10.1210/me.2011-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9:19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Chabtini L, Mfarrej B, Mounayar M, Zhu B, Batal I, Dakle PJ, Smith BD, Boenisch O, Najafian N, Akiba H, et al. TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J Immunol. 2013;190:88–96. doi: 10.4049/jimmunol.1202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, McGovern PG, Schlaff WD, Carr BR, Steinkampf MP, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–1272. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]
- Dalrymple A, Slater DM, Poston L, Tribe RM. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1 beta. J Clin Endocrinol Metab. 2004;89:1291–1300. doi: 10.1210/jc.2003-031428. [DOI] [PubMed] [Google Scholar]
- Denny KJ, Woodruff TM, Taylor SM, Callaway LK. Complement in pregnancy: a delicate balance. Am J Reprod Immunol. 2013;69:3–11. doi: 10.1111/aji.12000. [DOI] [PubMed] [Google Scholar]
- Dubowy RL, Feinberg RF, Keefe DL, Doncel GF, Williams SC, McSweet JC, Kliman HJ. Improved endometrial assessment using cyclin E and p27. Fertil Steril. 2003;80:146–156. doi: 10.1016/s0015-0282(03)00573-9. [DOI] [PubMed] [Google Scholar]
- Fukui K, Yoshimoto I, Matsubara K, Hori R, Ochi H, Ito M. Leukocyte function-associated antigen-1 expression on decidual natural killer cells in patients with early pregnancy loss. Mol Hum Reprod. 1999;5:1083–1088. doi: 10.1093/molehr/5.11.1083. [DOI] [PubMed] [Google Scholar]
- Grewal S, Carver JG, Ridley AJ, Mardon HJ. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc Natl Acad Sci USA. 2008;105:16189–16194. doi: 10.1073/pnas.0806219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinius L, Kessler C, Schroeder J, Handwerger S. Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J Endocrinol. 2006;189:179–187. doi: 10.1677/joe.1.06451. [DOI] [PubMed] [Google Scholar]
- Haouzi D, Mahmoud K, Fourar M, Bendhaou K, Dechaud H, De Vos J, Reme T, Dewailly D, Hamamah S. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod. 2009;24:198–205. doi: 10.1093/humrep/den360. [DOI] [PubMed] [Google Scholar]
- Hou ZC, Sterner KN, Romero R, Than NG, Gonzalez JM, Weckle A, Xing J, Benirschke K, Goodman M, Wildman DE. Elephant transcriptome provides insights into the evolution of eutherian placentation. Genome Biol Evol. 2012;4:713–725. doi: 10.1093/gbe/evs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Hara T, Okamoto E, Kusunoki Y, Ohama K. Characteristic changes of large granular lymphocytes that strongly express CD56 in endometrium during the menstrual cycle and early pregnancy. Hum Reprod. 1998;13:1036–1043. doi: 10.1093/humrep/13.4.1036. [DOI] [PubMed] [Google Scholar]
- Ledee N, Munaut C, Aubert J, Serazin V, Rahmati M, Chaouat G, Sandra O, Foidart JM. Specific and extensive endometrial deregulation is present before conception in IVF/ICSI repeated implantation failures (IF) or recurrent miscarriages. J Pathol. 2011;225:554–564. doi: 10.1002/path.2948. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Das A, Demayo FJ, Hornsby PJ, Young SL, Taylor RN, Bagchi MK, Bagchi IC. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion M, Bisio A, Tebaldi T, De Sanctis V, Menendez D, Resnick MA, Ciribilli Y, Inga A. Interaction between p53 and estradiol pathways in transcriptional responses to chemotherapeutics. Cell Cycle. 2013;12:1211–1224. doi: 10.4161/cc.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch VJ, Nnamani M, Brayer KJ, Emera D, Wertheim JO, Kosakovsky Pond SL, Grutzner F, Bauersachs S, Graf A, Kapusta A, et al. Lineage-specific transposons drove massive gene expression recruitments during the evolution of pregnancy in mammals. 2012 arXiv:1208.4639 [q-bio.PE] [Google Scholar]
- Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20:2104–2117. doi: 10.1093/humrep/dei051. [DOI] [PubMed] [Google Scholar]
- Nebigil CG, Launay JM, Hickel P, Tournois C, Maroteaux L. 5-hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci USA. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman R, Omar MH, Shan LP, Shafiee MN, Jamal R, Mokhtar NM. Microarray profiling of secretory-phase endometrium from patients with recurrent miscarriage. Reprod Biol. 2012;12:183–199. doi: 10.1016/s1642-431x(12)60085-0. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–1111. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simon C. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9:253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- Ruiz-Alonso M, Blesa D, Simon C. The genomics of the human endometrium. Biochim Biophys Acta. 2012;1822:1931–1942. doi: 10.1016/j.bbadis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24–29. [PubMed] [Google Scholar]
- Stephenson M, Kutteh W. Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol. 2007;50:132–145. doi: 10.1097/GRF.0b013e31802f1c28. [DOI] [PubMed] [Google Scholar]
- Stephenson MD, Kutteh WH, Purkiss S, Librach C, Schultz P, Houlihan E, Liao C. Intravenous immunoglobulin and idiopathic secondary recurrent miscarriage: a multicentered randomized placebo-controlled trial. Hum Reprod. 2010;25:2203–2209. doi: 10.1093/humrep/deq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibzadeh S, Mason JM, Shea W, Cai Y, Murray MJ, Lessey B. Dysregulated expression of ebaf, a novel molecular defect in the endometria of patients with infertility. J Clin Endocrinol Metab. 2000;85:2526–2536. doi: 10.1210/jcem.85.7.6674. [DOI] [PubMed] [Google Scholar]
- Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21:2334–2349. doi: 10.1210/me.2007-0058. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Tang M, Taylor HS, Tabibzadeh S. In vivo gene transfer of lefty leads to implantation failure in mice. Hum Reprod. 2005;20:1772–1778. doi: 10.1093/humrep/deh849. [DOI] [PubMed] [Google Scholar]
- Terzidou V, Blanks AM, Kim SH, Thornton S, Bennett PR. Labor and inflammation increase the expression of oxytocin receptor in human amnion. Biol Reprod. 2011;84:546–552. doi: 10.1095/biolreprod.110.086785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thie M, Denker HW. In vitro studies on endometrial adhesiveness for trophoblast: cellular dynamics in uterine epithelial cells. Cells Tissues Organs. 2002;172:237–252. doi: 10.1159/000066963. [DOI] [PubMed] [Google Scholar]
- Tinel H, Denker HW, Thie M. Calcium influx in human uterine epithelial RL95-2 cells triggers adhesiveness for trophoblast-like cells. Model studies on signalling events during embryo implantation. Mol Hum Reprod. 2000;6:1119–1130. doi: 10.1093/molehr/6.12.1119. [DOI] [PubMed] [Google Scholar]
- Wang JW, Howson JM, Ghansah T, Desponts C, Ninos JM, May SL, Nguyen KH, Toyama-Sorimachi N, Kerr WG. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 2002;295:2094–2097. doi: 10.1126/science.1068438. [DOI] [PubMed] [Google Scholar]
- Zhao J, Lei Z, Liu Y, Li B, Zhang L, Fang H, Song C, Wang X, Zhang GM, Feng ZH, et al. Human pregnancy up-regulates Tim-3 in innate immune cells for systemic immunity. J Immunol. 2009;182:6618–6624. doi: 10.4049/jimmunol.0803876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.