Abstract
Nutritional conditions in early life may affect adult health, but prior studies of mortality have been limited to small samples. We evaluated the relationship between pre-/perinatal famine exposure during the Dutch Hunger Winter of 1944–1945 and mortality through age 63 years among 41,096 men born in 1944–1947 and examined at age 18 years for universal military service in the Netherlands. Of these men, 22,952 had been born around the time of the Dutch famine in 6 affected cities; the remainder served as unexposed controls. Cox proportional hazards models were used to estimate hazard ratios for death from cancer, heart disease, other natural causes, and external causes. After 1,853,023 person-years of follow-up, we recorded 1,938 deaths from cancer, 1,040 from heart disease, 1,418 from other natural causes, and 523 from external causes. We found no increase in mortality from cancer or cardiovascular disease after prenatal famine exposure. However, there were increases in mortality from other natural causes (hazard ratio = 1.24, 95% confidence interval: 1.03, 1.49) and external causes (hazard ratio = 1.46, 95% confidence interval: 1.09, 1.97) after famine exposure in the first trimester of gestation. Further follow-up of the cohort is needed to provide more accurate risk estimates of mortality from specific causes of death after nutritional disturbances during gestation and very early life.
Keywords: cohort studies, Dutch Hunger Winter, famine, fetal origins, prenatal exposure delayed effects, undernutrition
Variations in maternal nutrition during pregnancy may result in changes in fetal structure and function that affect susceptibility to chronic disease in later life (1–3). This body of literature builds on ecological studies by Kermack et al. (4) and Forsdahl (5) linking circumstances around the time of birth with adult death from heart disease and other conditions. The so-called “fetal origins hypothesis” was popularized following Barker's studies on the relationship between size at birth and later health (6).
Birth weight is inversely associated with coronary heart disease (7–9) and with hypertension, insulin resistance, type 2 diabetes mellitus, and impaired glucose tolerance (10–17). Birth weight is also associated with mortality, both directly from cancer (18) and inversely from circulatory diseases (19, 20).
A well-recognized limitation of these studies is that birth weight is a poor measure of maternal nutrition during pregnancy. Even when pregnant women are exposed to extreme starvation, a decrease in infant birth weights is seen only after exposure in late pregnancy (21). Because exposure to severe famine in early gestation (but not in late gestation) is associated with hypomethylation in key regulatory enzyme pathways (22), it is clear that further research on the long-term consequences of fetal programming needs to move beyond studies of birth weight (23).
The circumstances of the Dutch Hunger Winter of 1944–1945, with civilian starvation caused by the conditions of World War II, offer a special opportunity to study the relationship between maternal nutrition in pregnancy and adult health (24, 25). The severity and widespread nature of the famine have been fully documented (24, 26–28). The famine ceased soon after the German surrender in May 1945, when Allied food supplies were rapidly distributed across the country (Figure 1).
Figure 1.
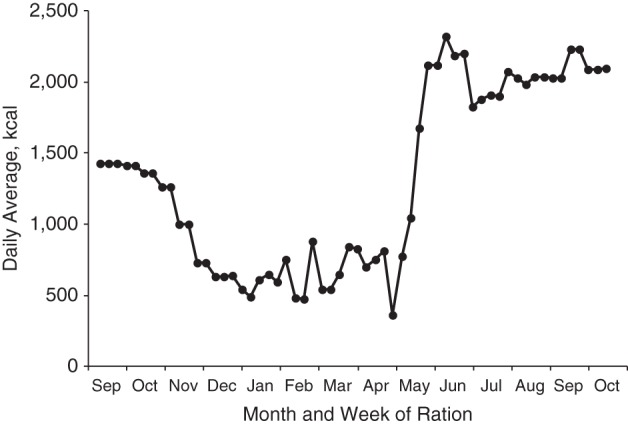
Food rations in the western Netherlands (daily average from weekly rations (26)) during the Dutch Hunger Winter, September 1944–October 1945.
Prior studies have shown an increase in body mass index (weight (kg)/height (m)2) and the prevalence of type 2 diabetes mellitus in both men and women after prenatal famine exposure (29–32), but results have been inconsistent with respect to cardiovascular disease (33, 34). In a small study, women with early-gestation famine exposure were found to have an increased risk of breast cancer (35). To date, mortality studies have been limited to fetal and infant mortality (36) or to small studies with few deaths (37).
We used the quasi-experimental circumstances of the Dutch famine to determine, in national male birth cohorts, whether famine exposure during gestation is associated with mortality from selected causes between the ages of 18 and 63 years. Our aim in this study was to collect reliable empirical evidence for any such association and to help formulate specific hypotheses for testing in later studies. We reported previously that famine exposure during the first trimester of gestation (but not in other periods of gestation) was associated with an increase in all-cause mortality (hazard ratio (HR) = 1.12, 95% confidence interval (CI): 1.01, 1.24) (38).
METHODS
Study population
As described previously, we studied male conscripts who had been born in the Netherlands between January 1, 1944, and December 31, 1947, and were examined for military service at age 18 years (38). These physical examinations include all male Dutch citizens aged 18 years who are listed in national population registers, except those living in psychiatric hospitals or in special institutions for the blind or the deaf-mute (0.6%). For those not examined, the military record still provides full demographic information and relevant medical diagnoses from the institution for complete mortality follow-up.
We considered all men born between November 1944 and March 1946 in any of the 6 most affected cities in the western Netherlands (Amsterdam, Haarlem, Rotterdam, The Hague, Leiden, and Utrecht) as likely to have had gestational famine exposure (n = 25,283). To select unexposed controls, we randomly sampled 15% of men born before November 1944 or after March 1946 in these same cities (n = 10,667) and 3% of men born between 1944 and 1947 in the remainder of the country (n = 9,087). This provided us with 45,037 physical examination records for mortality follow-up. Famine exposure was not related to hospitalization in psychiatric or special institutions.
Under confidentiality procedures approved by the Dutch military, identifying numbers from the examination records were linked to individual names by the Office of Registration and Information on Discharged Personnel at the Netherlands Ministry of Defence and further linked to population and death records at Statistics Netherlands. Additional details are given elsewhere (38). Study investigators had no access to individually identifiable information, and by determination of the Columbia University Medical Center Institutional Review Board, the US Department of Health and Human Services definition of “human subjects” did not apply to this study population. Follow-up continued through 2010 for all men, by which time the youngest survivors (those born in December 1947) were 63 years of age. The death records provide primary and secondary causes of death, classified according to the version of the International Classification of Diseases (ICD) that was in use at the time of death (see Web Table 1, available at http://aje.oxfordjournals.org/).
Famine exposure
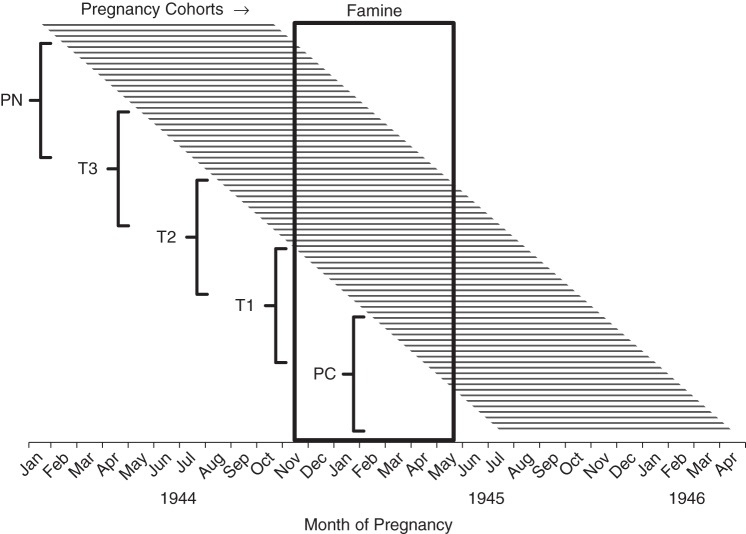
Famine exposure in any specific trimester of pregnancy was defined as a mean caloric ration of 900 kcal/day or less during that trimester (based on date of birth) in relation to distributed weekly food rations in the western Netherlands, assuming a uniform gestation period of 9 months (38). Accordingly, persons born between November 1, 1944, and March 31, 1945, were defined as having been exposed during the immediate postnatal period; persons born between February 1, 1945, and June 30, 1945, were defined as having been exposed during the third trimester of pregnancy (T3); persons born between May 1, 1945, and September 30, 1945, were defined as having been exposed during the second trimester of pregnancy (T2); persons born between August 1, 1945, and December 31, 1945, were defined as having been exposed during the first trimester of pregnancy (T1); and persons born between November 1, 1945, and March 31, 1946, were defined as having been exposed during the periconceptional period. We also considered exposure to famine at any time in gestation by defining as “exposed in T1–T3” any individual with famine exposure in T3, T2, or T1 (Figure 2).
Figure 2.
Famine exposure categories for 5 pregnancy cohorts among births taking place in the western Netherlands during the Dutch Hunger Winter, January 1944–April 1946. Famine exposure was defined as an average distributed food ration of 900 kcal/day or less during the exposure period of interest. PN, postnatal (exposed in the immediate postnatal period); T3, third trimester (exposed during the third trimester of pregnancy); T2, second trimester (exposed during the second trimester of pregnancy); T1, first trimester (exposed during the first trimester of pregnancy); PC, periconception (exposed just before the estimated date of conception).
Relevant characteristics from military examination interviews
We selected for further analysis specific information collected at the medical examination that reflected birth characteristics (father's occupation, religion) as one subgroup and characteristics that might plausibly mediate the relationship between famine exposure and mortality (education, body mass index, fitness for military service) as another subgroup (38). Educational level was classified in 4 categories (39): primary school (ages 6–12 years); lower vocational education (2 years post–primary school); lower secondary education (4 years post–primary school); and more advanced secondary education (≥6 years post–primary school). Men who did not complete primary school or who attended schools for the physically or mentally disabled were placed in a separate category.
Father's occupation was classified as nonmanual or manual. The nonmanual group included upper professional, lower professional, managerial, and clerical occupations, and the manual group included self-employed proprietors, craftsmen and foremen, shop assistants, operatives, process workers, domestic and other service workers, mine workers, and laborers. Farm workers and farm owners were classified separately in view of their likely easier access to food supplies during the famine. Unknown paternal occupations were also classified separately.
Religion was classified as Roman Catholic, Protestant (Dutch Reformed or Calvinist), other, or none, based on self-report. The number of siblings as reported by the examinee was used as an indication of family size. Religion was analyzed in view of the prevailing regional gradient in the Netherlands (Protestant north vs. Catholic south), its relationship to socioeconomic group, and the late fertility decline among Dutch Roman Catholics in comparison with other religious denominations (40).
Place of birth as recorded in the national population register was confirmed at military examination.
Examination results were summarized as a determination of status regarding fitness for military service (fit, almost fit, fairly fit, or unfit). Body mass index was calculated from measured weight and height and classified into 4 categories: <19, 19–20, 21–24, or ≥25.
Causes of death
Causes of death were coded according to the International Classification of Diseases, Tenth Revision (ICD-10), for deaths recorded during the years 1996–2010, the Ninth Revision of the ICD for deaths recorded in 1979–1995, the Eighth Revision for deaths recorded in 1969–1978, and the Seventh Revision for deaths recorded in 1963–1968. Following the primary study question, we first examined deaths from cancer (malignant neoplasms), heart disease, and other causes of death, including other natural causes of death and external causes of death. Codes from all previous versions of the ICD were translated to ICD-10 codes. Web Table 1 provides the specific codes used for each revision of the ICD. In subgroup analyses, we examined all ICD-10 categories that included 100 or more deaths.
Statistical analysis
We created binary variables (1 if exposed; 0 otherwise) for each of the 5 famine exposure categories (immediate postnatal period, T3, T2, T1, and periconceptional period) and for the combined exposure category T1–T3 (1 if exposed in T1, T2, or T3; 0 otherwise).
For each of the 5 exposure periods from immediate postnatal period to periconception, we estimated the period-specific mortality risk using all available controls after removing births that occurred in any of the other 4 exposure periods. For the combined exposure category T1–T3, we estimated risk after removing births with only immediate postnatal or periconceptional exposure from the model. Therefore, all exposure-specific risk estimates used the same control group. The study outcome was mortality from selected causes of death between the ages of 18 and 63 years.
Age at death was evaluated in Cox proportional hazards models (41) with age as the time scale, using the stcompadj procedure (42) in STATA (StataCorp LP, College Station, Texas) (43). Age at study entry was set at 18 years, and age at follow-up was determined by age at any of the following events: death, emigration, or loss to follow-up from other causes. The STATA stcompadj procedure allows for the analysis of competing risks (causes of death) using the Cox proportional hazards model. We confirmed the adequacy of the proportional hazards assumption by examining plots of Schoenfeld residuals (44). Two-tailed significance tests were used throughout. Statistical significance was declared at P < 0.05. The causes of death were also evaluated with Fine and Gray proportional hazards models for competing risks (45).
In sensitivity analyses, we compared the mortality estimates for each of the 5 exposure groups taken singly with estimates including additional adjustment for all other exposure groups. We also compared the estimates for selecting as the control group either births taking place in the famine cities before the famine (prefamine time controls) or after the famine (postfamine time controls), births occurring in the remainder of the country (place controls), or all controls combined. We also compared any changes resulting from adding to the analysis all men with missing death information under the assumption that they were still alive. The mortality estimates did not change in any of these scenarios.
RESULTS
Of the 45,037 men sampled for tracing, vital status in 2010 was ascertained for 41,096 (91.2%); 36,088 men (80.1%) were known to be alive at age 63 years. Follow-up status was incomplete (because of emigration and other right-censoring events) for 1,316 men (2.9%) and entirely unknown for 2,625 (5.8%). There were minor variations in follow-up status by exposure category or place of birth (Web Table 2).
The prefamine and postfamine time controls from the urbanized western part of the country and the place controls from the less-urbanized and rural areas in the north, south, and east differed somewhat in terms of characteristics at examination (Table 1).
Table 1.
Characteristics (%) of Dutch Male Military Conscripts Born in 1944–1946 and Examined at Age 18 Years, by Pre-/Perinatal Exposure to the Dutch Hunger Winter of 1944–1945
| Characteristic | Famine Exposure Status |
|||
|---|---|---|---|---|
| Total (n = 41,096) | Exposed to Famine (n = 22,952) | Unexposed to Famine |
||
| Time Controls (n = 9,745) | Place Controls (n = 8,399) | |||
| Educational level | ||||
| Special (less than primary school) | 6.2 | 5.7 | 6.4 | 7.6 |
| Primary school (6 years) | 13.6 | 13.1 | 13.9 | 14.6 |
| Primary school and 2 more years | 34.5 | 32.7 | 32.9 | 41.5 |
| Primary school and 4 more years | 30.8 | 32.9 | 31.0 | 24.9 |
| Primary school and ≥6 more years | 14.8 | 15.6 | 15.8 | 11.4 |
| Father's occupation | ||||
| Nonmanual | 42.6 | 45.9 | 45.2 | 30.5 |
| Manual | 45.4 | 44.5 | 45.3 | 48.0 |
| Farming | 4.4 | 1.6 | 1.6 | 15.2 |
| Unknown | 7.6 | 8.0 | 7.9 | 6.3 |
| Religion | ||||
| Roman Catholic | 32.8 | 30.0 | 28.4 | 45.7 |
| Protestant | 30.2 | 30.1 | 29.5 | 31.6 |
| Other religion | 8.4 | 7.8 | 7.6 | 11.1 |
| Without religious affiliation | 28.5 | 32.1 | 34.5 | 11.5 |
| Fitness for military service | ||||
| Fit | 80.3 | 81.5 | 77.6 | 80.0 |
| Almost fit | 6.5 | 6.0 | 7.5 | 6.5 |
| Fairly fit | 2.0 | 2.2 | 2.1 | 1.6 |
| Unfit | 7.9 | 7.4 | 9.1 | 8.0 |
| Unknown | 3.3 | 2.9 | 3.7 | 4.0 |
| Body mass indexa | ||||
| <19 | 2.3 | 2.2 | 2.4 | 2.3 |
| 19–20 | 41.7 | 41.3 | 42.9 | 41.5 |
| 21–24 | 49.2 | 49.6 | 47.8 | 49.7 |
| ≥25 | 6.9 | 7.0 | 6.9 | 6.4 |
a Weight (kg)/height (m)2.
The number of recorded deaths during the follow-up period was 5,011 (11.1%), after 1,853,023 person-years of observation. Cancer accounted for 38.7% of recorded deaths, heart disease for 20.8%, other natural causes for 28.3%, and external causes for 10.4% (Table 2). The most common deaths from other natural causes were from ill-defined conditions, diseases of the circulatory system (excluding heart diseases), and diabetes mellitus. The most common deaths from external causes were from transport accidents and intentional self-harm.
Table 2.
Causes of Death (Coded According to the International Classification of Diseases, Tenth Revision) Among Selected Dutch Male Military Conscripts Born in 1944–1946 and Followed From Age 18 Years to Age 63 Years
| Mortality Category and Cause of Deatha (ICD-10 Codes) | No. of Deaths | % of All Deaths |
|---|---|---|
| Malignant neoplasms (C00.0–C97.9) | ||
| Digestive organs (C15.0–C26.9) | 585 | |
| Respiratory and intrathoracic organs (C30.0–C39.9) | 565 | |
| Lymphoid, hematopoietic, and related tissue (C81.0–C96.9) | 185 | |
| Urinary tract (C64–C68.9) | 125 | |
| Ill-defined, secondary, and unspecified sites (C76.0–C80) | 100 | |
| Otherb | 378 | |
| Subtotal | 1,938 | 38.7 |
| Heart diseases (I20.0–I52.8) | ||
| Ischemic heart disease (I20.0–I25.9) | 704 | |
| Other forms of heart disease (I30.0–I52.8) | 315 | |
| Other | 21 | |
| Subtotal | 1,040 | 20.8 |
| Other natural causes | ||
| Ill-defined and unknown causes (R96–R99) | 309 | |
| Cerebrovascular diseases (I60.0–I69.8) | 167 | |
| Diseases of the liver (K70.0–K77.8) | 123 | |
| Diabetes mellitus (E10.0–E14.9) | 115 | |
| Other | 704 | |
| Subtotal | 1,418 | 28.3 |
| External causes (S00.0–Y98.9) | ||
| Transport accidents (V01.0–V89.9) | 189 | |
| Intentional self-harm (X60.0–X84.9) | 187 | |
| Other | 147 | |
| Subtotal | 523 | 10.4 |
| Missing data (uncoded diagnosis) | 92 | 1.8 |
| Total (all causes) | 5,011 | 100.0 |
Abbreviation: ICD-10, International Classification of Diseases, Tenth Revision.
a Causes for which there were at least 100 deaths.
b The “other” category includes all ICD-10 codes with fewer than 100 deaths.
Table 3 shows hazard ratios for selected causes of death among men born after famine exposure. After famine exposure, during trimesters T1–T3 of gestation, there was no increased mortality from cancer (HR = 0.98, 95% CI: 0.87, 1.11), from heart disease (HR = 1.07, 95% CI: 0.91, 1.26), from other natural causes of death (HR = 1.06, 95% CI: 0.93, 1.26), or from external causes of death (HR = 1.14, 95% CI: 0.90, 1.44). However, famine exposure during early gestation (T1) was associated with increased risks of mortality from other natural causes (HR = 1.24, 95% CI: 1.03, 1.49) and from external causes (HR = 1.46, 95% CI: 1.09, 1.97).
Table 3.
Risk of Death in Selected Prenatal Famine Exposure Groups (As Compared With Unexposed Controls) Among Dutch Male Military Conscripts Born in 1944–1946 and Followed From Age 18 Years to Age 63 Years, by Primary Cause of Death and Period of Famine Exposurea
| Cause of Death (ICD-10 Codes) and Famine Exposure Period | Hazard Ratio | 95% Confidence Interval |
|---|---|---|
| Cancer (C00.0–C97.9) | ||
| PNb | 1.05 | 0.92, 1.21 |
| T3c | 1.02 | 0.89, 1.17 |
| T2d | 0.97 | 0.84, 1.12 |
| T1e | 0.97 | 0.82, 1.14 |
| PCf | 0.85 | 0.74, 0.99 |
| T1–T3g | 0.98 | 0.87, 1.11 |
| Heart disease (I20.0–I52.8) | ||
| PN | 1.13 | 0.94, 1.36 |
| T3 | 1.19 | 0.99, 1.43 |
| T2 | 1.08 | 0.88, 1.32 |
| T1 | 0.89 | 0.70, 1.14 |
| PC | 0.94 | 0.77, 1.15 |
| T1–T3 | 1.07 | 0.91, 1.26 |
| Other natural causes | ||
| PN | 1.07 | 0.91, 1.26 |
| T3 | 0.98 | 0.83, 1.15 |
| T2 | 1.00 | 0.84, 1.19 |
| T1 | 1.24 | 1.03, 1.49 |
| PC | 1.12 | 0.96, 1.32 |
| T1–T3 | 1.06 | 0.93, 1.26 |
| External causes (S00.0–Y98.9) | ||
| PN | 1.00 | 0.76, 1.32 |
| T3 | 1.00 | 0.75, 1.32 |
| T2 | 1.22 | 0.92, 1.61 |
| T1 | 1.46 | 1.09, 1.97 |
| PC | 1.12 | 0.86, 1.49 |
| T1–T3 | 1.14 | 0.90, 1.44 |
Abbreviations: ICD-10, International Classification of Diseases, Tenth Revision; PC, periconception; PN, postnatal; T1, first trimester; T2, second trimester; T3, third trimester.
a Hazard ratios and 95% confidence intervals from Cox proportional hazards models with competing risks. All models adjusted for father's occupation, religion, education, body mass index, and fitness for military service as categorized in Table 1.
b Exposed during the immediate postnatal period.
c Exposed during the third trimester of pregnancy.
d Exposed during the second trimester of pregnancy.
e Exposed during the first trimester of pregnancy.
f Exposed just before the estimated date of conception.
g Any famine exposure in T3, T2, or T1.
The borderline elevated hazard ratio for heart disease (ICD-10 codes I20–I52) after famine exposure in the third trimester of pregnancy (HR = 1.19, 95% CI: 0.99, 1.43), as seen in Table 3, was not confirmed for the major subgroups “ischemic heart disease” (ICD-10 codes I20–I25) and “other forms of heart disease” (ICD-10 codes I30–I52) (Web Table 3).
By contrast, the reduced hazard ratio for malignant neoplasms after exposure during the periconceptional period (HR = 0.85, 95% CI: 0.74, 0.99), as seen in Table 3, reflects a trend in all of the 5 major subgroups, although in each group the number of cases and study power was too small for statistical significance (Web Table 3).
Within the other natural causes of death, the data suggested a similar relationship between early-gestation exposure and increased mortality for deaths from ill-defined and unknown causes (HR = 1.46, 95% CI: 1.02, 2.10), cerebrovascular diseases (HR = 1.55, 95% CI: 0.95, 2.51), and diabetes mellitus (HR = 1.61, 95% CI: 0.91, 2.86), although for some of these subgroups the number of cases and study power was again limited. Within the external causes of death, mortality from transport accidents and mortality from intentional self-harm appeared to show similar elevations after early-gestation famine exposure (Web Table 3).
We adjusted in all models for paternal occupation, religion, educational level, body mass index, and fitness for military service at age 18 years. The reported associations did not change with the removal of these covariates. Cox proportional hazards models with and without adjustment for competing risks gave the same mortality estimates as calculated to the second decimal point. Potential differences in cohort heterogeneity were evaluated with Gompertz models with or without a frailty (γ) parameter. The results from these models did not show detectable frailty within our defined clusters (with frailty variance components θ equal to 0 and associated P values equal to 1), and the mortality estimates did not differ from the reported estimates from the Cox models.
DISCUSSION
To our knowledge, this study was the first to quantify in national birth cohorts the relationship between exogenous nutritional challenges during well-defined periods of pregnancy and long-term mortality from selected causes of death. Our study advanced the field by using extreme variation in maternal nutrition during well-defined periods of pregnancy and by moving beyond the limitations of birth weight as a general indicator of nutrition in pregnancy. Because the study outcomes were based on death certificates, disease incidence could not be examined.
This study did not show a relationship between pre-/perinatal famine conditions and subsequent mortality from cancer or cardiovascular disease as we had expected. However, there were increases in deaths from other natural causes and from external causes, although the results reflected relatively small numbers of events, even in this national population.
We estimated mortality risks from individual models for each single famine exposure period and from aggregate models with simultaneous adjustment for all exposure periods. The estimates did not differ. The observed patterns were robust to adjustment for several individual characteristics, including father's occupation, religion, educational level, and health status, which are strongly associated with survival through age 63 years in the study population (38). Therefore, there was no suggestion of mediation of long-term mortality by characteristics observable among young adults that might be amenable to intervention.
The observed patterns were also robust to the use of different control groups. This suggests that early childhood exposure to the famine for subjects born just before the famine had no impact on long-term mortality in this population.
We do not think our results could be explained by selective losses to follow-up. The births that took place in the famine and control cities during the time period under study were fully accounted for by tracing the men from birth to age 18 years, when they were examined for military service (24), and our statistical analyses failed to show any frailty differences in comparisons of different exposure categories. The group lost to follow-up was also small (less than 6% of the study population) and was evenly distributed across exposure categories. Furthermore, a comparison of demographic and examination characteristics recorded at age 18 years among traced and untraced men showed no significant differences (Web Table 2). Mortality risk estimates for specific exposure periods did not change in sensitivity analyses that modeled men with missing death information as being still alive.
It is a limitation of our study that the military examination files do not include information on smoking habits. At the time of examination, 82% of men in the Netherlands were smokers (46), and the average age of smoking initiation was 16 years, with only 5% of men starting to smoke in adulthood (47). Therefore, it is likely that the majority of examinees were smokers. The lack of information on smoking is a potential concern. However, there was no relationship between prenatal famine exposure and smoking in our other studies of famine in the Netherlands, which followed men and women from birth to age 57 years (30, 34).
Another limitation is the fact that women were never called for conscript military service in the Netherlands. Therefore, it was not possible to compare sex-specific outcomes using our study design. The available estimates of mortality among women are based on small sample sizes with imprecise estimates (37). Cross-sectional studies could provide comparisons of current health among men and women born at the time of the famine, but in another study in the Netherlands no relationship of current health with early-life exposure to famine was seen (48). Comparisons may be biased by differential survival patterns, as was illustrated by the less adverse health outcomes in men compared with women after early-life exposure to the Great Chinese Famine of 1959–1961, which could largely be explained by increased mortality among men (49). Finally, the majority of the cohort is still alive, since it is not yet possible to ascertain deaths beyond age 63 years in this population. Further follow-up of the cohort will therefore be necessary in order to obtain definitive risk measures for deaths from specific causes after nutritional disturbances in gestation and very early life.
Several observations (mostly related to birth weight) have suggested a link between cancer and heart disease incidence and events occurring in the prenatal period. As an example, among men followed to age 30–45 years in a Swedish birth cohort, a 17% increase in cancer incidence was seen for each 1,000-g increase in birth weight, after adjustment for socioeconomic indicators at birth and in adulthood (18). Additionally, a prospective study of men screened at age 45–59 years and followed for 10 years showed the highest risk of fatal and nonfatal coronary heart disease events among men with the lowest birth weights, independent of adult body mass index (8). In a study in Hertfordshire, United Kingdom, men born in 1911–1930 with the lowest birth weights had the highest rates of death from ischemic heart disease (50).
These relationships were not seen in our Dutch conscript population. During the Dutch famine, there was a decrease in birth weight of about 300 g among infants exposed during the third trimester of pregnancy but no change in birth weight among infants exposed early in pregnancy (21, 24). Therefore, mechanisms related to birth weight appear to be unsatisfactory for explaining our study findings. In addition, our mortality findings, although they were adjusted for important socioeconomic indicators (such as paternal occupation and conscript's education and body mass index) as in these other studies, are not strictly comparable with findings from disease incidence studies.
The observation that events taking place in early life may affect later disease risk has also stimulated predictive adaptive response and mismatch theories (1). These theories provide a link between an adverse intrauterine environment and an integrated set of responses by the organism “that resets the developmental trajectory in expectation of poor postnatal conditions” (1, p. 1). Any mismatch between the expected postnatal conditions and actual postnatal conditions is then predicted to have adverse health consequences. Although they are potentially attractive, these theories need further development for the formulation of testable hypotheses linking specific health outcomes to changes in the uterine environment at different stages of pregnancy.
In conclusion, we found no increase in mortality from cancer or cardiovascular disease after prenatal exposure to the Dutch famine of 1944–1945. There was, however, an increase in deaths from other natural causes and from external causes among those men with early-gestation exposure. Because the men in our study population were 63 years of age at follow-up, they will now be entering a period of rapidly increasing mortality. This will provide significantly more study power in the future to detect associations between famine exposure by stage of gestation and more narrowly defined causes of death. Because of findings from other studies, we continue to be interested in a possible relationship with mortality from cardiovascular disease, as well as glucose-insulin dysregulation.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Netherlands Interdisciplinary Demographic Institute, Royal Netherlands Academy of Arts and Sciences/University of Groningen, The Hague, the Netherlands (Peter Ekamper, Frans van Poppel, Govert E. Bijwaard); Department of Cultural Anthropology and Sociology, Faculty of Social and Behavioural Sciences, Utrecht University, Utrecht, the Netherlands (Frans van Poppel); Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Aryeh D. Stein); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (L. H. Lumey); and Department of Molecular Epidemiology, Leiden University Medical Center, Leiden, the Netherlands (L. H. Lumey).
This study was supported by US National Institutes of Health grant RO1-AG028593 (Principal Investigator: Dr. L. H. Lumey).
We thank Drs. Diana Kuh, George Davey Smith, and the late Mervyn Susser for study advice and Ingeborg Deerenberg (Statistics Netherlands), Drs. Frans Willekens and Leo van Wissen (Netherlands Interdisciplinary Demographic Institute), Gerard van der Horst (Administration Personnel Office, Netherlands Ministry of Defence), Dr. Bert Lever (Netherlands Central Bureau for Genealogy), and Jan Groenewegen (Office of Registration and Information on Discharged Personnel, Netherlands Ministry of Defence) for their cooperation in the execution of the study.
Conflict of interest: none declared.
REFERENCES
- 1.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19(1):1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 2.Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. New York, NY: Oxford University Press; 1997. [Google Scholar]
- 3.Langley-Evans SC. Fetal Nutrition and Adult Disease: Programming of Chronic Disease Through Fetal Exposure to Undernutrition. Wallingford, United Kingdom: CABI Publishing; 2004. [Google Scholar]
- 4.Kermack WO, McKendrick AG, McKinlay PL. Death-rates in Great Britain and Sweden. Some general regularities and their significance. Lancet. 1934;223(5770):698–703. doi: 10.1093/ije/30.4.678. [DOI] [PubMed] [Google Scholar]
- 5.Forsdahl A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? Br J Prev Soc Med. 1977;31(2):91–95. doi: 10.1136/jech.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, editor. Fetal and Infant Origins of Adult Disease. London, United Kingdom: BMJ Publishing Group; 1992. [Google Scholar]
- 7.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankel S, Elwood P, Sweetnam P, et al. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348(9040):1478–1480. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 9.Rich-Edwards JW, Stampfer MJ, Manson JE, et al. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315(7105):396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen JO, Borch-Johnsen K, Pedersen O. Relation between birth weight and the insulin sensitivity index in a population sample of 331 young, healthy Caucasians. Am J Epidemiol. 1997;146(1):23–31. doi: 10.1093/oxfordjournals.aje.a009188. [DOI] [PubMed] [Google Scholar]
- 11.Curhan GC, Willett WC, Rimm EB, et al. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 12.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303(6809):1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn HS, Narayan KM, Williamson DF, et al. Relation of birth weight to lean and fat thigh tissue in young men. Int J Obes Relat Metab Disord. 2000;24(6):667–672. doi: 10.1038/sj.ijo.0801211. [DOI] [PubMed] [Google Scholar]
- 14.Leger J, Levy-Marchal C, Bloch J, et al. Reduced final height and indications for insulin resistance in 20 year olds born small for gestational age: regional cohort study. BMJ. 1997;315(7104):341–347. doi: 10.1136/bmj.315.7104.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lithell HO, McKeigue PM, Berglund L, et al. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ. 1996;312(7028):406–410. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips DI, Barker DJ, Hales CN, et al. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37(2):150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 17.Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130(4)):278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 18.McCormack VA, dos Santos Silva I, Koupil I, et al. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115(4):611–617. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- 19.Leon DA, Lithell HO, Vâgerö D, et al. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915–29. BMJ. 1998;317(7153):241–245. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syddall HE, Sayer AA, Simmonds SJ, et al. Birth weight, infant weight gain, and cause-specific mortality: the Hertfordshire Cohort Study. Am J Epidemiol. 2005;161(11):1074–1080. doi: 10.1093/aje/kwi137. [DOI] [PubMed] [Google Scholar]
- 21.Stein AD, Zybert PA, van de Bor M, et al. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J Epidemiol. 2004;33(4):831–836. doi: 10.1093/ije/dyh083. [DOI] [PubMed] [Google Scholar]
- 22.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein ZA, Susser M, Saenger G, et al. Famine and Human Development: The Dutch Hunger Winter of 1944–1945. New York, NY: Oxford University Press; 1975. [Google Scholar]
- 25.Lumey LH, Van Poppel F. The Dutch famine of 1944–45 as a human laboratory: changes in the early life environment and adult health. In: Lumey LH, Vaiserman AM, editors. Early Life Nutrition and Adult Health and Development. New York, NY: Nova Science Publishers; 2013. pp. 59–76. [Google Scholar]
- 26.Burger GCE, Drummond JC, Sandstead HR. Malnutrition and Starvation in Western Netherlands, September 1944 to July 1945. The Hague, the Netherlands: Staatsuitgeverij; 1948. [Google Scholar]
- 27.Lumey LH, Van Poppel FW. The Dutch famine of 1944–45: mortality and morbidity in past and present generations. Soc Hist Med. 1994;7(2):229–246. doi: 10.1093/shm/7.2.229. [DOI] [PubMed] [Google Scholar]
- 28.Trienekens G. The food supply in the Netherlands during the Second World War. In: Smith DF, Phillips J, editors. Food, Science, Policy and Regulation in the Twentieth Century: International and Comparative Perspectives. London, United Kingdom: Routledge; 2001. pp. 117–133. [Google Scholar]
- 29.Ravelli AC, van Der Meulen JH, Osmond C, et al. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 30.Stein AD, Kahn HS, Rundle A, et al. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85(3):869–876. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- 31.Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351(9097):173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 32.Lumey LH, Stein AD, Kahn HS. Food restriction during gestation and impaired fasting glucose or glucose tolerance and type 2 diabetes mellitus in adulthood: evidence from the Dutch Hunger Winter Families Study. J Dev Orig Health Dis. 2009;1(suppl 1):S164. [abstract] [Google Scholar]
- 33.Roseboom TJ, van der Meulen JH, Osmond C, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84(6):595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumey LH, Martini LH, Myerson M, et al. No relation between coronary artery disease or electrocardiographic markers of disease in middle age and prenatal exposure to the Dutch famine of 1944–5. Heart. 2012;98(22):1653–1659. doi: 10.1136/heartjnl-2012-302419. [DOI] [PubMed] [Google Scholar]
- 35.Painter RC, De Rooij SR, Bossuyt PM, et al. A possible link between prenatal exposure to famine and breast cancer: a preliminary study. Am J Hum Biol. 2006;18(6):853–856. doi: 10.1002/ajhb.20564. [DOI] [PubMed] [Google Scholar]
- 36.Hart N. Famine, maternal nutrition and infant mortality: a re-examination of the Dutch Hunger Winter. Popul Stud (Camb) 1993;47(1):27–46. doi: 10.1080/0032472031000146716. [DOI] [PubMed] [Google Scholar]
- 37.Painter RC, Roseboom TJ, Bossuyt PM, et al. Adult mortality at age 57 after prenatal exposure to the Dutch famine. Eur J Epidemiol. 2005;20(8):673–676. doi: 10.1007/s10654-005-7921-0. [DOI] [PubMed] [Google Scholar]
- 38.Ekamper P, van Poppel F, Stein AD, et al. Independent and additive association of prenatal famine exposure and intermediary life conditions with adult mortality between age 18–63 years. Soc Sci Med. 2014;119(10):232–239. doi: 10.1016/j.socscimed.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doornbos G, Kromhout D. Educational level and mortality in a 32-year follow-up study of 18-year-old men in The Netherlands. Int J Epidemiol. 1990;19(2):374–379. doi: 10.1093/ije/19.2.374. [DOI] [PubMed] [Google Scholar]
- 40.Van Poppel FWA. Late fertility decline in the Netherlands: the influence of religious denomination, socio-economic group and region. Eur J Popul. 1985;1(4):347–373. doi: 10.1007/BF01797148. [DOI] [PubMed] [Google Scholar]
- 41.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187–220. [with discussion] [Google Scholar]
- 42.Coviello E. Chestnut Hill, MA: Department of Economics, Boston College; 2009. STCOMPADJ: Stata module to estimate the covariate-adjusted cumulative incidence function in the presence of competing risks. (Statistical Software Components, no. S457063) http://ideas.repec.org/c/boc/bocode/s457063.html. Accessed November 14, 2012. [Google Scholar]
- 43.StataCorp LP. College Station, TX: StataCorp LP; 2009. Stata statistical software, release 11. [Google Scholar]
- 44.Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980;67(1):145–153. [Google Scholar]
- 45.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 46.Van Reek J. Smoking behaviour in the Netherlands and the United Kingdom: 1958–1982. Rev Epidemiol Sante Publique. 1984;32(6):383–390. [PubMed] [Google Scholar]
- 47.Gadourek I. Risky Habits and Concern About Individual Wellbeing. Groningen, the Netherlands: JB Wolters; 1963. [in Dutch] [Google Scholar]
- 48.Portrait F, Teeuwiszen E, Deeg D. Early life undernutrition and chronic diseases at older ages: the effects of the Dutch famine on cardiovascular diseases and diabetes. Soc Sci Med. 2011;73(5):711–718. doi: 10.1016/j.socscimed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Mu R, Zhang X. Gender Difference in the Long-Term Impact of Famine. Washington, DC: International Food Policy Research Institute; 2008. http://www.ifpri.org/sites/default/files/publications/ifpridp00760.pdf . Accessed December 10, 2013. [Google Scholar]
- 50.Barker DJ, Winter PD, Osmond C, et al. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.