Abstract
Kaposiform hemangioendothelioma (KHE) is a locally aggressive vascular tumor that may be complicated by Kasabach-Merritt phenomenon (KMP), a profound thrombocytopenia resulting from platelet trapping within a vascular tumor, either KHE or tufted angioma (TA). Typical features also include low fibrinogen and elevated D-dimers. It is well known that KMP is not caused by infantile hemangiomas. Management of vascular tumors complicated by KMP is challenging, and it is common for referral centers to receive patients in critical medical condition after multimodality treatment failure of vascular anomalies. Our aim is to communicate the importance of early diagnosis and treatment of KHE associated with KMP. A full-term male newborn with KHE complicated by KMP is reported. Treatment with vincristine, aspirin and ticlopidine normalized the coagulation parameters within one week, requiring a total of six doses of vincristine, seven months of ticlopidine and 17 months of aspirin. Early diagnosis and treatment of KHE complicated by KMP may allow the administration of fewer doses of vincristine and avoid the use of corticosteroids.
Keywords: kaposiform hemangioendothelioma, Kasabach-Merritt phenomenon, vincristine, ticlopidine, aspirin
Case report
A full-term male newborn was admitted to our hospital with diagnosis of right latero-cervical vascular mass present at birth (Figure 1). He was transferred to our center one week after his birth due to a progressive increase of tumor size. No prior therapy was given. Physical exam revealed a 12×10 cm mixed (deep and superficial) plaque-like lesion with ecchymosis. A severe coagulopathy with profound thrombocytopenia (27,000×109/L) and elevated D-dimer level (84,369 mcg/L) were observed, all of which supported the diagnosis of Kasabach-Merritt phenomenon (KMP). A Doppler ultra-sound study and magnetic resonance imaging (MRI) revealed a multichanneled fast-flowing vascular tumor. Clinical appearance of the tumor, radiological findings, and the coexisting KMP supported the diagnosis of kaposiform hemangioendothelioma (KHE). Treatment was according to SEOP (Spanish Society of Pediatric Oncology) guidelines for vascular tumors complicated by KMP and included vincristine, aspirin and ticlopidine (VAT therapy). The patient received VAT therapy with vincristine: 0.05 mg/kg weekly; aspirin: 10 mg/kg/day; and ticlopidine: 10 mg/kg/day. Treatment response was based on platelet count and classified as good response (increase of >50% of platelet count) observed in obtained blood tests no longer than three weeks since the treatment was started and repeated every two weeks. Coagulation parameters normalized within one week, along with the decrease in size of the tumor. The patient required a total of six doses of vincristine, seven months of ticlopidine and 17 months of aspirin. Twelve months after discontinuation of aspirin, the patient remains asymptomatic with no tumor regrowth and normal platelet count.
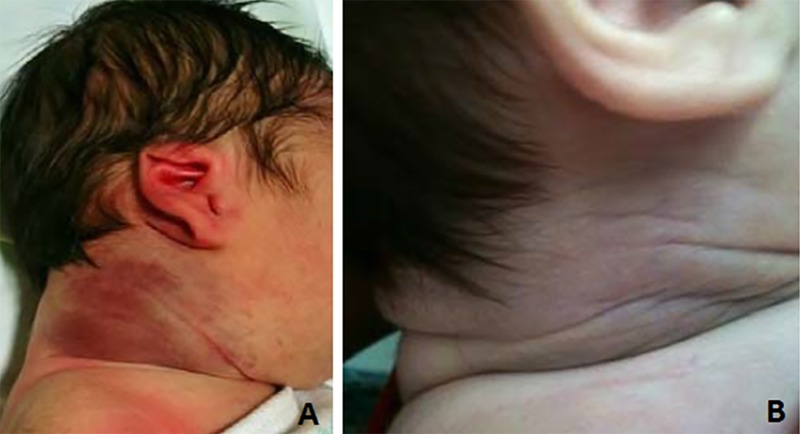
Figure 1.
Right latero-cervical kaposiform hemangioendothelioma at birth (A) and 17 months later, after completion of therapy (B). (Copyright: ©2015 Vivas-Colmenares et al.)
Discussion
KHE involves a spectrum of lesions from small, superficial tumors without KMP to large, infiltrative lesions with life-threatening complications including KMP [1–5]. In an extensive review of KHE, Croteau et al [6] reported that superficial tumors, tumors isolated to bone, or presentation at an older age are each a characteristic that is associated with decreased frequency of KMP. Patients with KHE with fascial involvement but not deeper invasion of muscle or bone seem to present with few complications of KMP. The consensus-derived practice standards plan for complicated KHE was published in 2013 and reported that 64% of experts’ centers did not think that a tissue biopsy was necessary to confirm diagnosis of KMP [7]. As in our case, most of the patients present are diagnosed based on clinical, imaging and laboratory findings (profound thrombocytopenia and elevated serum D-dimer).
Diagnosis of KHE associated with KMP is usually delayed, and several factors may be implicated such as lack of experience in clinical diagnosis of vascular anomalies, deep tumor location (mediastinum, retroperitoneum) and/or the coagulation abnormalities that makes the biopsy challenging. Differential diagnosis with other tumors that may cause coagulopathy should be performed. Baselga et al [8] described that rapidly involuting congenital hemangioma (RICH) may present with thrombocytopenia and coagulopathy similar to mild KMP early in the neonatal period. However, in contrast to true KMP, these abnormal laboratory findings are self-limited and are usually not complicated by bleeding problems. RICH is fully formed at birth and it does not enlarge within the first weeks of life. Clinical appearance of RICH is characterized by superficial prominent veins and is encircled by a pale halo, while KHE is a plaque-like lesion with ecchymosis. A biopsy would have been useful to obtain a histological diagnosis in our case, but the patient presented a profound thrombocytopenia and severe abnormalities in coagulation parameters, which made the biopsy a high-risk bleeding procedure. On imaging studies (MRI, Doppler-US), both tumors are highly vascularized lesions with high-flow, but KHE has a more infiltrative pattern compared to RICH.
Evaluation of treatment outcomes is difficult and based on personal opinions, isolated case reports and small series. Most of the patients receive multimodality therapy, and some delays of initiation of therapy may cause significant morbidity and mortality. The literature describes a variable response to corticosteroid therapy (20–70%), and depending on the dosage can cause several adverse side effects such as severe hypertension, growth restriction, and osteoporosis [9,10]. Although case reports have described the efficacy of propranolol for the treatment of KHE, Chiu et al [11] found that propranolol was ineffective in seven of their eleven patients and recommend clinicians to proceed cautiously before treating these life-threatening conditions with propranolol. Recently, sirolimus has been described to be effective and safe in patients with life-threatening KHE and may represent a promising tool in treating refractory KHE [12]. Vincristine is usually used in combination with other therapies for severe, life-threatening cases because of its good tolerance and response. Several studies used vincristine monotherapy for KMP with good response rates of 86–100% [13,14]. In our protocol, antiaggregant therapy (aspirin and ticlopidine) in combination with vincristine is used as primary treatment for KMP associated with KHE (VAT therapy). This therapy is continued until normal platelet count is obtained; then, vincristine is generally discontinued and antiaggregant agents are continued, although the optimal duration of treatment has not been defined.
The interest of the case lies in the benefit of the early management of KHE complicated by KMP, avoiding the use of corticosteroids and allowing the administration of fewer doses of vincristine. However, some discussion should be highlighted; early referral of patients with KHE to centers with expertise in vascular anomalies is uncommon and diagnosis is delayed in most of the cases. Also, there are potential side effects associated with early treatment in younger patients. Vincristine may cause neuropathy and antiaggregant agents may cause bleeding. Chronic use of aspirin has been associated with the development of Reye syndrome, but the current recommendation is to discontinue the use of aspirin only during suspected influenza or varicella infections [15].
Footnotes
Competing interests: The authors have no conflicts of interest to disclose.
Funding: None.
References
- 1.Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. An aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321–28. doi: 10.1097/00000478-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach-Merritt phenomenon) is associated with kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg. 1997;100:1377–86. doi: 10.1097/00006534-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kelly M. Kasabach-Merritt phenomenon. Pediatr Clin North Am. 2010;57:1085–89. doi: 10.1016/j.pcl.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar M, Mulliken JB, Kozakewich HPW, et al. Thrombocytopenic coagulopathy (Kasabach-Merritt 129 phenomenon) is associated with kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg. 1997;100:1377–86. doi: 10.1097/00006534-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Enjolras O, Wassef M, Mazoyer E, et al. Infants with Kasabach-Merritt syndrome do not have “true” hemangiomas. J Pediatr. 1997;130:631–40. doi: 10.1016/s0022-3476(97)70249-x. [DOI] [PubMed] [Google Scholar]
- 6.Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform Hemangioendothelioma: atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142–47. doi: 10.1016/j.jpeds.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drolet BA, Trenor CC, Brandão LR, et al. Consensusderived practice standards plan for complicated Kaposiform hemangioendothelioma. J Pediatr. 2013;163(1):285–91. doi: 10.1016/j.jpeds.2013.03.080. [DOI] [PubMed] [Google Scholar]
- 8.Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363. doi: 10.1111/j.1365-2133.2008.08546.x. [DOI] [PubMed] [Google Scholar]
- 9.Fahrtash F, McCahon E, Arbuckle S. Successful treatment of kaposiform hemangioendothelioma and tufted angioma with vincristine. J Pediatr Hematol Oncol. 2010;32(6):506–10. doi: 10.1097/MPH.0b013e3181e001a9. [DOI] [PubMed] [Google Scholar]
- 10.Hall GW. Kasabach-Merritt syndrome: pathogenesis and management. Br J Haematol. 2001 Mar;112(4):851–62. doi: 10.1046/j.1365-2141.2001.02453.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiu YE, Drolet BA, Blei F, et al. Variable response to propranolol treatment of kaposiform hemangioendothelioma, tufted angioma, and Kasabach-Merritt phenomenon. Pediatr Blood Cancer. 2012;59(5):934–8. doi: 10.1002/pbc.24103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kai L, Wang Z, Yao W, Dong K, Xiao X. Sirolimus, a promising treatment for refractory Kaposiform hemangioendothelioma. J Cancer Res Clin Oncol. 2014;140(3):471–76. doi: 10.1007/s00432-013-1549-3. [DOI] [PubMed] [Google Scholar]
- 13.Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33(4):171–73. doi: 10.1097/MPH.0b013e3182152e4e. [DOI] [PubMed] [Google Scholar]
- 14.Haisley-Royster C, Enjolras O, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24(6):459–62. doi: 10.1097/00043426-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6Suppl):887S–968S. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]