Abstract
STUDY QUESTION
What is the role of microRNA-451 (miR-451) in human endometriotic tissue?
SUMMARY ANSWER
miR451 expression was elevated in endometriotic lesion tissue. MiR451 modulated the expression of macrophage migration inhibitory factor and limited cell survival.
WHAT IS KNOWN ALREADY
microRNAs are post-transcriptional regulators of gene expression which have been reported to be mis-expressed in endometriotic tissue. The exact pattern of expression and role of miR451 in endometriosis is currently unknown.
STUDY DESIGN, SIZE, DURATION
Thirty women with endometriosis are included in the study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Matched eutopic (N = 30) and endometriotic lesion tissue (N = 43) were collected. miR-451, macrophage migration inhibitory factor (MIF), cyclin E1 (CCNE) and phosphatase and tensin homolog (PTEN) mRNA expression were examined by quantitative real-time (qRT)-PCR while MIF protein expression was evaluated by western blot analysis. miR-451 regulation of MIF in vitro translation was confirmed by 3′untranslated region (UTR) reporter assays and western blot analysis. The effect of miR-451 on cell survival was assessed using a human endometrial epithelial cell line (HES).
MAIN RESULTS AND THE ROLE OF CHANCE
Compared with eutopic endometrium, both MIF mRNA and protein were significantly (P < 0.05) decreased in endometriotic lesions and this was associated with a significant (P < 0.05) increase in miR-451 expression. Transfection of HES cells with luciferase reporter constructs for MIF revealed that miR-451 specifically bound to the 3′UTR to regulate expression. Further, forced expression of miR-451 induced a significant (P < 0.05) down-regulation of both MIF mRNA and protein in HES cells which was associated with a significant (P < 0.05) reduction in cell survival. Inhibition of MIF using a specific antagonist verified that reduction of MIF contributes to HES cell survival.
LIMITATIONS, REASONS FOR CAUTION
miR-451 and MIF expression were only examined in tissue from women with endometriosis.
WIDER IMPLICATIONS OF THE FINDINGS
Our data support the hypothesis that miR-451 is elevated in endometriotic tissue and, through regulating MIF expression, may function to limit endometriotic lesion cell survival.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by the National Institutes of Health/NICHD by grant NIH HD069043 to W.B.N. The authors have no competing interests.
Keywords: endometriosis, miRNA, macrophage migration inhibitory factor
Introduction
Endometriosis is a debilitating disease which affects as many as 10% of women of reproductive age and is characterized by primary complaints of pelvic pain, dysmenorrhea and infertility (Giudice and Kao, 2004). Defined as the presence of ectopic endometrial stromal and glandular tissue, endometriosis is thought to develop via reverse menstruation of viable endometrial tissue into the peritoneal cavity. However, because almost all women of reproductive age exhibit some degree of retrograde menstruation (Halme et al., 1984; Liu and Hitchcock, 1986), it is postulated that additional, yet unidentified, factors must contribute to the development and progression of the disease.
MicroRNAs (miRNAs) have been proposed to play a role in the pathogenesis of endometriosis but beyond initial characterization, our understanding on their role in this disease is just beginning to evolve. Defined as a class of small non-coding regulatory RNAs that regulate gene expression post-transcriptionally (Bartel, 2004; Vasudevan et al., 2007), miRNAs have been implicated to play a vital role in cellular events, many of which are conducive to endometriosis development, including cell proliferation, invasion and apoptosis (Erson and Petty, 2008). miRNA expression profiles have been established for endometriosis in both the disease tissue and eutopic endometrium as well as control patients (Pan et al., 2007; Ohlsson Teague et al., 2009; Filigheddu et al., 2010; Hawkins et al., 2011; Braza-Boïls et al., 2014).
Of those miRNAs mis-expressed in endometriotic tissue, miR-451 is of specific interest. Based upon bioinformatic programs such as TargetScan and Miranda which predict miRNA targets, one of the proposed targets of miR-451 is macrophage migration inhibitory factor (MIF). MIF is a cytokine which is secreted by endometriotic cells in vitro and exhibits mitogenic activity promoting the growth of endothelial cells (Yang et al., 2000) as well as the ability to stimulate prostaglandin E2, cyclooxygenase-2 (Carli et al., 2009), vascular endothelial growth factor, interleukin-8 and monocyte chemotactic protein-1 (Veillat et al., 2010). These factors are all associated with a proliferative and angiogenic phenotype conducive to endometriotic establishment and/or growth. Further, in vivo evidence suggests that MIF is predominantly expressed in glandular epithelial cells of both eutopic endometrium (Arcuri et al., 2001; Kats et al., 2005) and robustly expressed in epithelium of active and early/stage I endometriotic lesions (Kats et al., 2005) with focal stromal staining in both tissue types. MIF levels are also elevated in the peritoneal fluid (Kats et al., 2002a,b) and peripheral blood (Morin et al., 2005) of women with endometriosis and MIF secretion is enhanced in peritoneal macrophages from women with the disease (Akoum et al., 2002). Using mouse experimental models for endometriosis, we (Nothnick et al., 2011) and others (Khoufache et al., 2012) have demonstrated that the MIF antagonist, ISO-1 reduces endometriotic lesion size. Collectively, these data strongly suggest that MIF is associated with the pathogenesis of endometriosis.
Although expressed by endometriotic lesion tissue, the expression and function of miR-451 as related to endometriosis remains controversial. miR-451 was initially reported (Pan et al., 2007) as one of the most differentially expressed miRNAs between normal eutopic endometrium and both eutopic and ectopic endometrial tissue from women with endometriosis (∼2-fold lower in endometriosis subjects). However, a more recent study by Hawkins et al. (2011) reported that miR-451 expression was elevated in ovarian endometriomas compared with eutopic endometrium. Lastly, a recent study from our group (Nothnick et al., 2014) reported that absence of miR-451 is associated with a reduced ability of endometrial tissue to establish ectopically in a murine experimental model for endometriosis. Thus, there is uncertainty with respect to the expression and function of miR-451 in the pathogenesis of endometriosis. The objective of the current study was to examine the expression of miR-451 in endometriotic tissue and matching eutopic endometrium in a group of women with endometriosis as well as explore the relationship with its target transcript MIF. Further we evaluated the functional role of miR-451 in mediating MIF expression and cell proliferation.
Materials and Methods
Human subjects and tissue acquisition
The study was approved by the institutional review boards of both the University of Kansas Medical Center and Cleveland Clinic. Written informed consent was obtained prior to surgical removal of endometriotic lesion tissue and endometrial biopsies.
Women with endometriosis who presented with pelvic pain due to failed previous endometriosis treatment and were undergoing surgical removal of endometriotic lesion tissue were enrolled. A total of 30 subjects were enrolled (Table I) with an average age of 33.7 years (range 21–40; median 34 years of age). Of these, 8 subjects were classified with stage I/II endometriosis (1 in the menstrual stage of the menstrual cycle, 3 in the proliferative phase, 1 in the secretory phase and 3 not exhibiting cycles) while the remaining 22 were classified with stage III/IV (1 in the menstrual phase of the menstrual cycle, 3 in the proliferative phase, 13 in the secretory phase and 5 not exhibiting menstrual cycles). Endometriosis classification was done according to the American Society for Reproductive Medicine guidelines (Canis et al., 1997).
Table I.
Study subject characteristics.
| Subject | Age | Cycle stage | Endometriosis stage | Lesion typea | Medicationsb |
|---|---|---|---|---|---|
| 12-0709B | 32 | No cycle | I/II | Perit | LNorg, OA, DS, I, F |
| 12-1130 | 25 | No cycle | I/II | Bl | None |
| 13-0705 | 40 | No cycle | I/II | Perit | None |
| 12-1112 | 34 | No cycle | III/IV | Perit | OA, DS |
| 12-1116 | 34 | No cycle | III/IV | Perit | LNorg |
| 13-0708 | 28 | No cycle | III/IV | Perit | OA, DS, I |
| 13-0830 | 31 | No cycle | III/IV | Perit, Vag | LNorg |
| 13-1025 | 23 | No cycle | III/IV | Perit | OA, DS, I |
| 12-0508 | 37 | Menstrual | I/II | Perit | R, P |
| 12-1029 | 37 | Menstrual | III/IV | Perit; Ov | OA, DS |
| 12-0817 | 34 | Proliferative | I/II | Perit | OA, DS |
| 12-1005 | 33 | Proliferative | I/II | Perit | MV, GC, I |
| 12-1029B | 30 | Proliferative | I/II | Perit | Pro, I |
| 12-0427 | 35 | Proliferative | III/IV | Perit | Na, DS, PreV |
| 12-0611B | 38 | Proliferative | III/IV | Perit | DS, H, M |
| 13-0301 | 35 | Proliferative | III/IV | Perit (2), Ov | P, L |
| 12-0518 | 22 | Secretory | I/II | Perit | OA, DS, I |
| 12-0611 | 34 | Secretory | III/IV | Perit (2) | OA, DS, I |
| 12-0615 | 28 | Secretory | III/IV | Perit | LNorg, OA, DS, I |
| 12-0820B | 37 | Secretory | III/IV | Ov | OA, DS, I, Oxy, PG |
| 12-1015 | 37 | Secretory | III/IV | Perit, R, Ov | DS, I |
| 12-1019 | 32 | Secretory | III/IV | Perit | DS, I, Mg(OH)2 |
| 13-0318 | 39 | Secretory | III/IV | Perit | None |
| 13-0419 | 34 | Secretory | III/IV | Perit (2), Ov (2) | A, Pro |
| 13-0419B | 31 | Secretory | III/IV | Perit (2) | I |
| 13-0520 | 21 | Secretory | III/IV | Perit | DS, I |
| 13-0617 | 33 | Secretory | III/IV | Perit (2) | OA, DS, I |
| 13-0708 | 28 | Secretory | III/IV | Perit | OA, DS, I |
| 13-0819 | 35 | Secretory | III/IV | Perit, Ov | I |
| 13-1005 | 36 | Secretory | III/IV | Perit | OA, DS, I, Ond |
aPerit, peritoneal lesion; Bl, bladder; Ov, ovarian endometrioma; R, rectal; (2), 2 separate lesions.
bLNorg, L-norgestimate + ethinyl estradiol; R, ranitidine; P, prednisone; OA, oxycodone-acetaminophen; DS, docusate sodium; I, ibuprofen; F, fluoxetine; MV, multivitamins; GC, glucosamine-chondroitin; Pro, promethazine; Na, naproxen; PreV, prenatal vitamins; H, hydromorphone; M, meclofenamate; L, loratadine; Oxy, oxybutynin; PG, polyethylene glycol; A, albuterol; Ond, ondansetron.
The majority (28/30) of patients were currently taking (or had taken within the past 3 months) anti-inflammatory regimes for pain while the remaining 2 of the 30 patients were on no medications. No subjects had taken GnRH analogs within the previous 3 months prior to surgery. A total of 30 endometrial biopsies (eutopic endometrium) and 43 endometriotic lesions were collected. The number of lesions obtained from the same subject ranged from 1 to 4. When multiple endometriotic lesions were obtained from the same patient, these lesions were treated as independent observations as endometriotic lesions are postulated to be heterogeneous within the same patient (Howell et al., 1994). All specimens were collected by the same surgeon (TF) at Cleveland Clinic with emphasis on minimizing sample contamination from underlying/surrounding non-endometriotic lesion tissue. To do so, endometriotic lesions were excised and sent to pathology for confirmation of endometriosis, which was defined as the presence of endometrial glands and stroma. Tissue was excised using sharp scissors with no energy. During the excision the underlying tissue was separated from the lesion tissue. A portion of the same sample lesion which was sent for endometriosis confirmation by a pathologist was utilized for research. Location of the site from which the endometriotic lesion(s) was/were excised was noted (Table I). Research samples were immediately snap-frozen, stored at −80°C and then shipped to the University of Kansas Medical Center. Samples were subjected to RNA and protein extraction followed by quantitative real-time (qRT)-PCR and Western analysis, respectively, as described below. As no difference in MIF mRNA, protein or miR-451 expression was noted among stages of the menstrual cycle, stages of endometriosis or influenced by medications, data were collapsed and analyzed as ectopic versus eutopic tissue for MIF mRNA and protein as well as miR-451 expression.
mRNA and miRNA qRT–PCR
QRT–PCR was performed as previously described (Nothnick and Healy, 2010; Nothnick et al., 2011, 2014). Briefly, total RNA was isolated using Tri-Reagent (Sigma Chemical Co., St. Louis, MO, USA) according to recommendations of the manufacturer. Total RNA (1 µg in 20 µl) was reverse transcribed using reverse transcription (RT) kits (Applied Biosystems; Foster City, CA, USA) following the manufacturer's protocol. Primers for macrophage migration inhibitory factor (MIF), cytokeratin 18 (variants 1 and 2; KRT18v1 and KRT18v2, respectively), phosphatase and tensin homolog (PTEN) and cyclin E1 (CCNE1) were designed using Primer-Blast and synthesized by Integrated DNA Technology (IDT, Coralville, IA). Sequences for the human MIF (NM_002415) primers were: forward, 5′-GCGCCTGCGCATCAG-3′ and reverse, 5′-CGCGTTCATGTCGTAATAGTTGA-3′, human KRT18v1(NM_000224): forward, 5′-GAGGGCTCAGATCTTCGCAA-3′ and reverse, 5′-AGCCCATGGATGTCGTTCTC-3′, KRT18v2(NM_199187): forward, 5′-AGCCTCGAGGGCCAACAAC-3′ and reverse, 5′-GTGAAGCTCATGCCCCCAGAA-3′, human PTEN (NM_000314): forward, 5′-AAGACATTATGACACCGCCAAA-3′ and reverse, 5′-GTGGGTTATGGTCTTCAAAAGGA-3′, human CCNE1 (NM_001238): forward, 5′- F – CAGGGAGCGGGATGCG-3′, and reverse, 5′- GGTCACGTTTGCCTTCCTCT-3′. Resulting material was then used for independent qRT–PCR. qRT–PCR was carried out on an Applied Biosystems HT7900 Sequence Detector. To account for differences in starting material, human 18S primers and probe reagents were used for MIF and values were expressed as fold change from the indicated control.
To assess miR-451 expression, miRNA kits for miR-451 (now designated miR-451a) were purchased from Applied Biosystems. Total RNA (250 ng in 5 µl) was reverse transcribed using RT kits (Applied Biosystems) following the manufacturer's protocol with the following modifications. Briefly, miRNAs were reverse transcribed in a single reaction using 2 µl of each miRNA specific 5X RT primers. Resulting material was then used for independent qRT–PCR for each miRNA. To normalize for starting material, a reverse snRNA U6 was included in the miRNA RT reactions and qRT–PCR of U6 was performed. qRT–PCR reactions were completed on a 7900 HT Sequence Detection System (Applied Biosystems). All samples were run in triplicate and the average value used in subsequent calculations. The 2-delta-delta CT method was used to calculate the fold-change values among samples as previously described by our group (Nothnick and Healy, 2010; Nothnick et al., 2011, 2014). All data are displayed as the mean ± SEM.
Western analysis
Total protein was extracted from frozen endometrial biopsies (N = 22/30) and lesion samples (N = 33/43) using RIPA buffer (1X RIPA, Catalog #9806, Cell Signaling Technologies [CST], Danvers, MA, USA). Protein concentration in each sample was determined using the Bio-Rad Protein Assay ([Catalog 3500-0006], Bio-Rad Laboratories, Richmond, CA, USA). The same amount of protein (10 μg) was subjected to 12% Bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane (w/v) gel electrophoresis and electroblotted onto PVDF membranes (Invitrogen). Rabbit anti-MIF (1:250; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and goat anti-rabbit secondary antibody (1:5000; GE Healthcare/Fisher Scientific, Pittsburgh, PA, USA) were used. Stripping and reprobing for β-actin (Abcam, Cambridge, MA, USA) was conducted to normalize MIF protein expression levels. Immunodetection was carried out using an enhanced chemiluminescence (ECL) kit (Thermo Scientific, Waltham, MA, USA).
Cell culture and transfection for reporter assays
The human endometrial epithelial cell line (HES) was provided by Dr. Douglas Kniss (The Ohio State University, Columbus, OH, USA; Kniss et al., 1997). HES cells were selected as the experimental model due to their expression of MIF mRNA and protein, relatively low level of miR451 expression (allowing minimization of competition with endogenous miR451 in our experiments) and transfection efficiency (unpublished observations). HES cells were cultured in phenol red-free Dulbecco's Minimum essential medium (DMEM)/Ham's F12 (Sigma Chemical Co.) + 10% charcoal stripped FBS (Atlanta Biologicals, Atlanta, GA, USA) + Pen-Strep (Sigma Chemical Co.; media referred herein as complete media) in T75 flasks and seeded at 1 × 106 cells/ml of media until ∼90% confluency. Cells were then passed and plated in 6- or 24-well plates at a density of 1 × 105 cells/ml in DMEM/Ham's F12 media lacking FBS and Pen-Strep. The next day, cells were transfected as described below for each specific experiment.
To assess the impact of miR-451 on MIF protein expression, HES cells were transfected with 30 nM of pre-miR451 (pre451; Life Technologies/Ambion, Inc., Grand Island, NY, USA) or a non-targeting mimic (NT-mimic, negative control; Life Technologies/Ambion, Inc.) using Lipofectamine 2000 (Life Technologies, Inc.) according to the recommendations of the manufacturer. Cells were cultured for 24 h and 48 h after which an aliquot of cells were prepared for miRNA isolation and miR-451 expression was verified by qRT–PCR as described above. Remaining cells were prepared for Western analysis of MIF protein as described above with the exception that cell lysis buffer (catalog #9803 from CST) was used in place of RIPA buffer.
Luciferase reporter assays
To determine if miR-451 binds to the 3′ untranslated region (UTR) of the MIF transcript to regulate MIF translation, luciferase reporter assays were performed. Wild-type and mutant Renilla luciferase constructs for MIF 3′UTR were kindly provided by Dr. Eva Bandres (University of Navarra, Pamplona, Spain; Bandres et al., 2009). Wild-type Renilla-MIF-3′untranslated region (UTR) constructs contained the following oligonucleotide sequences:
MIF-3′UTR-F: 5′-CTAGAGCCCACCCCAACCTTCTGGTGGGGAGAAATAAACGGTTTAGAGACTGC-3′, and MIF-3′UTR-R: 5′-GGCCGCAGTCTCTAAACCGTTTATTTCTCCCCACCAGAAGGTTGGGGTGGGCT-3′, while the mutated constructs contained: MIF-3′UTR-Mut-F: 5′-CTAGAGCCCACCCCAACCTTCTGGTGGGGAGAAATAGGTACTGAAGAGACTGC-3′, and MIF-3′UTR-Mut-R: 5′-GGCCGCAGTCTCTTCAGTACCTATTTCTCCCCACCAGAAGGTTGGGGTGGGCT-3′.
HES cells were co-transfected with the Renilla-MIF 3′UTR vector (0.2 µg) or the Renilla-MIF-3′UTR-mutant, a control vector containing firefly luciferase (0.05 µg; pGL3-Promoter; Promega Corp., Madison, WI, USA) plus either pre-miR-451 mimic oligonucleotide (451 mimic) or non-targeting (NT) mimic controls (30 nM each) using Lipofectamine 2000. All cells were cultured in 24-well plates for 24 h at 37°C after which both Renilla (reporter) and firefly (control for transfection efficiency) luciferase activity was determined using the Dual Luciferase reporter assay system following the protocol supplied by Promega.
Assessment of cell proliferation/cell survival
To determine if miR-451 modulates endometrial epithelial cell survival, HES cells were reverse transfected with pre-NT or pre-miR-451 mimics using siPORT™ NeoFX™ transfection agent (Life Technologies/Ambion, Inc.). Briefly, mimics (30 nM final concentration) were mixed with transfection reagent and placed into wells of 6-well plates. HES cells (1 × 105 cells in 1.8 ml of complete media) were over-layed onto the transfection reagent (0.2 ml volume) and then cultured for 24–48 h in 6-well plates. At the indicated time point, medium was removed and the percent of dead (non-attached) cells was assessed. Attached (viable) cells were harvested by mild trypsin digestion and an aliquot was subjected to trypan blue dye exclusion to assess cell viability and total cell number. All assessments were in duplicate. A separate group of cells which were transfected with pre-miR-451, pre-miR-NT mimic or media alone were subjected to RNA isolation and qRT–PCR quantification to assess miR-451 transfection and liberation of mature miR-451 expression.
To further demonstrate that the miR-451 target, MIF, may be at least partially responsible for mediating endometrial epithelial cell survival, we conducted an additional experiment in which HES cells were treated with increasing doses (1, 5, 10, 50 or 100 µM) of the MIF antagonist, ISO-1 (R&D Systems, Inc./Tocris Chemicals, Minneapolis, MN, USA) or vehicle (DMSO; Sigma Chemical Co.).
Statistical analysis
All data were analyzed using GraphPad Instat 3 (GraphPad Software, Inc., La Jolla, CA, USA). All data from human eutopic endometrium and endometriotic lesion samples were expressed as fold change from matched eutopic endometrial controls (ectopic/eutopic). Matched eutopic control values were expressed as the mean ± SEM which was determined by averaging baseline expression of each eutopic endometrial sample. Once the mean values were calculated for eutopic and ectopic end-points, data were then subjected to normality tests and those which were not considered normally distributed were analyzed using non-parametric tests. Specific data analysis methods are provided in each figure legend for lesion and eutopic endometrium (unpaired t-test or Mann–Whitney test). For in vitro cell culture studies, one-way ANOVA was used for comparison across treatment groups. When an F test indicated statistical significance, post hoc analysis was made using the Tukey honest significant difference procedure. Significance was set at P < 0.05 for all comparisons.
Results
MIF mRNA and protein expression in endometriotic tissue
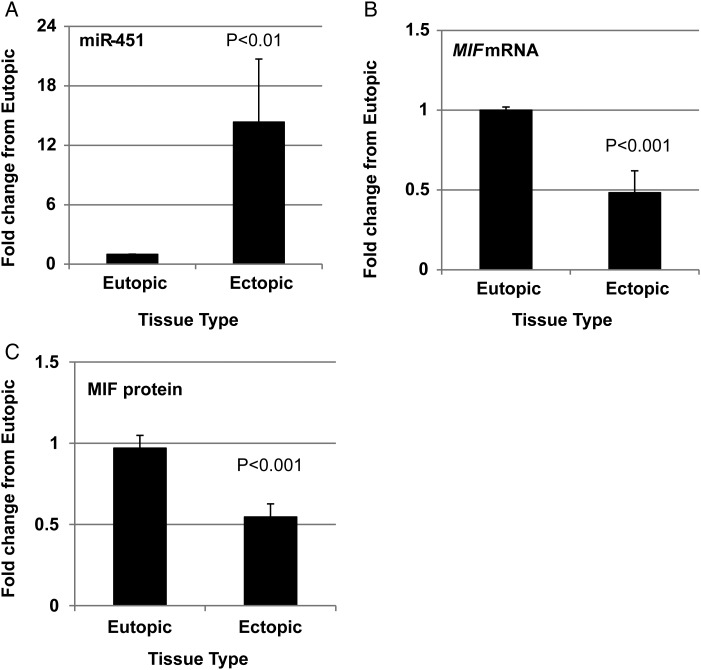
We first analyzed miR-451, MIF mRNA and MIF protein expression based upon stage of menstrual cycle, stage of endometriosis and current medications. In all assessments, data did not display normal distribution and were therefore analyzed using non-parametric tests (as specified in each table). No significant differences in miR-451, MIF mRNA, or MIF protein expression were detected based upon stage of the menstrual cycle (Supplementary Table SI), stage of endometriosis (Supplementaryl Table SII) or influence of medications such as oral contraceptives or anti-inflammatory agents (Supplementary Table SIII). Based upon these analyses, data were collapsed and analyzed as ectopic versus eutopic tissue for miR-451, MIF mRNA and MIF protein expression. miR-451, MIF mRNA and MIF protein expression was variable not only among patients but also in ectopic lesion tissue within the same subject. Overall, compared with eutopic endometrial tissue, ectopic tissue exhibited significantly higher levels of miR-451 (Fig. 1A) which was associated with both lower levels of MIF mRNA (Fig. 1B) and MIF protein (Fig. 1C) expression. To verify that changes in MIF or miR-451 transcript levels in endometriotic lesion could not be attributed to differences in the proportion of epithelial cells (the predominant source of both MIF and miR-451) among lesions and corresponding endometrial biopsies, we examined the expression of cytokeratin 18 (KRT18 variant 1; KRT18 variant 2 was expressed at very low levels and therefore not evaluated). There was no significant difference in the level of KRT18v1 expression between eutopic endometrium and corresponding ectopic tissue (P > 0.05; data not shown). The observation that KRT18v1 levels were essentially similar between tissue types and that the pattern of miR-451 (increased) and MIF (decreased) was not associated with the expression pattern of KRT18v1 expression suggested to us that the significant changes in MIF and miR-451 expression in lesion tissue were not due to an enrichment or reduction in the proportion of epithelial cell content compared with eutopic tissue.
Figure 1.
Endometriotic lesion microRNA-451 (miR-451) expression is elevated and inversely related to macrophage migration inhibitory factor (MIF) mRNA and protein expression compared with matched eutopic endometrium. Matched endometriotic lesion and corresponding eutopic endometrial tissue was processed for RNA isolation and (A) miR-451 and (B) MIF mRNA were examined by qRT–PCR while (C) MIF protein was evaluated by western blot analysis as described under ‘Materials and Methods’. Data are displayed as the mean ± SEM and P-values are indicated for each assessment. In total, 30 eutopic endometrial samples and 43 corresponding endometriotic lesion samples were analyzed for miR-451 and MIF mRNA, while 29 eutopic endometrial samples and 40 corresponding endometriotic lesion samples were analyzed for MIF protein expression. Data for miR-451, MIF mRNA and MIF protein did not pass normality testing and were therefore analyzed using the non-parametric Mann–Whitney test.
The observation that miR-451 over-expression was associated with significantly lower levels of MIF mRNA and MIF protein suggested that miR-451 may target MIF transcript to modulate translation as we originally proposed. To begin to test this hypothesis, we first evaluated if miR-451 could bind to the 3′ UTR of MIF.
miR-451 binds to the 3′ UTR of MIF
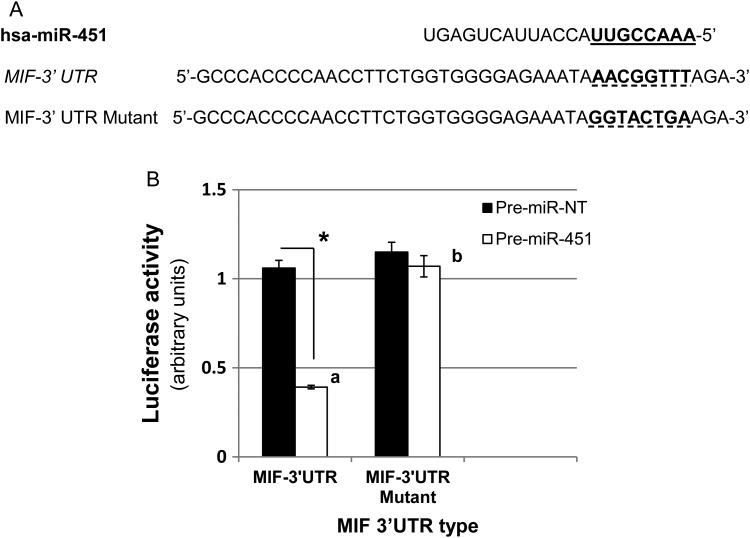
As MIF is predominantly expressed in endometrial/endometriotic epithelial cells (with endometrial stromal cells expressing little if any MIF [Arcuri et al., 2001; Kats et al., 2005]), we utilized an immortalized human endometrial epithelial cell line which would allow for efficient transfection studies. To determine if miR-451 regulates MIF translation in endometrial epithelial cells we first assessed if miR-451 was capable of binding to the 3′ UTR of the human MIF transcript using luciferase reporter assays. Immortalized human endometrial epithelial (HES) cells were transfected with Renilla reporters that contained either a wild-type (MIF-3′ UTR) or mutated miR-451 target sequence in the 3′ UTR (MIF-3′ UTR Mutant; Fig. 2A) as well as pre-miR-451 mimics. Analysis of Renilla luciferase activity indicated that ectopic expression of miR-451 inhibited the expression of the reporter vector containing wild-type MIF 3′ UTR but not the reporter vector containing the mutation of the seed miR-451 binding site (Fig. 2B). Forced expression of the negative control pre-miR-NT mimic had no effect on luciferase activity in cells transfected with either the wild-type or mutant MIF-3′ UTR constructs (Fig. 2B) demonstrating specificity of the effect.
Figure 2.
miR-451 targets the 3′UTR of MIF to regulate its expression. (A) Sequence alignment of human miR-451 with the 3′ UTR of MIF. Seed sequence of miR-451 which corresponds to the binding site within the 3′ UTR of MIF is highlighted in bold and underlined. This sequence was mutated in the 3′ UTR of MIF to provide a negative control demonstrating specificity of binding (mutated sequence is highlighted in bold and broken underline). (B) Luciferase assays were conducted as described in ‘Materials and Methods’. Pre-miR-451 significantly reduced Renilla luciferase activity in cells transfected with wild-type 3′UTR MIF construct, but not in cells transfected with the mutated 3′ UTR of MIF. Pre-miR-NT had no effect on Renilla luciferase activity in cells transfected with either the wild-type or mutant 3′ UTR MIF reporter constructs. Data are displayed as the mean ± SEM and are representative of four separate experiments (N = 4). Different letters indicate statistically significant different means within miR between 3′ UTR type while asterisk (*) indicates statistically significant different means within 3′ UTR type between pre-miRs. Data were analyzed using unpaired t-tests.
miR-451 down-regulates MIF protein levels by decreasing MIF transcript
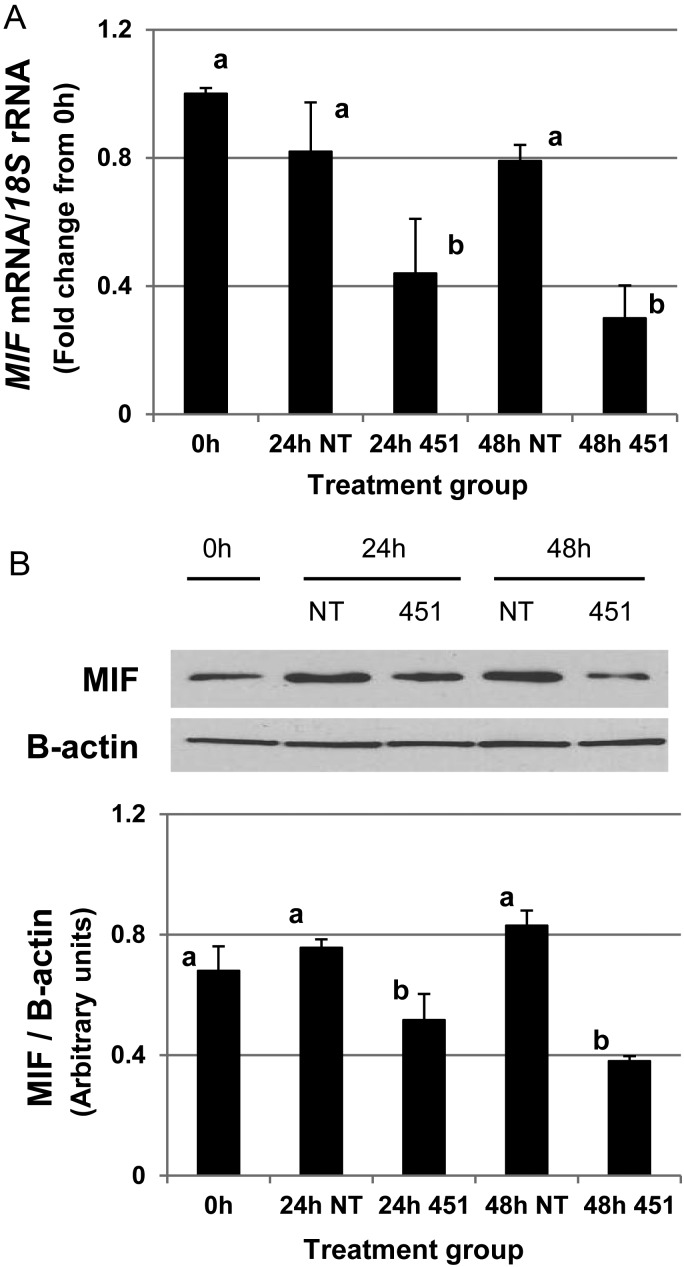
As the reporter assays (Fig. 2) demonstrated that miR-451 bound the 3′UTR of MIF, we next wished to determine if this binding led to reduced MIF protein expression/translation. To test the hypothesis, HES cells were transfected with either pre-miR-451 or pre-miR-NT mimics and MIF transcript and protein expression were evaluated. Forced expression of miR-451 but not that of the NT mimic was associated with a significant decrease in MIF transcript (Fig. 3A) and protein expression (Fig. 3B). Transfection of HES cells with pre-miR-451, but not with non-targeting mimics (NT-mimic) led to increased miR-451 expression as confirmed by qRT–PCR (data not shown). Collectively, these data suggested that MIF is a direct target of miR-451 and that the down-regulation of MIF may occur via inhibition of translation through degradation/reduction in transcript expression.
Figure 3.
miR-451 reduces MIF protein expression. Human endometrial epithelial cells (HES) were transfected as described in ‘Materials and Methods’ with either a non-targeting pre-miR mimic (NT) or a pre-miR-451 mimic (451). (A) qRT–PCR analysis of MIF expression in response to cell transfection with NT mimic and 451 mimic demonstrates a significant reduction in MIF transcript expression at both 24 and 48 h post-transfection. Data were normalized to the level of 18S expression and are expressed as the fold change from control (0 h; pre-transfection time point) ± SEM. (B) Western analysis of MIF protein in response to transfection with NT mimic (NT) or 451 mimic (451) demonstrates that forced expression of miR-451 results in a significant reduction in MIF protein expression. Bar graph represents normalized values of MIF/β-actin and data are displayed as the mean ± SEM. Different letters indicate statistically significant different means and are representative of four separate experiments (N = 4). Transfection with pre-miR-451 (451) but not with pre-miR-NT (NT) mimics results in a significant increase in miR-451 expression level at both 24 and 48 h post-transfection (data not shown). Data were analyzed by one-way ANOVA.
miR-451 reduces cell survival which may be attributed to MIF levels
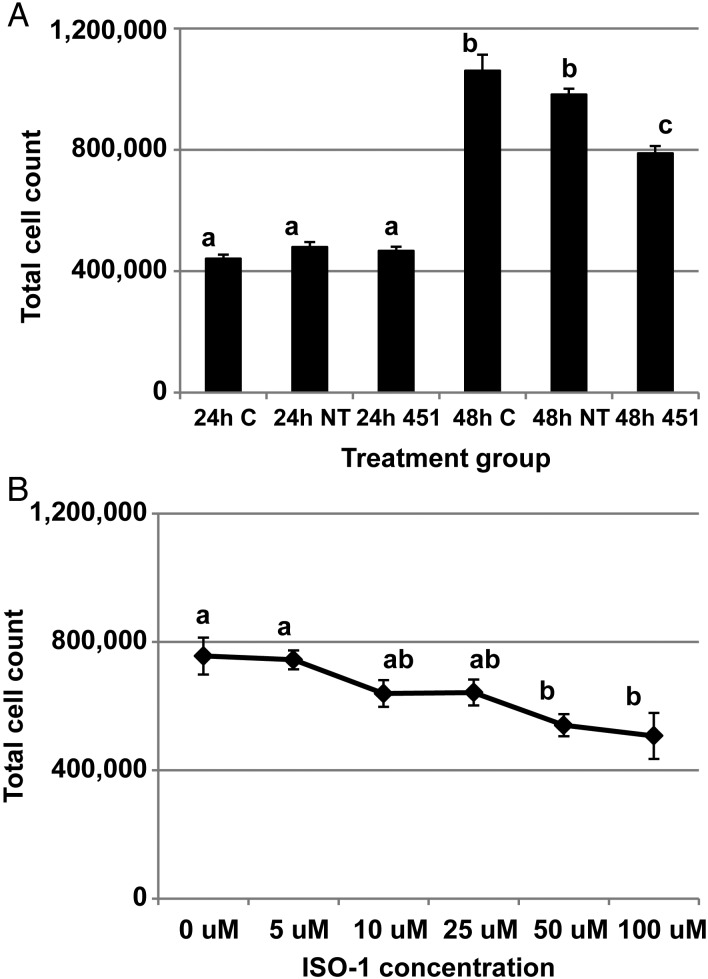
To this point we had established that human endometriotic lesion expresses reduced levels of MIF transcript and protein and that the down-regulation may be due to elevated miR-451 expression. As MIF has been proposed to be a cell survival factor and miR-451 has been associated with inhibition of proliferation, we next wished to determine if the elevated miR-451 expression in ectopic endometriotic tissue may be an endogenous mechanism to minimize lesion survival and growth. To test this hypothesis, HES cells were transfected with pre-miR-451 and cell survival/proliferation was assessed. We elected to perform cell count/trypan blue exclusion assays to allow concurrent assessment of viable and dead cells in the same sample. Forced expression of miR-451 was associated with a reduction in total cell number 48 h after transfection (Fig. 4A). To confirm that MIF was at least in part responsible for the miR-451 modulated cell survival, we performed an additional experiment in which HES cells were cultured for 48 h in the presence of increasing concentrations (0, 5, 10, 25, 50 or 100 µM) of the MIF antagonist, ISO-1. As our current study indicated that HES cells express high levels of MIF (upwards of 75 ng/ml by ELISA assessment) in culture, we postulated that inhibition using the MIF antagonist, ISO-1, may be the most efficient way to evaluate the ability of MIF to regulate cell survival as opposed to adding recombinant MIF protein to the cell cultures. As depicted in Fig. 4B, ISO-1 administration significantly decreased cell survival/total cell numbers at both the 50 and 100 µM concentrations. Taken together, we interpret these data to suggest that miR-451, through suppression of MIF translation, minimized cell survival and that elevated expression of miR-451 by endometriotic lesion tissue may be a compensatory mechanism to limit lesion tissue/epithelial cell survival in vivo.
Figure 4.
miR-451 targets macrophage migration inhibitory factor to regulate cell survival. (A) HES cells were transfected with no miRNA mimic (control = C), a non-targeting pre-miRNA (NT) or pre-miR-451 (451) and total cell count was assessed at 24 and 48 h after transfection. Different letters indicate statistical significance among the treatment groups. (B) MIF antagonist ISO-1 reduces cell survival. HES cells were cultured and treated with increasing doses of ISO-1 and cell survival was assessed 48 h post-treatment. Different letters indicate statistically significant differences among the doses. For both experiments, data are displayed as the mean ± SEM. Different letters indicate statistically significant different means and are representative of four separate experiments (N = 4). Transfection with pre-miR-451 (451) but not with pre-miR-NT mimics (NT) results in a significant increase in miR-451 expression level at both 24 and 48 h post-transfection (data not shown). Data were analyzed by one-way ANOVA.
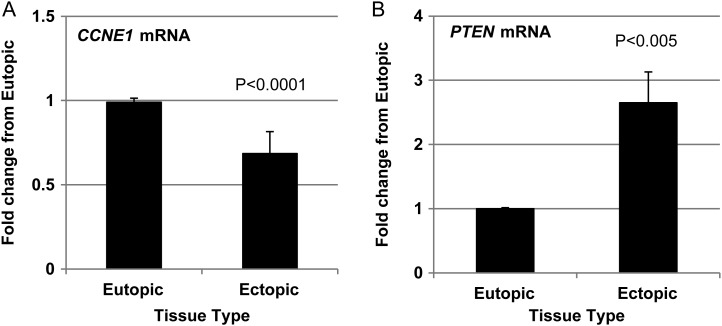
Cyclin E1 and PTEN are inversely expressed in endometriotic lesion tissue and their expression pattern is indicative of repressed proliferation
Based upon the in vitro observation that miR-451 and MIF are associated with cell proliferation/survival (Figs. 3 and 4), we examined lesion expression of cell proliferation marker cyclin E1 (CCNE1) and tumor suppressor, PTEN in those samples that were initially analyzed for miR-451 and MIF expression (Fig. 1). It should be noted that neither CCNE1 nor PTEN are postulated direct targets of miR-451 and as such, we selected these as independent markers of cell survival (Nakayama et al., 2010) and apoptosis (Yin and Shen, 2008), respectively. CCNE1 expression was significantly lower in endometriotic lesion tissue compared with that of matched endometrial samples (Fig. 5A). In contrast, expression of the pro-apoptotic factor, PTEN was significantly elevated in endometriotic lesion tissue compared with eutopic endometrium (Fig. 5B). This pattern of expression is consistent with a reduction in cell proliferation as is the earlier observations of increased miR-451 expression (Fig. 1A) and reduced MIF expression (Fig. 1B and C).
Figure 5.
Endometriotic lesion cell proliferation marker expression suggests reduced proliferative status of lesion tissue. Matched endometriotic lesion and corresponding eutopic endometrial tissue was processed for RNA isolation and (A) CCNE1 and (B) PTEN mRNA were examined by qRT–PCR for the same samples analyzed and displayed in Fig. 1. Data are displayed as the mean ± the standard error of the mean (SEM) and P-values are indicated for each assessment for 30 eutopic endometrial samples and 43 corresponding endometriotic lesion samples. Data for CCNE1 and PTEN mRNA did not pass normality testing and were therefore analyzed using the non-parametric Mann–Whitney test.
Discussion
miR-451 is a microRNA which is viewed largely as a tumor suppressor, preventing cell migration, proliferation and survival of carcinoma cells of human glioma (Nan et al., 2010) as well as those of gastrointestinal, nasopharyngeal, breast, liver and lung origin (Bandres et al., 2009; Wang et al., 2011; Bergamaschi and Katzenellenbogen, 2012; Liu et al., 2013, 2014a,b; Lv et al., 2014). The first characterization of miRNA expression in endometriotic lesion tissue suggested that miR-451 expression was significantly reduced in endometriotic lesions compared with eutopic endometrium (Pan et al., 2007). However, a more recent study indicated the opposite, with miR-451 being higher in lesion tissue (Hawkins et al., 2011). Potential sources of the discrepancy may be due to the type of lesion assessed, with only ovarian endometriomas being assessed in the study by Hawkins et al. (2011), while lesion type was not specified in the study by Pan et al. (2007). Further, the study by Hawkins and colleagues compared ovarian endometriomas to eutopic endometrium from a separate set of control eutopic endometrium (not matched to the same patient), where the study by Pan et al. (2007) compared lesion expression to eutopic endometrium to matched controls. In our study, we compared ectopic expression to matched eutopic endometrium from the same patient and assessed as well as specified the type (peritoneal, ovarian endometrioma) of lesion for each patient. We found no difference in miR-451 or MIF expression based upon type (peritoneal, ovarian endometrioma) of lesion. Based upon our current study, we propose that endometriotic lesion miR-451 is heterogeneous and reflects the proliferative state of the lesion (discussed in the following paragraphs).
One of the first reported targets of miR-451 that was shown to influence cell proliferation/survival was macrophage migration inhibitory factor (MIF; Bandres et al., 2009). MIF is expressed primarily in endometrial epithelial cells in eutopic endometrium (Arcuri et al., 2001; Kats et al., 2002a,b) and epithelium of active and early/stage I endometriotic lesions (Kats et al., 2002a,b). While expression of MIF in early stage, active lesions support the notion that MIF has a positive influence on cell proliferation/survival, this study did not compare MIF expression to a control tissue for reference such as eutopic endometrium. A functional role for MIF in survival of ectopic endometriotic lesions was more recently supported using experimental rodent models for endometriosis which incorporated the MIF antagonist, ISO-1 (Nothnick et al., 2011; Khoufache et al., 2012). These observations, coupled with data from the literature, suggest that MIF may play a role in endometriotic lesion cell survival; a concept which is further supported by both the in vitro and in vivo data obtained in the current study.
In the current study our in vitro experiments demonstrated that both reduction of MIF expression (by pre-miR-451 transfection) and function (using the MIF antagonist, ISO-1) suggest that MIF contributes to cell survival. In vivo assessment of endometriotic lesion MIF expression, compared with matched eutopic endometrium, revealed that the relative level of MIF expression was inversely related to the level of miR-451 expressed and that this pattern of expression was consistent with suppressed proliferation/survival as CCNE1 (proliferation/survival) was decreased and PTEN (suppressor of proliferation) was increased. It should be noted that neither CCNE1 nor PTEN are direct targets of miR-451 (based upon TargetScan 6.2 and other computational modeling systems). We hypothesized that analysis of these markers would allow for an independent assessment of the state of lesion survival/apoptosis independent of miR-451 expression.
The mechanisms which lead to altered miR-451 and/or MIF expression in endometriotic tissue remain largely unknown. In the current study we included all subjects which underwent surgical removal of endometriosis due to persistent and/or failed pain management. As such, our study population included subjects on oral contraceptives (OCPs) and/or non-steroidal anti-inflammatory drugs (NSAIDs) for pain management. Initial analysis of data by treatment (OCPs, NSAIDs, or no medications) did not reveal a statistically significant impact on our end-points based upon treatment group. This observation may suggest that these factors do not influence miR-451 and/or MIF in vivo or, that in these patients these medications are ineffective for modulating expression of these factors as well as the pain associated with the disease.
With respect to OCPs which contain synthetic estradiol and/or progestins, miR-451 has been shown to be up-regulated in murine endometrium in vivo by both estrogen and progesterone (Nothnick and Healy, 2010) and this induction can be blocked with estrogen and progesterone receptor antagonists (Nothnick and Healy, 2010). Similarly, the estrogen receptor antagonist, tamoxifen suppresses miR-451 expression in breast cancer cells in vitro (Bergamaschi and Katzenellenbogen, 2012), while the ability of progesterone to modulate miR451 expression in human cells or tissue is yet to be reported. Beyond these two reports, very little is known with respect to steroidal regulation of miR-451 expression either in vivo or in vitro.
In contrast to miR-451, regulation of MIF by estrogen is well-established with the majority of the research coming from the field of wound healing. Estrogen was first reported by Ashcroft et al. (2003) to down-regulate MIF expression in wounded tissue and a similar effect has been described in colon (Houdeau et al., 2007). Progesterone has been reported to increase MIF expression in rat colon (Houdeau et al., 2007) as well. Collectively, steroidal regulation of miR-451 and to a lesser extent, MIF, lacks detailed assessment. Currently, we are assessing steroidal regulation of both endometriotic lesion cell miR-451 and MIF in vitro to further define the mechanisms by which these factors are regulated in endometriotic lesion cells and tissue.
The ability of NSAIDs to modulate MIF expression and/or action appears to vary by experimental model. In vitro, NSAIDs such as aspirin and NS398 have been shown to decrease both MIF mRNA and protein in Eca-109 esophageal squamous carcinoma cells (Xia et al., 2005), while acetaminophen reduces MIF (tautomerization) activity (Altinoz and Korkmaz, 2004). Indomethacin, in contrast, increased gastric MIF expression in BALB/c mice in vivo (Ohkawara et al., 2011) while celecoxib treatment of patients with depression did not alter MIF levels compared with placebo treatment (Musil et al., 2011). While it is recognized that MIF is induced by, and associated with inflammation, (Asare et al., 2013), the ability of anti- inflammatory agents to modulate MIF expression appears inconclusive at this point.
The role of miR-451 in inflammatory conditions is less well understood as is its modulation by inflammatory mediators. Zhang and colleagues reported that miR-451 is one of several miRNAs whose expression is up-regulated in activated (polarized) murine macrophages (Zhang et al., 2013), but a more recent study examining neutrophils derived from patients with autoimmune arthritis revealed significantly lower levels of miR-451 compared with neutrophils from healthy control patients (Murata et al., 2014). Rosenberger and colleagues (Rosenberger et al., 2012) recently reported that miR-451 is up-regulated in murine dendritic cells infected with influenza. Further treatment of primary splenic dendritic cells with miR-451 antagomirs resulted in significantly greater secretion of inflammatory cytokines such as interleukin-6, tumor necrosis factor alpha, RANTES and MIP-1 alpha, suggesting that miR-451 may functionally regulate their expression. Information pertaining to NSAIDs regulation of miR-451 is limited to a single report which demonstrated that NSAIDs can partially off-set miR-451 induced cardiomyocyte survival (Zhang et al., 2010). Thus, when we take into account the information in the literature coupled with the current findings in our study (no significant differences in miR-451 or MIF expression in those patients taking OCPs or NSAIDs), it appears that the detected differences in these end-points are due to inherent differences in the endometriotic tissue and not associated with modulation of steroidal or inflammatory pathways which may be respectively associated with OCP and NSAIDs use.
In summary, endometriotic lesion miR-451 expression was significantly elevated while MIF expression was significantly lower compared with matched eutopic endometrial tissue. In vitro analysis revealed that miR-451 reduced MIF mRNA levels and protein translation by targeting the 3′ UTR of MIF. Forced expression of miR-451 resulted in reduced MIF protein levels and cell survival. The role of MIF in cell survival was confirmed independently using a MIF antagonist. Lastly, elevated miR-451 and reduced MIF was associated with reduced cell proliferation as CCNE1 expression was reduced and PTEN elevated in endometriotic lesion tissue. From these observations, we propose that within the context of endometriosis pathogenesis, elevated miR-451 may function to regulate MIF expression in an attempt to curtail endometriotic lesion tissue/cell survival. In contrast, lesions which express low levels of miR-451 and elevated MIF may be more apt to survive. The potential of a switch from low level to elevated expression of miR-451 and modulation of MIF expression as a mechanism to limit survival during the lifespan of endometriotic lesions is currently being explored.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
A.G.: Performed the experiments, analyzed the data and wrote and edited the manuscript. T.F.: Oversaw collection of and provided human specimens and wrote and edited the manuscript. W.B.N.: conceived the ideas, designed the experiments, performed experiments, performed statistical analysis and edited the manuscript.
Funding
This study was funded by the National Institutes of Health/NICHD by grant NIH HD069043 to W.B.N.
Conflict of interest
None declared.
Supplementary Material
References
- Akoum A, Kong J, Metz C, Beaumont MC. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil Steril. 2002;77:989–994. doi: 10.1016/s0015-0282(02)03082-0. [DOI] [PubMed] [Google Scholar]
- Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51:239–247. [PubMed] [Google Scholar]
- Arcuri F, Ricci C, Ietta F, Cintorino M, Tripodi SA, Cetin I, Garzia E, Schatz F, Klemi P, Santopietro R, et al. Macrophage migration inhibitory factor in the human endometrium: expression and localization during the menstrual cycle and early pregnancy. Biol Reprod. 2001;64:1200–1205. doi: 10.1095/biolreprod64.4.1200. [DOI] [PubMed] [Google Scholar]
- Asare Y, Schmitt M, Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb Haemost. 2013;109:391–398. doi: 10.1160/TH12-11-0831. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–1318. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, et al. MicroRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bergamaschi A, Katzenellenbogen BS. Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene. 2012;31:39–47. doi: 10.1038/onc.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braza-Boïls A, Marí-Alexandre J, Gilabert J, Sánchez-Izquierdo D, España F, Estellés A, Gilabert-Estellés J. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod. 2014;29:978–988. doi: 10.1093/humrep/deu019. [DOI] [PubMed] [Google Scholar]
- Canis M, Donnez JG, Guzick DS, Halme JK, Rock JA, Schenken RS, Vernon MW. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150:3128–3137. doi: 10.1210/en.2008-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erson AE, Petty EM. MicroRNAs in development and disease. Clin Genet. 2008;74:296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- Filigheddu N, Gregnanin I, Proporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol. 2010:369549. doi: 10.1155/2010/369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional MircoRNA involved in endometriosis. Mol Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdeau E, Moriez R, Leveque M, Salvador-Cartier C, Waget A, Leng L, Bueno L, Bucala R, Fioramonti J. Sex steroid regulation of macrophage migration inhibitory factor in normal and inflamed colon in the female rat. Gastroenterology. 2007;132:982–993. doi: 10.1053/j.gastro.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Howell RJ, Dowsett M, Edmonds DK. Oestrogen and progesterone receptors in endometriosis: heterogeneity of different sites. Hum Reprod. 1994;9:1752–1758. doi: 10.1093/oxfordjournals.humrep.a138788. [DOI] [PubMed] [Google Scholar]
- Kats R, Collette T, Metz CN, Akoum A. Marked elevation of macrophage migration inhibitory factor in the peritoneal fluid of women with endometriosis. Fertil Steril. 2002a;78:69–76. doi: 10.1016/s0015-0282(02)03189-8. [DOI] [PubMed] [Google Scholar]
- Kats R, Metz CN, Akoum A. Macrophage migration inhibitory factor is markedly expressed in active and early-stage endometriotic lesions. J Clin Endocrinol Metab. 2002b;87:883–889. doi: 10.1210/jcem.87.2.8260. [DOI] [PubMed] [Google Scholar]
- Kats R, Al-Akoum M, Guay S, Metz C, Akoum A. Cycle-dependent expression of macrophage migration inhibitory factor in the human endometrium. Hum Reprod. 2005;20:3518–3525. doi: 10.1093/humrep/dei234. [DOI] [PubMed] [Google Scholar]
- Khoufache K, Bazin S, Girard K, Guillemette J, Roy MC, Verreault JP, Al-Abed Y, Foster W, Akoum A. Macrophage migration inhibitory factor antagonist blocks the development of endometriosis in vivo. PLoS One. 2012;7:e37264. doi: 10.1371/journal.pone.0037264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss DA, Zimmerman PD, Garver CL, Fertel RH. Interleukin-1 receptor antagonist blocks interleukin-1-induced expression of cyclooxygenase-2 in endometrium. Am J Obstet Gynecol. 1997;177:559–567. doi: 10.1016/s0002-9378(97)70146-7. [DOI] [PubMed] [Google Scholar]
- Liu DTY, Hitchcock A. Endometriosis: Its association with retrograde menstruation, dysmenorrhea, and tubal pathology. Br J Obstet Gynaecol. 1986;93:859–886. doi: 10.1111/j.1471-0528.1986.tb07995.x. [DOI] [PubMed] [Google Scholar]
- Liu N, Jiang N, Guo R, Jiang W, He QM, Xu YF, Li YZ, Tang LL, Mao YP, Sun Y, et al. MiR-451 inhibits cell growth and invasion by targeting MIF and is associated with survival in nasopharyngeal carcinoma. Mol Cancer. 2013;12:123. doi: 10.1186/1476-4598-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liu C, Wang X, Ingvarsson S, Chen H. MicroRNA-451 suppresses tumor growth by down-regulating IL6R gene expression. Cancer Epidemiol. 2014a;38:85–92. doi: 10.1016/j.canep.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang X, Xiang J, Lv Y, Shi J. miR-451: potential role as tumor suppressor of human hepatoma cell growth and invasion. Int J Oncol. 2014b;45:739–745. doi: 10.3892/ijo.2014.2446. [DOI] [PubMed] [Google Scholar]
- Lv G, Hu Z, Tie Y, Du J, Fu H, Gao X, Zheng X. MicroRNA-451 regulates activating transcription factor 2 expression and inhibits liver cancer cell migration. Oncol Rep. 2014;32:1021–1028. doi: 10.3892/or.2014.3296. [DOI] [PubMed] [Google Scholar]
- Morin M, Bellehumeur C, Therriault MJ, Metz C, Maheux R, Akoum A. Elevated levels of macrophage migration inhibitory factor in the peripheral blood of women with endometriosis. Fertil Steril. 2005;83:865–872. doi: 10.1016/j.fertnstert.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Murata K, Yoshitomi H, Furu M, Ishikawa M, Shibuya H, Ito H, Matsuda S. MicroRNA-451 down-regulates neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol. 2014;66:549–559. doi: 10.1002/art.38269. [DOI] [PubMed] [Google Scholar]
- Musil R, Schwarz MJ, Riedel M, Dehning S, Cerovecki A, Spellmann I, Arolt V, Müller N. Elevated macrophage migration inhibitory factor and decreased transforming growth factor-beta levels in major depression—no influence of celecoxib treatment. J Affect Disord. 2011;134:217–225. doi: 10.1016/j.jad.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Nakayama K, Shaimima Y, Ishikawa M, Katagiri A, Iida K, Miyazaki K. Gene amplification CCNE1 is related to poor survival and potential therapeutic target in ovarian cancer. Cancer. 2010;116:2621–2634. doi: 10.1002/cncr.24987. [DOI] [PubMed] [Google Scholar]
- Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Zhong Y, Kang C. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- Nothnick WB, Healy CA. Estrogen induces distinct patterns of microRNA expression within the mouse uterus. Reprod Sci. 2010;17:987–994. doi: 10.1177/1933719110377472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnick WB, Colvin A, Cheng KF, Al-Abed Y. The macrophage migration inhibitory factor antagonist, ISO-1 suppresses endometriotic lesion size in mice with experimentally induced disease. J Endometr. 2011;3:135–142. [PMC free article] [PubMed] [Google Scholar]
- Nothnick WB, Graham A, Holbert J, Weiss MJ. miR-451 deficiency is associated with altered endometrial fibrinogen alpha chain expression and reduced endometriotic lesion establishment in an experimental mouse model. PLoS One. 2014;9:e100336. doi: 10.1371/journal.pone.0100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara T, Takeda H, Ohnishi S, Kato M, Nishihira J, Asaka M. Macrophage migration inhibitory factor contributes to development of nonsteroidal anti-inflammatory drugs-induced gastric injury in mice. Int Immunopharmacol. 2011;11:418–423. doi: 10.1016/j.intimp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23:265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- Rosenberger CM, Podyminogin RL, Navarro G, Zhao GW, Askovich PS, Weiss MJ, Aderem A. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189:5965–5975. doi: 10.4049/jimmunol.1201437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Veillat V, Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. 2010;95:403–412. doi: 10.1210/jc.2010-0417. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang ZX, Yang JS, Pan X, De W, Chen LB. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14) Oncogene. 2011;30:2644–2658. doi: 10.1038/onc.2010.642. [DOI] [PubMed] [Google Scholar]
- Xia HH, Zhang ST, Lam SK, Lin MC, Kung HF, Wong BC. Expression of macrophage migration inhibitory factor in esophageal squamous cell carcinoma and effects of bile acids and NSAIDs. Carcinogenesis. 2005;26:11–15. doi: 10.1093/carcin/bgh279. [DOI] [PubMed] [Google Scholar]
- Yang Y, Degranpré P, Kharfi A, Akoum A. Identification of macrophage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Metab. 2000;85:4721–4727. doi: 10.1210/jcem.85.12.7003. [DOI] [PubMed] [Google Scholar]
- Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008;27:5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, Jegga AG, Fan G-C. Synergistic effect of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell Cardiol. 2010;49:841–850. doi: 10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med. 2013;31:797–802. doi: 10.3892/ijmm.2013.1260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.