Abstract
Pseudorhabdosynochus regius n. sp. is described from the gills of the mottled grouper Mycteroperca rubra caught off Senegal, Tunisia and Libya (type-locality: off Dakar, Senegal). The species is distinguished from its congeners by the structure of its sclerotised vagina (length 26–35 μm), which exhibits a trumpet in continuity with the primary canal, a straight primary canal, and primary and secondary chambers included in a common sclerotised mass along the primary canal. The species is also characterised by small squamodiscs (length 20–40 μm) with 10–11 rows of rodlets. Its closest relatives (based on the structure of the sclerotised vagina) are species mostly found in the Mediterranean Sea and parasites on species of Mycteroperca. A second species of Pseudorhabdosynochus Yamaguti, 1958 is reported from the same host and localities but not described. A list of diplectanids from groupers in the Mediterranean Sea is provided. We point out that a recent article was not compliant with the new Article 8.5.3 of the International Code of Zoological Nomenclature; for this reason, three species, P. nhatrangensis Dang, Bristow, Schander & Berland, 2013, P. vietnamensis Dang et al., 2013 and P. brunei Dang et al., 2013, are invalid.
Keywords: Diplectanidae, Grouper, Mycteroperca rubra, Mediterranean Sea, Eastern Atlantic, ICZN Article 8.5
Abstract
Pseudorhabdosynochus regius n. sp. est décrit des branchies du mérou royal Mycteroperca rubra pêché au Sénégal, en Tunisie et en Libye (localité-type: au large de Dakar, Sénégal). L’espèce se distingue de ses congénères par la structure de son vagin sclérifié (longueur 26-35 μm), qui présente une trompette en continuité avec le canal primaire, un canal primaire droit, et des chambres primaire et secondaire incluses dans une masse sclérifiée commune le long du canal primaire. L’espèce est également caractérisée par de petits squamodisques (longueur 20-40 μm) avec 10-11 rangées de bâtonnets. Ses plus proches parents (sur la base de la structure du vagin sclérifié) sont pour la plupart des espèces trouvées dans la mer Méditerranée et parasites sur des espèces de Mycteroperca. Une seconde espèce de Pseudorhabdosynochus Yamaguti, 1958 est signalée dans le même hôte et même localités mais non décrite. Une liste des Diplectanidae de mérous en mer Méditerranée est fournie. Nous signalons qu’un article récent n’était pas conforme avec le nouvel article 8.5.3 du Code International de Nomenclature Zoologique; pour cette raison, trois espèces, P. nhatrangensis Dang, Bristow, Schander & Berland, 2013, P. vietnamensis Dang et al., 2013 et P. brunei Dang et al., 2013 ne sont pas valides.
Introduction
The mottled grouper Mycteroperca rubra inhabits a rocky environment in the Mediterranean Sea and along the coast of the Eastern Atlantic Ocean [8]; it is common off Senegal but rare along the North African coast [8, 41]. Groupers generally harbour numerous diplectanid monogenean parasites [18, 19] and those from the Mediterranean Sea are no exception (Table 1).
Table 1.
Groupers in the Mediterranean (according to [7, 8]) and their diplectanid species. The placement of certain host species in Mycteroperca follows recent molecular results [4, 40]; some of these species were previously classified within Epinephelus.
| Host species | Diplectanid species, authorship of description and current combination, additional references |
|---|---|
| Native Mediterranean species | |
| Epinephelus aeneus (Geoffroy Saint-Hilaire) | Pseudorhabdosynochus hargisi (Oliver & Paperna, 1984) Santos, Buchmann & Gibson, 2000 [3, 36]. Redescription: [45] |
| P. americanus (Price, 1937) Kritsky & Beverley-Burton, 1986 [25, 37]. Redescriptions: [6, 36, 45] | |
| Mycteroperca marginata (Lowe, 1834) = E. marginatus | P. riouxi (Oliver, 1986) Santos, Buchmann & Gibson, 2000 [33, 38]. |
| Redescriptions: [34, 45] | |
| P. beverleyburtonae (Oliver, 1984) Kritsky & Beverley-Burton, 1986 [25, 32]. | |
| Redescriptions: [35, 38] | |
| Echinoplectanum echinophallus (Euzet & Oliver, 1965) Justine & Euzet, 2006 [6, 21] | |
| M. costae (Steindachner) = E. costae | P. bouaini Neifar & Euzet, 2007 [31] |
| P. dolicocolpos Neifar & Euzet, 2007 [31] | |
| P. enitsuji Neifar & Euzet, 2007 [31] | |
| P. sinediscus Neifar & Euzet, 2007 [31] | |
| P. sosia Neifar & Euzet, 2007 [31] | |
| M. canina (Valenciennes) = E. caninus | None recorded in the Mediterranean |
| M. rubra (Bloch) | P. regius n. sp. (this paper) |
| P. sp. (this paper) | |
| Hyporthodus haifensis (Ben-Tuvia) | P. sp. (unpublished) |
| Introduced Lessepsian species | |
| E. coioides (Hamilton) | None recorded in the Mediterranean; several diplectanids in its native range. South China Sea: [3, 27, 28, 45, 46] |
| E. malabaricus (Bloch & Schneider) | None recorded in the Mediterranean; several diplectanids in its native range. New Caledonia: [23] |
| E. fasciatus (Forsskål) | None recorded in the Mediterranean; several diplectanids in its native range. Red Sea: [36]; New Caledonia: [9, 14, 20] |
| Introduced Atlantic species | |
| Cephalopholis taeniops (Valenciennes) | None recorded in the Mediterranean (unidentified diplectanids present off Dakar, Senegal; unpublished) |
| M. fusca (Lowe) | None recorded in the Mediterranean |
| Introduced species – aquarium escapee | |
| E. merra (Bloch) | None recorded in the Mediterranean; several diplectanids in its native range. Fiji: [26]; New Caledonia: [9, 14] |
We found two species of Pseudorhabdosynochus Yamaguti, 1958 on the gill filaments of M. rubra from Senegal, Tunisia and Libya; these are the first monogeneans reported from this fish. In this paper, we describe the most abundant of these species. The other species, which was rare, will be described when more material enables a full study.
Materials and methods
Five Mycteroperca rubra were obtained from fish markets, including two specimens at Ouakam, Dakar, Senegal (February 2003), two at Sfax, Tunisia (January 2005) and one at Tripoli, Libya (June 2013). In all cases, the fish were dead, but, although their monogenean parasites were in suboptimal condition, they were considered suitable for study. The specimens collected from the fish gills were examined in Petri dishes containing seawater, using a stereomicroscope with incident light. These monogeneans were prepared according to three methods: (a) mounted in ammonium picrate-glycerine [29] (designated as “p” with regard to their measurements; (b) mounted in Berlese (designated “b”); (c) fixed unflattened in ethanol on the gills, then later rehydrated for examination, dehydrated in an ethanol series, stained with carmine, cleared with clove oil and mounted in Canada balsam (unflattened carmine, designated “uc”).
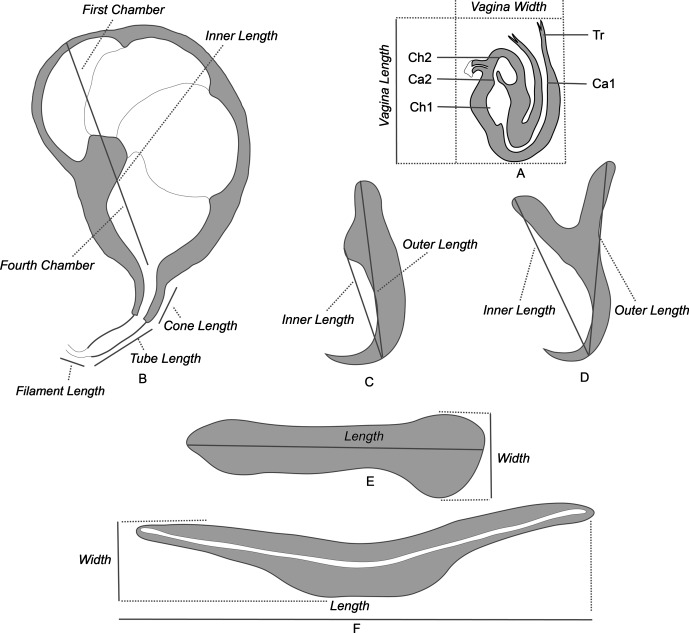
Monogeneans were drawn with the aid of an Olympus BH2 microscope equipped with a drawing apparatus and DIC optics. The sclerotised parts were measured and designated according to Figure 1. The measurements of the right-hand haptoral hard-parts and left-hand equivalents were pooled. All measurements on the drawings were taken with the help of a custom-made transparent rule and are in micrometres. Measurements of the holotype (h) are given separately. Drawings were scanned and redrawn on a computer using Adobe Illustrator.
Figure 1.
Methods of measurements and nomenclature of the sclerotised organs: A, sclerotised vagina: Tr, trumpet, Ca1, primary canal, Ch1, primary chamber, Ch2, secondary chamber, Ca2, secondary canal. B, male quadriloculate organ. C, ventral hamulus. D, dorsal hamulus. E, lateral (dorsal) bar. F, ventral bar.
Pseudorhabdosynochus regius n. sp.
urn:lsid:zoobank.org:act:19502FF9-544E-4364-B8D3-D139BB726BCC
Type-host: Mycteroperca rubra (Bloch) (Perciformes, Serranidae).
Site of infection: Gills.
Type-locality: Off Dakar (Senegal), February 2003.
Other localities: Off Sfax (Tunisia), January 2005; off Tripoli (Libya), June 2013.
Material examined: 43 specimens, including 5 “unflattened carmine” (uc), 8 “picrate” (p), 30 “berlese” (b).
Prevalence: 80%.
Type-specimens: Holotype and paratypes deposited in the Muséum national d’Histoire Naturelle, Paris (MNHN) as HEL516-517.
Etymology: The species name regius (a Latin adjective meaning “royal”) reflects the French name of the host, “Mérou royal”.
Description (Figs. 2–3)
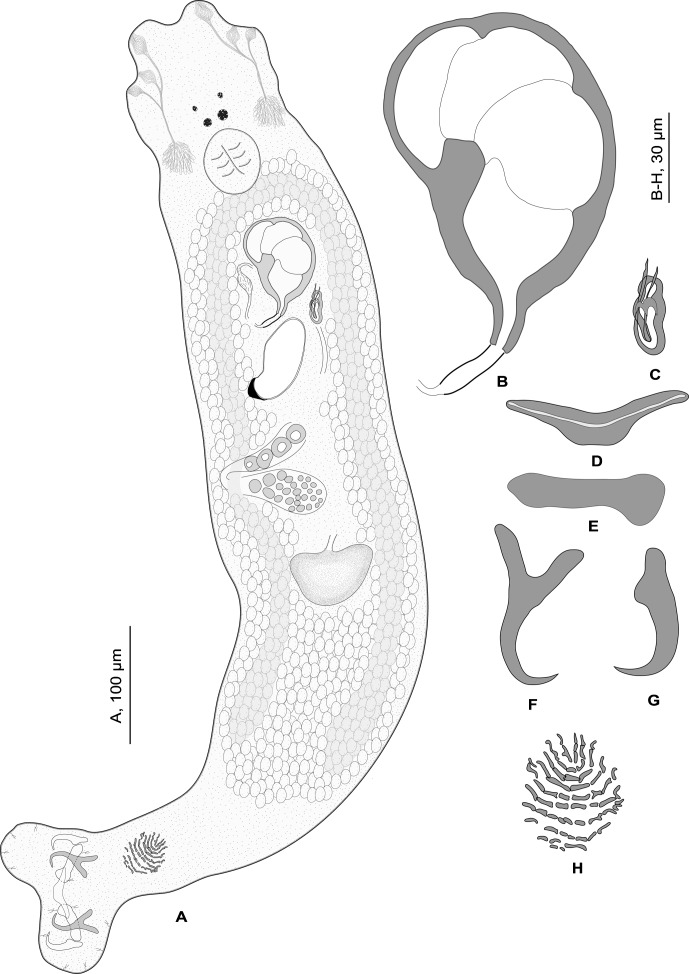
Figure 2.
Pseudorhabdosynochus regius n. sp. from Mycteroperca rubra. A, composite (mainly from the holotype). B, male quadriloculate organ. C, sclerotised vagina. D, ventral bar. E, dorsal bar. F, ventral hamulus. G, dorsal hamulus. H, ventral squamodisc (paratype) A–H, carmine.
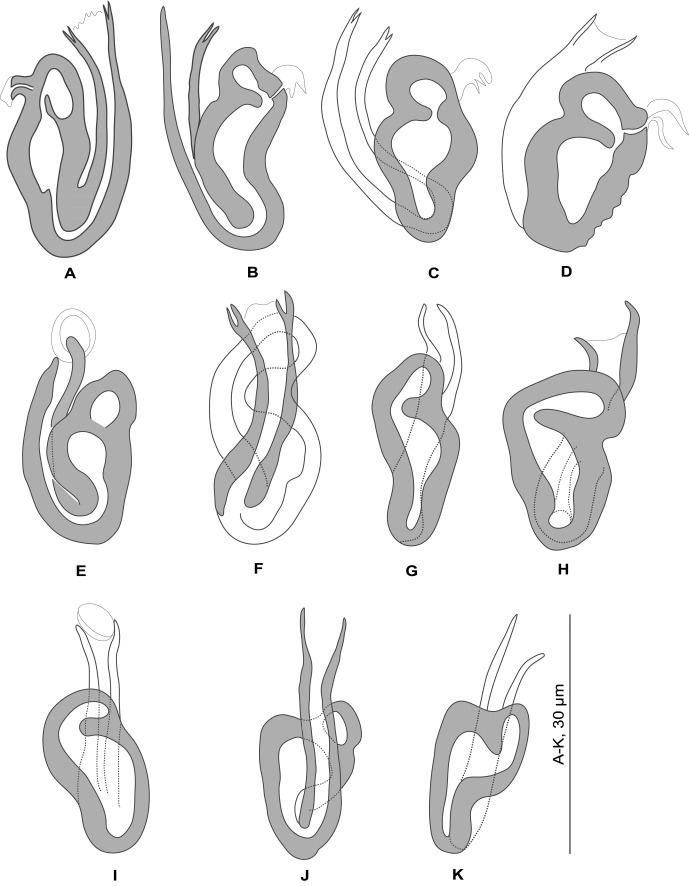
Figure 3.
Pseudorhabdosynochus regius n. sp. from Mycteroperca rubra. Sclerotised vagina, variations according to different specimens, orientation and preparation. A-H, Berlese; I-K, carmine.
Body length h 860, uc 898 (600–1300, n = 4), p 824 (650–1000, n = 7), b 909 (650–1200, n = 10) including haptor; maximum width h 140, b 157 (150–170, n = 3) at level of ovary. Tegument smooth. Anterior region with three pairs of head organs and two pairs of eye-spots. External width of anterior eye-spot pair h 30, uc 34 (30–41, n = 4), p 38 (30–45, n = 7), b 35 (20–52, n = 18), of posterior pair h 23, uc 28 (23–38, n = 4), p 32 (25–43, n = 7), b 30 (15–38, n = 16). Pharynx median, subspherical, length h 52, uc 51 (45–55, n = 5), p 42 (32–50, n = 7), b 45 (33–55, n = 11), width h 52, uc 52 (50–55, n = 5), p 46 (38–53, n = 7), b 45 (32–60, n = 11). Haptor differentiated from the rest of body, width h 140, provided with 2 squamodiscs, 2 pairs of lateral hamuli, 3 bars and 14 marginal hooklets (7 pairs). Dorsal and ventral squamodiscs round with 10–11 rows of rodlets; 2–3 innermost rows V-shaped. Ventral squamodisc, length uc 34 (28–40, n = 2), p 27 (20–33, n = 2), b 25 (22–30, n = 3), width uc 31 (28–33, n = 2), p 27 (20–33, n = 2), b 21 (20–23, n = 3); dorsal squamodisc, length uc 32 (30–33, n = 2), b 33 (33–33, n = 2),width uc 25 (22–28, n = 2), b 25 (23–26, n = 2). Ventral hamulus with handle and distinct guard, outer length uc 44 (40–52, n = 6), p 45 (41–48, n = 16), b 46 (41–53, n = 36), inner length h 44, uc 40 (36–45, n = 9), p 42 (37–46, n = 16), b 42 (36–47, n = 39). Dorsal hamulus with indistinct guard, outer length uc 40 (38–42, n = 7), p 41 (39–43, n = 16), b 40 (22–45, n = 34), inner length uc 23 (22–24, n = 8), p 24 (22–25, n = 16), b 25 (22–66, n = 30). Lateral bar with wide flattened medial extremity and cylindrical lateral extremity, length h 46, uc 46 (43–53, n = 10), p 48 (44–50, n = 16), b 55 (44–70, n = 46), width h 15, uc 13 (10–16, n = 10), p 15 (12–20, n = 16), b 17 (12–22, n = 46).Ventral bar with small, constricted, median portion and blunt ends, length h 62, uc 62 (56–68, n = 5), p 65 (58–71, n = 8), b 71 (30–85, n = 23), width h 10, uc 10 (9–13, n = 5), p 10 (8–11, n = 8), b 13 (8–20, n = 23).
Testis subspherical, intercaecal. Quadriloculate organ with large sclerotised bulb divided into four chambers; internal length h 80, uc 77 (71–83, n = 5), p 98 (94–100, n = 4), b 85 (70–100, n = 25); fourth chamber ends in short sclerotised cone, length h 16, uc 16 (13–17, n = 4), p 13 (10–16, n = 5), b 16 (10–22, n = 25), prolonged by thin sclerotised tube; tube length h 21, uc 20 (18–21, n = 3), p 19 (18–20, n = 3), b 18 (15–20, n = 24); tube diameter h 4.5, uc 5 (4–5, n = 3), p 5 (4.5–5, n = 3), b 5 (4–5, n = 25); end of tube prolonged by short filament, not always visible, length h 10, uc 0–10 (n = 3), p 0–10 (n = 2), b 0–10 (n = 25).
Ovary dextral, loops dorsoventrally around right intestinal caecum. Vitelline follicles lateral, coextensive with intestinal caeca and contiguous posterior to testis. Egg inside genital tract, without filament, length uc 108–130 (n = 2), b 100–145 (n = 10).
Sclerotised vagina (nomenclature of parts according to Justine [15]) comprises anterior trumpet, primary canal, and distal sclerotised part, along primary canal, including both primary and secondary chambers. Trumpet in continuity with primary canal, with diameter slightly greater than canal. Primary canal straight, heavily sclerotised, curves just prior to entering primary chamber. Primary chamber heavily sclerotised, with its entrance posterior (i.e. its orientation is opposite to that of primary canal); secondary canal very short; secondary chamber, anterior to and smaller than primary chamber, with same heavily sclerotised structure. Accessory structure not seen. Sclerotised vagina length (measured from tip of trumpet to exterior of bend of primary canal) h 30, uc 29 (27–31, n = 5), p 31 (28–33, n = 7), b31 (26–35, n = 29).
Differential diagnosis
The identification of species of Pseudorhabdosynochus is primarily based on the sclerotised vagina [14, 15, 17, 30]. However, the quadriloculate organ and the haptoral parts, including the squamodiscs, also provide characters useful for species identification [13, 14].
The general structure of the sclerotised vagina of P. regius n. sp. has the following characters: the trumpet in continuity with the linear primary canal, the orientation of the primary chamber opposite that of the primary canal, and the primary and secondary chambers grouped within a single heavily sclerotised structure along the primary canal. Other Pseudorhabdosynochus species which have a vaginal structure similar to P. regius are:
Pseudorhabdosynochus sosia Neifar & Euzet, 2007 (from Mycteroperca costae). This species can be differentiated by the shape of its trumpet (diameter similar to primary canal in P. regius vs. well differentiated in P. sosia), the shape of the anterior part of its primary canal (straight in P. regius vs. curved in P. sosia), the shape of its primary chamber and the length of the secondary canal (very short in P. regius vs. long in P. sosia). In addition, the two species have very different squamodiscs (central rows V-shaped in P. regius, circular in P. sosia).
Pseudorhabdosynochus beverleyburtonae (Oliver, 1984) Kritsky & Beverley-Burton, 1986 (from M. marginata). This species is close to P. sosia and can, therefore, be differentiated from P. regius based on the same characters.
Pseudorhabdosynochus enitsuji Neifar & Euzet, 2007 (from M. costae). Its sclerotised secondary chamber is larger than that in P. regius. In addition, the squamodiscs of P. enitsuji have numerous rows of rodlets (14–16).
Pseudorhabdosynochus sinediscus Neifar & Euzet, 2007 (from M. costae). The general structure is strikingly similar in this species, although the secondary chamber is not differentiated in the original description. P. sinediscus is differentiated from all other species of the genus, including P. regius, by the absence of squamodiscs.
Pseudorhabdosynochus riouxi (Oliver, 1986) Santos, Buchmann & Gibson, 2000 (from M. marginata). The general structure seems similar in this species, but the shape of the chambers (redescribed by Yang et al. [45]) is different. In addition, P. riouxi has squamodiscs with numerous rows of rodlets (11–22 in the original description).
Pseudorhabdosynochus pai Justine & Vignon, 2009 (from E. tauvina (Forsskål) in French Polynesian waters). The general structure is similar, but the primary canal is shorter in P. regius and the shape of the secondary chamber is different and complex (i.e. several secondary chambers) [24]. In addition, P. pai has a huge quadriloculate organ (72–144 inner length) and larger squamodiscs with numerous rows of rodlets (13–14). The hosts are also widely separated (Central Pacific vs. Atlantic/Mediterranean).
Discussion
We describe a new species of Pseudorhabdosynochus from Mycteroperca rubra in this paper, but also found an additional species. Identification of the second species, which is close to P. sosia and P. beverleyburtonae, will require more material and the examination of comparative material.
Species of Pseudorhabdosynochus are mainly parasites of members of the family Epinephelidae (groupers), including species of Epinephelus, Cephalopholis, Mycteroperca, Variola and Alphestes [19, 30] but not Plectropomus [21]. A few species are parasites of members of the closely related Serranidae, including members of Serranus [44], Paralabrax [2], and of a member of the Polyprionidae [15]. A record on a member of the Chaetodontidae [1], never confirmed, is probably due to a mistake in the fish identification. Species of Pseudorhabdosynochus tend to be strictly host-specific, with species often restricted to a single host fish [19], but a few exceptions are known [17, 22, 30, 39]. An interesting aspect of our comparisons of P. regius with similar species, mainly based on the sclerotised vagina, is that most of the closely comparable species are parasitic on members of Mycteroperca (Table 1). This suggests that a group of Pseudorhabdosynochus species, with common vaginal characteristics, parasitises species of Mycteroperca, a genus which was found to be monophyletic in recent molecular studies [4, 40]. In contradiction to this hypothesis, P. capurroi Vidal-Martinez & Mendoza-Franco, 1998 from M. bonaci, P. morrhua Justine, 2008 and P. variabilis Justine, 2008, both from M. morrhua, do not share the same vaginal structure [16, 43]. Another hypothesis is that the three species of Mycteroperca with similar species of Pseudorhabdosynochus are all from the Mediterranean Sea (and Eastern Atlantic), suggesting that the close relationships of these parasite species reflect a common geographical origin, with the differentiation of various monogenean species in the groupers from the same area. However, a similar vaginal structure is found in P. pai, a parasite of Epinephelus tauvina in the Pacific [24] (i.e. neither a species of Mycteroperca nor Mediterranean), thus negating the two hypotheses mentioned above. We conclude that the relationships of the various species of Pseudorhabdosynochus in the world are complex and resistant to a simple analysis.
M. rubra is common off Senegal but rare along the North African coast [8, 41]. Mediterranean populations of groupers were affected by the last glacial period. The presence of the same Pseudorhabdosynochus species in specimens of M. rubra from the Mediterranean Sea and the Atlantic Ocean suggests a new model, which merits investigation using molecular methods.
According to Table 1, the biodiversity of diplectanids in groupers of the Mediterranean appears lower than that observed in groupers from warmer seas and coral reefs [9, 15, 23, 42], where the number of species can reach up to 12 per fish species. However, not all grouper species of the Mediterranean have been studied in detail in terms of their parasite fauna (Table 1).
Nomenclatural validity of three recently described species of Pseudorhabdosynochus
While comparing our specimens with other species, our attention was drawn to a recent paper [5] describing new species of Pseudorhabdosynochus which we checked in terms of its nomenclatural validity.
The recent amendment [11, 12] of the International Code of Zoological Nomenclature [10] concerns electronic publication. According to Article 8.5, “to be considered published, a work issued and distributed electronically must:
8.5.1. have been issued after 2011,
8.5.2. state the date of publication in the work itself, and
8.5.3. be registered in the Official Register of Zoological Nomenclature (ZooBank) […] and contain evidence in the work itself that such registration has occurred [10].”
The descriptions of three new species of Pseudorhabdosynochus from groupers of Vietnam were published in 2013 [5]. The paper was published in the journal International Journal of Aquatic Sciences. The website of this journal (http://www.journal-aquaticscience.com) claims that it is an electronic publication (expressed as “e-journal”) and no mention of a printed version appears on the website or on the papers themselves. Therefore, the nomenclatural validity of the species described in this journal depends upon compliance with Article 8.5 of the Code, reproduced above. Articles 8.5.1 (after 2011) and 8.5.2 (date of publication indicated in the work itself, as 27 June 2013) are satisfied. However, Article 8.5.3 is not satisfied: there is no mention of a ZooBank number associated with this work. Article 8.5.3.3. describes the cases in which an error can be admitted: “An error in stating the evidence of registration does not make a work unavailable, provided that the work can be unambiguously associated with a record created in the Official Register of Zoological Nomenclature before the work was published” (the emboldened “before” is our own). We checked (2 December 2014) and found that neither these species nor the paper is indexed in ZooBank. According to the examples illustrating Article 8.5.3.3., even if the registration in ZooBank was performed now or later, the work would be unavailable.
Furthermore, to have its paper registered in ZooBank, the journal needs to meet the criteria enumerated in Articles 8.5.3.1 and 8.5.3.2. Article 8.5.3.1 requires “the name and Internet address of an organization other than the publisher that is intended to permanently archive the work in a manner that preserves the content and layout, and is capable of doing so”: we found no such repository mentioned on the journal website. This means that if the publisher closes its website, all papers will be lost. Article 8.5.3.2 requires “an ISSN for the journal containing the work”: the journal does mention an ISSN (2008–8019) but we could not retrieve this ISSN number from online systems such as WorldCat (http://www.worldcat.org/). Although these details do not specifically concern the case of the species dealt with in the present work, they show, more generally, that the journal itself (International Journal of Aquatic Sciences) could not publish a valid species according to the new ICZN Article 8.5.
Article 11 of the ICZN lists the criteria that make a name available. “Article 11. Requirements. To be available, a name or, where relevant, a nomenclatural act must satisfy the following provisions: 11.1. Publication. The name or nomenclatural act must have been published, in the meaning of Article 8, after 1757 [10].” In other words, a work which does not fulfil the criteria of Article 8 (especially, in the case of an electronic publication, Article 8.5. and its new amendment) does not satisfy Article 11 and thus is not published, and the new names of taxa in this work are not available.
To make things clearer, the paper itself [5] is “published” in terms of the general vocabulary used for publications, but the work is not published according to the International Code of Zoological Nomenclature [10]. The names of the three new species mentioned in the paper are unavailable, i.e. have not been published according to the International Code of Zoological Nomenclature. They are not valid and cannot be used in any publication which respects the ICZN – i.e. normally, all scientific journals.
Finally, we give here a list of the three names which are unavailable, for the reasons stated above: Pseudorhabdosynochus nhatrangensis Dang, Bristow, Schander & Berland, 2013; P. vietnamensis Dang et al., 2013; P. brunei Dang et al., 2013.
Conflict of Interest
The Editor-in-Chief of Parasite is one of the authors of this manuscript. COPE (Committee on Publication Ethics, http://publicationethics.org), to which Parasite adheres, advises special treatment in these cases. In this case, the peer review process was handled by an Invited Editor, Dominique Vuitton.
Acknowledgments
Alain Dubois (MNHN) clarified some points of nomenclature and kindly read a draft of the part dealing with the ICZN. Lobna Boudaya (FSS) kindly helped in drawing figures.
Cite this article as: Chaabane A, Neifar L & Justine J-L: Pseudorhabdosynochus regius n. sp. (Monogenea, Diplectanidae) from the mottled grouper Mycteroperca rubra (Teleostei) in the Mediterranean Sea and Eastern Atlantic. Parasite, 2015, 22, 9
References
- 1. Aljoshkina LD. 1984. New species of the family Diplectanidae (Monogenea) in the South-West Atlantic. Zoologicheskii Zhurnal, 63, 1253–1256 (In Russian). [Google Scholar]
- 2. Bravo-Hollis M. 1954. Diplectanum amplidiscatum n. sp., Trématode monogénétique des branchies d’un poisson marin. Annales de Parasitologie Humaine et Comparée, 24 (1–2), 37–41. [PubMed] [Google Scholar]
- 3. Bu SSH, Leong TS, Wong SY, Woo YSN, Foo RWT. 1999. Three diplectanid monogeneans from marine finfish (Epinephelus spp.) in the Far East. Journal of Helminthology, 73, 301–312. [Google Scholar]
- 4. Craig MT, Hastings PA. 2007. A molecular phylogeny of the groupers of the subfamily Epinephelinae (Serranidae) with a revised classification of the Epinephelini. Ichthyological Research, 54, 1–17. [Google Scholar]
- 5. Dang BT, Bristow GA, Schander C, Berland B. 2013. Three new species of Pseudorhabdosynochus (Monogenea: Diplectanidae) from Vietnamese grouper (Epinephelus spp.) (Perciformes: Serranidae). International Journal of Aquatic Science, 4, 44–58. [Google Scholar]
- 6. Euzet L, Oliver G. 1965. Diplectanidae (Monogenea) de Téléostéens de la Méditerranée occidentale. II. Parasites d’Epinephelus gigas (Brünnich, 1768). Annales de Parasitologie Humaine et Comparée, 40, 517–523. [PubMed] [Google Scholar]
- 7. Golani D, Orsi-Relini L, Massutui E, Quignard J-P 2014. CIESM Atlas of Exotic Species in the Mediterranean. Vol. 1 – Fishes Online publication at: http://www.ciesm.org/online/atlas/index.htm. [Google Scholar]
- 8. Heemstra PC, Randall JE. 1993. FAO Species Catalogue. Vol. 16. Groupers of the world (Family Serranidae, Subfamily Epinephelinae). An annotated and illustrated catalogue of the grouper, rockcod, hind, coral grouper and lyretail species known to date, FAO Fisheries Synopsis. Vol. No. 125, Volume 16, FAO: Rome, 382 pp. [Google Scholar]
- 9. Hinsinger DD, Justine J-L. 2006. The “Pseudorhabdosynochus cupatus group” (Monogenea: Diplectanidae) on Epinephelus fasciatus, E. howlandi, E. rivulatus and E. merra (Perciformes: Serranidae) off New Caledonia, with descriptions of Pseudorhabdosynochus cyathus n. sp. and P. calathus n. sp. Systematic Parasitology, 64, 69–90. [DOI] [PubMed] [Google Scholar]
- 10. International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature: The International Trust for Zoological Nomenclature. pp. 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. International Commission on Zoological Nomenclature. 2012. Amendment of Articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa, 3450, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Commission on Zoological Nomenclature. 2012. Amendment of Articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. ZooKeys, 219, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Justine J-L. 2005. Pseudorhabdosynochus hirundineus n. sp. (Monogenea: Diplectanidae) from Variola louti (Perciformes: Serranidae) off New Caledonia. Systematic Parasitology, 62, 39–45. [DOI] [PubMed] [Google Scholar]
- 14. Justine J-L. 2005. Species of Pseudorhabdosynochus Yamaguti, 1958 (Monogenea: Diplectanidae) from Epinephelus fasciatus and E. merra (Perciformes: Serranidae) off New Caledonia and other parts of the Indo-Pacific Ocean, with a comparison of measurements of specimens prepared using different methods, and a description of P. caledonicus n. sp. Systematic Parasitology, 62, 1–37. [DOI] [PubMed] [Google Scholar]
- 15. Justine J-L. 2007. Parasite biodiversity in a coral reef fish: twelve species of monogeneans on the gills of the grouper Epinephelus maculatus (Perciformes: Serranidae) off New Caledonia, with a description of eight new species of Pseudorhabdosynochus (Monogenea: Diplectanidae). Systematic Parasitology, 66, 81–129. [DOI] [PubMed] [Google Scholar]
- 16. Justine J-L. 2008. Two new species of Pseudorhabdosynochus Yamaguti, 1958 (Monogenea: Diplectanidae) from the deep-sea grouper Epinephelus morrhua (Val.) (Perciformes: Serranidae) off New Caledonia. Systematic Parasitology, 71, 145–158. [DOI] [PubMed] [Google Scholar]
- 17. Justine J-L. 2009. A redescription of Pseudorhabdosynochus epinepheli (Yamaguti, 1938), the type-species of Pseudorhabdosynochus Yamaguti, 1958 (Monogenea: Diplectanidae), and the description of P. satyui n. sp. from Epinephelus akaara off Japan. Systematic Parasitology, 72, 27–55. [DOI] [PubMed] [Google Scholar]
- 18. Justine J-L. 2010. Parasites of coral reef fish: how much do we know? With a bibliography of fish parasites in New Caledonia. Belgian Journal of Zoology, 140 (Suppl.), 155–190. [Google Scholar]
- 19. Justine J-L, Beveridge I, Boxshall GA, Bray RA, Moravec F, Trilles J-P, Whittington ID. 2010. An annotated list of parasites (Isopoda, Copepoda, Monogenea, Digenea, Cestoda and Nematoda) collected in groupers (Serranidae, Epinephelinae) in New Caledonia emphasizes parasite biodiversity in coral reef fish. Folia Parasitologica, 57, 237–262. [DOI] [PubMed] [Google Scholar]
- 20. Justine J-L, Dupoux C, Cribb TH. 2009. Resolution of the discrepant host-specificity of Pseudorhabdosynochus species (Monogenea, Diplectanidae) from serranid fishes in the tropical Indo-Pacific. Acta Parasitologica, 54, 119–130. [Google Scholar]
- 21. Justine J-L, Euzet L. 2006. Diplectanids (Monogenea) parasitic on the gills of the coralgroupers Plectropomus laevis and P. leopardus (Perciformes, Serranidae) off New Caledonia, with the description of five new species and the erection of Echinoplectanum n. g. Systematic Parasitology, 64, 147–172. [DOI] [PubMed] [Google Scholar]
- 22. Justine J-L, Henry É. 2010. Monogeneans from Epinephelus chlorostigma (Val.) (Perciformes: Serranidae) off New Caledonia, with the description of three new species of diplectanids. Systematic Parasitology, 77, 81–105. [DOI] [PubMed] [Google Scholar]
- 23. Justine J-L, Sigura A. 2007. Monogeneans of the malabar grouper Epinephelus malabaricus (Perciformes, Serranidae) off New Caledonia, with a description of six new species of Pseudorhabdosynochus (Monogenea: Diplectanidae). Zootaxa, 1543, 1–44. [Google Scholar]
- 24. Justine J-L, Vignon M. 2009. Monogeneans of the grouper Epinephelus tauvina (Perciformes, Serranidae) off Moorea, French Polynesia, with a description of Pseudorhabdosynochus pai n. sp. (Monogenea: Diplectanidae). Systematic Parasitology, 72, 113–125. [DOI] [PubMed] [Google Scholar]
- 25. Kritsky DC, Beverley-Burton M. 1986. The status of Pseudorhabdosynochus Yamaguti, 1958, and Cycloplectanum Oliver, 1968 (Monogenea: Diplectanidae). Proceedings of the Biological Society of Washington, 99, 17–20. [Google Scholar]
- 26. Laird M. 1958. Parasites of South Pacific fishes II. Diplectanum melanesiensis n. sp., a monogenetic trematode from Fiji and the New Hebrides. Canadian Journal of Zoology, 36, 167–173. [Google Scholar]
- 27. Luo YF, Yang TB. 2010. Seasonal population dynamics of the monogeneans Pseudorhabdosynochus coioidesis and P. serrani on wild versus cultured groupers in Daya Bay, South China Sea. Helminthologia, 47, 241–250. [Google Scholar]
- 28. Luo YF, Yang TB. 2012. Seasonal patterns in the community of gill monogeneans on wild versus cultured orange-spotted grouper, Epinephelus coioides Hamilton, 1822 in Daya Bay, South China Sea. Aquaculture Research, 43, 1232–1242. [Google Scholar]
- 29. Malmberg G. 1957. Om förekomsten av Gyrodactylus på svenska fiskar (in Swedish). Skrifter Utgivna av Södra Sveriges Fiskeriförening, Årsskrift, 1956, 19–76. [Google Scholar]
- 30. Mendoza-Franco EF, Violante-González J, Rojas Herrera AA. 2011. Six new and one previously described species of Pseudorhabdosynochus (Monogenoidea, Diplectanidae) infecting the gills of groupers (Perciformes, Serranidae) from the Pacific coasts of Mexico and Panama. Journal of Parasitology, 97, 20–35. [DOI] [PubMed] [Google Scholar]
- 31. Neifar L, Euzet L. 2007. Five new species of Pseudorhabdosynochus (Monogenea: Diplectanidae) from the gills of Epinephelus costae (Teleostei: Serranidae). Folia Parasitologica, 54, 117–128. [DOI] [PubMed] [Google Scholar]
- 32. Oliver G. 1984. Description de deux nouvelles espèces du genre Cycloplectanum Oliver, 1968 (Monogenea, Monopisthocotylea, Diplectanidae). Annales de Parasitologie Humaine et Comparée, 59, 31–39. [PubMed] [Google Scholar]
- 33. Oliver G. 1986. Cycloplectanum riouxi n. sp., une nouvelle espèce de Diplectanidae (Monogenea, Monopisthocotylea) parasite d’Epinephelus guaza (Linnaeus, 1758) (Pisces, Serranidae). Systematic Parasitology, 8, 317–322. [Google Scholar]
- 34. Oliver G. 1987. Les Diplectanidae Bychowsky, 1957 (Monogenea, Monopisthocotylea, Dactylogyridea). Systématique. Biologie. Ontogénie. Écologie. Essai de phylogenèse. Thèse d’État. Académie de Montpellier, Université des Sciences et Techniques du Languedoc. [Google Scholar]
- 35. Oliver G. 1992. Ectoparasites branchiaux du mérou, Epinephelus guaza (Linnaeus, 1758) (Pisces, Serranidae), des côtes de Corse (Méditerranée occidentale). Travaux Scientifiques du Parc National Régional de la Réserve Naturelle de Corse, France, 37, 101–112. [Google Scholar]
- 36. Oliver G, Paperna I. 1984. Diplectanidae Bychowsky, 1957 (Monogenea, Monopisthocotylea), parasites de Perciformes de Méditerranée orientale, de la Mer Rouge et de l'Océan Indien. Bulletin du Muséum National d’Histoire Naturelle, Paris, 4° série, 6, section A(1), 49–65. [Google Scholar]
- 37. Price EW. 1937. North American Monogenetic Trematodes. I. The superfamily Gyrodactyloidea. Journal of the Washington Academy of Sciences, 27, 146–164. [Google Scholar]
- 38. Santos CP, Buchmann K, Gibson DI. 2000. Pseudorhabdosynochus spp. (Monogenea: Diplectanidae) from the gills of Epinephelus spp. in Brazilian waters. Systematic Parasitology, 45, 145–153. [DOI] [PubMed] [Google Scholar]
- 39. Schoelinck C, Cruaud C, Justine J-L. 2012. Are all species of Pseudorhabdosynochus strictly host specific? A molecular study. Parasitology International, 61, 356–359. [DOI] [PubMed] [Google Scholar]
- 40. Schoelinck C, Hinsinger DD, Dettaï A, Cruaud C, Justine J-L. 2014. A phylogenetic re-analysis of groupers with applications for ciguatera fish poisoning. PLoS ONE, 9, e98198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siau Y, Bouain A. 1994. Preliminary indications on growth and reproduction in the protogynous grouper Mycteroperca rubra (Pisces, Serranidae). Journal of African Zoology, 108, 353–359. [Google Scholar]
- 42. Sigura A, Justine J-L. 2008. Monogeneans of the speckled blue grouper, Epinephelus cyanopodus (Perciformes, Serranidae), from off New Caledonia, with a description of four new species of Pseudorhabdosynochus and one new species of Laticola (Monogenea: Diplectanidae), and evidence of monogenean faunal changes according to the size of fish. Zootaxa, 1695, 1–44. [Google Scholar]
- 43. Vidal-Martínez VM, Mendoza-Franco EF. 1998. Pseudorhabdosynochus capurroi sp. n. (Monogenea: Diplectanidae) from the gills of Mycteroperca bonaci (Pisces: Serranidae) of the Yucatan Peninsula, Mexico. Folia Parasitologica, 45, 221–224. [PubMed] [Google Scholar]
- 44. Yamaguti S. 1953. Parasitic worms mainly from Celebes. Part 2. Monogenetic trematodes of fishes. Acta Medicinae Okayama, 8, 203–256 + 9 Pl. [Google Scholar]
- 45. Yang TB, Gibson DI, Zeng BJ. 2005. Pseudorhabdosynochus summanoides n. sp (Monogenea: Diplectanidae) from Epinephelus coioides in Dapeng Bay, South China Sea, with observations on several similar species of Pseudorhabdosynochus Yamaguti, 1958. Systematic Parasitology, 62, 221–239. [DOI] [PubMed] [Google Scholar]
- 46. Yang TB, Zeng BJ, Gibson DI. 2005. Description of Pseudorhabdosynochus shenzhenensis n. sp. (Monogenea: Diplectanidae) and redescription of P. serrani Yamaguti, 1953 from Epinephelus coioides off Dapeng Bay, Shenzhen, China. Journal of Parasitology, 91, 808–813. [DOI] [PubMed] [Google Scholar]