Abstract
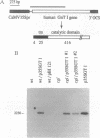
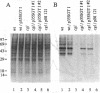
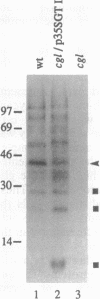
N-Acetylglucosaminyltransferase I (EC 2.4.1.101) initiates the conversion of high-mannose asparagine-linked glycans to complex asparagine-linked glycans in plant as well as in animal cells. This Golgi enzyme is missing in the cgl mutant of Arabidopsis thaliana, and the mutant cells are unable to synthesize complex glycans. Transformation of cells from the mutant plants with the cDNA encoding human N-acetylglucosaminyltransferase I restores the wild-type phenotype of the plant cells. Fractionation of the subcellular organelles on isopycnic sucrose gradients shows that the human enzyme in the complemented cells bands at the same density, 1.14 g/cm3, typical of Golgi cisternae, as the enzyme in the wild-type plant cells. These results demonstrate that complementation results from the presence of the human enzyme in the plant Golgi apparatus, where it is functionally integrated into the biosynthetic machinery of the plant cell. In addition, given the evolutionary distance between plants and mammals and the great diversity of glycoproteins that are modified in each, there is probably no specific recognition between this Golgi enzyme and the polypeptide domains of the proteins it modifies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranski T. J., Cantor A. B., Kornfeld S. Lysosomal enzyme phosphorylation. I. Protein recognition determinants in both lobes of procathepsin D mediate its interaction with UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase. J Biol Chem. 1992 Nov 15;267(32):23342–23348. [PubMed] [Google Scholar]
- Burke J., Pettitt J. M., Schachter H., Sarkar M., Gleeson P. A. The transmembrane and flanking sequences of beta 1,2-N-acetylglucosaminyltransferase I specify medial-Golgi localization. J Biol Chem. 1992 Dec 5;267(34):24433–24440. [PubMed] [Google Scholar]
- Cantor A. B., Baranski T. J., Kornfeld S. Lysosomal enzyme phosphorylation. II. Protein recognition determinants in either lobe of procathepsin D are sufficient for phosphorylation of both the amino and carboxyl lobe oligosaccharides. J Biol Chem. 1992 Nov 15;267(32):23349–23356. [PubMed] [Google Scholar]
- Driouich A., Faye L., Staehelin L. A. The plant Golgi apparatus: a factory for complex polysaccharides and glycoproteins. Trends Biochem Sci. 1993 Jun;18(6):210–214. doi: 10.1016/0968-0004(93)90191-o. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Glycoprotein processing and glycoprotein processing inhibitors. Plant Physiol. 1988 Jun;87(2):291–295. doi: 10.1104/pp.87.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991 Dec;5(15):3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Faye L., Gomord V., Fitchette-Lainé A. C., Chrispeels M. J. Affinity purification of antibodies specific for Asn-linked glycans containing alpha 1-->3 fucose or beta 1-->2 xylose. Anal Biochem. 1993 Feb 15;209(1):104–108. doi: 10.1006/abio.1993.1088. [DOI] [PubMed] [Google Scholar]
- Gomez L., Chrispeels M. J. Tonoplast and Soluble Vacuolar Proteins Are Targeted by Different Mechanisms. Plant Cell. 1993 Sep;5(9):1113–1124. doi: 10.1105/tpc.5.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull E., Sarkar M., Spruijt M. P., Höppener J. W., Dunn R., Schachter H. Organization and localization to chromosome 5 of the human UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I gene. Biochem Biophys Res Commun. 1991 Apr 30;176(2):608–615. doi: 10.1016/s0006-291x(05)80227-x. [DOI] [PubMed] [Google Scholar]
- Höfte H., Hubbard L., Reizer J., Ludevid D., Herman E. M., Chrispeels M. J. Vegetative and Seed-Specific Forms of Tonoplast Intrinsic Protein in the Vacuolar Membrane of Arabidopsis thaliana. Plant Physiol. 1992 Jun;99(2):561–570. doi: 10.1104/pp.99.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Chrispeels M. J. Substrate Specificities of N-Acetylglucosaminyl-, Fucosyl-, and Xylosyltransferases that Modify Glycoproteins in the Golgi Apparatus of Bean Cotyledons. Plant Physiol. 1987 Aug;84(4):1301–1308. doi: 10.1104/pp.84.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M. J., Johnson K. D., Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989 Jul;90(3):1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pownall S., Kozak C. A., Schappert K., Sarkar M., Hull E., Schachter H., Marth J. D. Molecular cloning and characterization of the mouse UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I gene. Genomics. 1992 Apr;12(4):699–704. doi: 10.1016/0888-7543(92)90297-6. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Nothwehr S. F., Stevens T. H. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992 Oct;119(1):69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., Hull E., Sarkar M., Simpson R. J., Moritz R. L., Höppener J. W., Dunn R. Molecular cloning of human and rabbit UDP-N-acetylglucosamine: alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I. Biochem Soc Trans. 1991 Aug;19(3):645–648. doi: 10.1042/bst0190645. [DOI] [PubMed] [Google Scholar]
- Sturm A., Johnson K. D., Szumilo T., Elbein A. D., Chrispeels M. J. Subcellular localization of glycosidases and glycosyltransferases involved in the processing of N-linked oligosaccharides. Plant Physiol. 1987 Nov;85(3):741–745. doi: 10.1104/pp.85.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. L., Wong S. H., Low S. H., Hong W. The transmembrane domain of N-glucosaminyltransferase I contains a Golgi retention signal. J Biol Chem. 1992 May 15;267(14):10122–10126. [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. A., Redding K., Wright R., Fuller R. S. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol Biol Cell. 1992 Dec;3(12):1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yet M. G., Shao M. C., Wold F. Effects of the protein matrix on glycan processing in glycoproteins. FASEB J. 1988 Jan;2(1):22–31. doi: 10.1096/fasebj.2.1.3275561. [DOI] [PubMed] [Google Scholar]
- von Schaewen A., Sturm A., O'Neill J., Chrispeels M. J. Isolation of a mutant Arabidopsis plant that lacks N-acetyl glucosaminyl transferase I and is unable to synthesize Golgi-modified complex N-linked glycans. Plant Physiol. 1993 Aug;102(4):1109–1118. doi: 10.1104/pp.102.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]