Abstract
Extremely low birth weight infants (ELBW) are born at a time when the fetus is undergoing rapid intrauterine brain and body growth. Continuation of this growth in the first several weeks postnatally during the time these infants are on ventilator support and receiving critical care is often a challenge. These infants are usually highly stressed and at risk for catabolism. Parenteral nutrition is needed in these infants because most cannot meet the majority of their nutritional needs using the enteral route. Despite adoption of a more aggressive approach with amino acid infusions, there still appears to be a reluctance to use early intravenous lipids. This is based on several dogmas that suggest that lipid infusions may be associated with the development or exacerbation of lung disease, displace bilirubin from albumin, exacerbate sepsis, and cause CNS injury and thrombocytopena. Several recent reviews have focused on intravenous nutrition for premature neonate, but very little exists that provides a comprehensive review of intravenous lipid for very low birth and other critically ill neonates. Here, we would like to provide a brief basic overview, of lipid biochemistry and metabolism of lipids, especially as they pertain to the preterm infant, discuss the origin of some of the current clinical practices, and provide a review of the literature, that can be used as a basis for revising clinical care, and provide some clarity in this controversial area, where clinical care is often based more on tradition and dogma than science.
Keywords: intravenous lipids (IL), preterm infant
Introduction
The capability to provide intravenous fluids and nutrition, for critically ill neonates, has existed for almost 40 years. Despite vast improvements from the original formulations and optimization by means of administration, considerable confusion remains about optimal usage, and much still needs to be learned, to optimize this form of therapy. Several recent reviews have focused on intravenous nutrition for premature neonates, but few have provided comprehensive review of intravenous lipid, for very low birth weight (VLBW) neonates, and other critically ill neonates.
Based on recent studies in the last few years that were catalyzed by conferences at National Institutes of Health, many centers started to use early more aggressive parenteral nutrition (continuous glucose infusion at a rate of 6–8 mg/kg/day; protein 3 mg/kg/day), starting within the first 1–2 hours after birth. Despite the adoption of a more aggressive approach with amino acid infusions, there is still reluctance to the early use of intravenous lipids. This is based on several dogmas that suggest that lipid infusions may be associated with the development or exacerbation of lung disease, can displace bilirubin from albumin, can cause central nervous system (CNS) injury, can cause thrombocytopenia, and can exacerbate sepsis. In reality, the data for these are nonexistent, misinterpreted, or lacking. Furthermore, there is clear evidence nowadays that fat emulsions can be tolerated well by VLBW and extremely low birth weight (ELBW) infants starting from the first day, and even from the first 1–2 hours of life.1–10 Many dogmas still exist, and appear to be the responsible for not to use 2–3 g/kg/d of intravenous lipids by many neonatologist in VLBW and ELBW infants from the first day of life. Here, we would like to provide a brief basic overview of lipid biochemistry and metabolism of lipids, especially as they pertain to the preterm infant, discuss the origin of some of the current clinical practices, and provide a review of the literature that can be used as a basis for revising clinical care and provide some clarity in this controversial area, where clinical care is often based more on tradition and dogma.
Biochemistry and Metabolism of Lipids
Composition of lipid emulsions
Lipid emulsions are composed of three elements: triglycerides (TGs), phospholi-pids (PGs), and glycerol.
Triglycerides
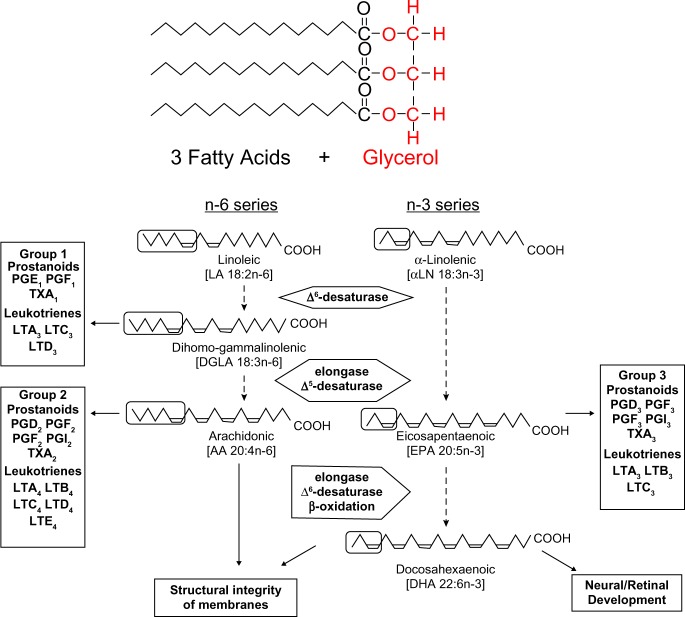
These provide the substrate, as a source of energy and essential fatty acids. Triglycerides are formed by combining glycerol with three molecules of fatty acids. The glycerol molecule has three hydroxyl (HO–) groups (Fig. 1).
Figure 1.
Structure of triglycerides.
Fatty acids are categorized according to the number of carbon atoms and the position and number of any double bonds into saturated, monounsaturated, and polyunsaturated fatty acids. Both saturated and monounsaturated fatty acids can be synthesized from acetyl CoA derived from the metabolism of fat, carbohydrate, or protein. Linoleic acid (9,12-octadecadienoic, C 18:2ω-6) and α-linolenic acid (9,12,15-octadecatrienoic, C 18:3ω-3) are considered essential fatty acids. Both linoleic and α-linolenic acids undergo further metabolism in the liver to form more unsaturated and longer chain omega-6 and omega-3 fatty acids. The most important metabolite of linoleic acid is 5,8,11,14-Eicosatetraenoic (arachidonic acid [AA]) (C 20:4ω-6); 5,8,11,14,17-eicosapentaenoic acid [EPA] (20:5ω-3); and 4,7,10,13,16,19-Docosahexaenoic acid [DHA] (22:6ω-3), are formed from linolenic acid.
The triglycerides that are used are either soya or safflower oil triglycerides, which are rich in long-chain C16–C18 fatty acids (long-chain triglycerides [LCTs]). However, they are also rich in polyunsaturated fatty acids, of the omega-6 series; medium-chain triglycerides (MCTs) made up of C8–C10 fatty acids; olive oil triglycerides with monounsaturated fatty acids; or fish oil derivatives. Linoleic acid, EPA), and DHA belong to the omega-3 series.
Phospholipids
These are emulsifying agents. Phospholipids form the structural bilayer of all cellular and subcellular membranes, regulating the movement of ions and nutrients and facilitating communication of intracellular metabolic events in response to external stimuli. Phospholipids also have important roles in lung surfactants, bile lipids, and plasma lipoproteins (Fig. 2).
Figure 2.
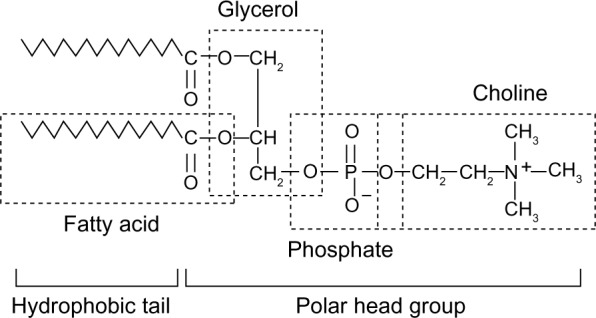
Structure of phospholipids.
Glycerol
This is added to make the emulsion isotonic (HOCH2-CHOH-CH2OH). The commercially available mixtures are lipid emulsions composed exclusively of LCT and rich in omega-6 fatty acids, a mixture of LCT and MCT in different proportions, and structured lipids in which the triglyceride has both long-chain fatty acids (LCFA) and medium-chain fatty acids (MCFA) esterified with the same glycerol molecule.
Lipid peroxidation and free radicals
Lipid peroxidation is a natural metabolic process under normal conditions and refers to the oxidative degradation of lipids. It is the process whereby free radicals “steal” electrons from the lipids in the cell membranes, resulting in cell damage. Antioxidants (different molecules that speed up termination by catching free radicals, and therefore protect the cell membrane) such as vitamin E, superoxidase dismutase, catalase, and peroxidase enzymes are made within the body. It is important to remember that the activity of antioxidants is decreased under stress conditions (anoxia, hypoxia, starvation, and hypothermia), which are common in ELBW infants. Other factors that can lead to increased free radicals formation in ELBW infants are vitamin E deficiency,11 the degree of light that the intravenous lipid is exposed to,12–16 phototherapy,17 blood transfusions,18 and the increase of the carbohydrate–fat ratio of the ELBW infant supplementation.19
The tolerance and clearance of lipid infusion
The triglyceride portion of lipid emulsion particles is hydrolyzed by heparin-activated endothelial lipoprotein lipase (LPL) or hepatic triglyceride lipase. The liver rapidly removes lipid emulsion particle remnants. Free fatty acids (FFAs) can be captured by the adjacent tissues, or circulate bound to albumin, for use in other tissues or uptake by the liver. The activity of endothelial LPL increases with gestational age, as well as with postnatal age, and is inhibited by stress (sepsis and surgery), theophylline, and malnutrition, ie, small for gestational age (SGA).20 Insulin is found to increase the endothelial LPL activity.
The tolerance of lipid infusions is based on the ability to hydrolyze the intravenous fat. Infants less than 33 weeks of gestational age cleared fat at a rate of 0.16 g/kg/h, and those who were older than 33 weeks cleared fat at a rate of 0.3 g/kg/h.21,22 While the clearance of lipid infusions is based on the rate and the interval of infusion, multiple studies showed that rate not exceeding 0.25 g/kg/h over 24 hours in full-term infants and 0.15 g/kg/h over 24 hours in VLBW infants is well tolerated and is not associated with increased plasma lipid values.3,6,10,23–26 Different serum TG concentration norms are recommended by different authors (eg, 100–150, <200, <250 mg/dL). Empirically the authors monitored the plasma TG levels and did not allow them to climb above 300 mg/dL because the clearance of lipid emulsion is saturated at concentrations above 400 mg/dL or 4.5 mmol/L.27 A study by Adamkin et al.1 suggested that premature infants receiving continuous infusions of intravenous fat emulsion over a 20- to 24-hour period could tolerate serum triglyceride levels of <250 mg/dL, without any undesirable consequences.
Lipids of 20% compared to lipids of 10% have half the amount of phospholipids relative to the same amount of triglycerides (provide a lower phospholipid–triglyceride ratio) making it a more efficient clearance of triglycerides, even at higher infusion rates than 10% solution.28,29 It is a well-known fact that structured lipids (SLs) are cleared more slowly than MCT, but faster than LCT, and the LCFA in SL is oxidized more rapidly than in a mixture of MCT/LCT.30 Thus, there is a greater tendency toward hypertriglyceridemia with LCT than MCT emulsions.31
It was found that serum total triglyceride concentrations in >32- and <32-week gestation premature infants ranged between ∼50 and 130 mg/dL when provided with up to 3 g/kg/day as a continuous infusion. However, when provided in 8-hour regimen, levels of over 300 mg/dL were seen, especially in the <32-week premature infants.24 The practice of increasing intravenous lipid infusion in increments is questionable and based on tradition rather than science.32 There is no evidence to support the common practice of gradually increasing the daily lipid intake to induce further lipid clearance.26 Studies using a higher starting dose of 2–4 g/kg/day of intravenous lipids in newborn (term, VLBW and ELBW) infants3,6,10,22,25 showed that these doses are well tolerated, with no significant increase in serum total triglycerides; stepwise increase in intravenous lipid infusion in VLBW and ELBW infants does not improve lipid clearance or tolerance; and more rapid infusions of intravenous lipid were found to be safe. Heparin is another factor that enhances lipid clearance by facilitating LPL activity. Although most authors still recommend the use of heparin dosing at 0.5–1 units/mL of Parenteral nutrition (PN) solution, with a maximum of 137 units/day to improve the intravenous lipid tolerance, and to stabilize serum triglyceride values, some authors still believe that the increase in LPL activity by heparin leads to an increase in FFAs that may exceed the infant’s ability to clear the products of lipolysis, and may weaken the binding of LPL to the endothelium (heparin induces release of LPL from the endothelium into the circulation); hence, they do not recommend the routine addition of heparin with the intravenous lipid infusion.33–35 It was found that, stimulation of LPL activity with heparin in “bolus” injection is ineffectual, while slow continuous administration reduces the serum TG concentration, which is useful in VLBW infants.8,36 Another factor is carnitine, as in vitro studies have suggested that fatty acid oxidation is impaired when the tissue carnitine levels fall below 10% of normal, but a meta-analysis by Cairns et al.37 showed no benefit of parenteral carnitine supplementation on lipid tolerance. Moreover, a retrospective study by Martin et al.38 showed that patients who received lipids delivered in plastic bags are more likely to have hypertriglyceridemia than those who received lipids from glass bottles. This is possible because of a higher proportion of large-diameter fat globules in plastic bags. Based on the above discussion, we believe that it does not make sense to evaluate serum triglycerides before, during, and after each change in lipid infusion. Twice a week and even weekly evaluation of serum triglycerides in otherwise stable premature infants on intravenous lipid infusion is fair enough.
Lipids and protein accretion and energy
Increase in protein mass is a measure of true growth, and protein accretion rate comparable to that of the age-matched fetus should be the primary goal of neonatal nutrition.26 ELBW infants on supplemental glucose only lose approximately 1%–1.5% of protein stores daily (approximately 1.2–1.8 g protein/kg body weight), while they should have been accumulating protein at a rate of 2% per day.39 Although rates of protein synthesis increased to the same degree in ELBW and term infants, rates of protein breakdown remained high in ELBW infants,26 which meant a full term on 90-kcal/kg/day and 2.5 g protein/kg/day is in a positive nitrogen balance, but ELBW infants on 80 kcal/kg/day and 3 g protein/kg/day are still in a negative nitrogen balance.
The minimal glucose requirement to provide basal metabolic needs for the ELBW infant and to maintain adequate energy for the brain is approximately 6 mg/kg/min. In addition to that, 25 kcl/kg or about 2–3 mg/min pre kilogram of glucose per gram of protein intake is necessary to support protein deposition all that will be equal 12–15 mg/kg/min.26 The upper limit of glucose delivery should be less than the maximal glucose oxidative capacity (approximately 18 mg/kg/min); otherwise, excess glucose will be converted to fat, and thus increase energy expenditure, increase oxygen consumption, increase carbon dioxide production, increase lipogenesis, and may lead to fatty liver. Of concern is hyperglycemia, which is quite common in ELBW infants (20%–85% of cases after the second day of life). Glucose as dextrose is commonly initiated at the endogenous hepatic glucose production, and a utilization rate of 8–10 in ELBW infants provides 40–50 kcal/kg/day and preserves carbohydrate stores,40 but most authors recommend starting with 6–8 mg/kg/min, which provides around 40 kcal/kg/day, so the 1,000-g ELBW infants still need around 30 kcal of energy for growth and positive nitrogen balance, which is 2.7–3 g of 20% lipids (at a rate of 0.112–0.125 mg/kg/day) and is even less than the recommended safe rate for lipid infusion. It is almost equal to 40% of the composition of the recommended total nonprotein calories (and not above the maximum fat oxidation) and still can be provided on the first day of life, with no side effects as mentioned above. Moreover, increased energy intake by adding lipid to parenteral nutrition improves nitrogen retention and utilization significantly, resulting in positive nitrogen balance,3,41,42 decreases energy expenditure and glucose utilization by reducing lipogenesis,2,3 reduces both carbon dioxide production and oxygen consumption,2,3,43 provides higher energy storage, increases the net fat storage, and most importantly, prevents essential fatty acid deficiency.
The requirement for lipids to meet energy requirements of ELBW infants
Assume a premature infant weighing 1 kg and requiring at least 80–90 kcal/kg/day for growth when on exclusive parenteral nutrition (Table 1).
Table 1.
Calorie calculation.
| Calorie calculation (Josef Neu) | The total of Kcal/kg/d = Glucose Kcal/kg/d + Amino Acids Kcal/Kg/d + Lipids Kcal/kg/d 90 Kcal/kg/d = (8 mg/kg/min ~ 39 Kcal) + (4 g/kg/d = 16 Kcal) + Lipids Kcal/kg/d 90–39–16 = Lipids Kcal/kg/d → Lipids Kcal/kg/d ~ 35 Kcal 35 Kcal/d lipids = (35 Kcal X mL/2.2 Kcal) × 0.2 g/mL = 3 g of lipids/d. |
| Recommended linoleic acid 4–5% and linolenic acid 1% of total calories calculation (Elizabeth Brine) | Based on a minimal intake of 80 kcal/kg/d, a fat intake of 0.6 to 0.8 g fat/kg/d will meet linoleic acid recommendations and 1.0 g fat/kg/d will meet linolenic acid recommendations from a solution that is 100% soy. Because the 50% soy and 50% safflower mixture provides less linolenic acid, the amount required to meet recommendations is 2.0 g fat/kg/d. In case of 100–120 Kcal/kg/d that will be equal 2.5–3 g fat/kg/d. |
| Recommendation that fat should provide not more than 40–60% of daily non protein calorie (Ghassan Salama) | Total calorie/d = Protein calorie + Non protein calorie 90 = 16 + Non protein calorie 90–16 = Non protein calorie → 74 calorie/d. While 3 grams of 20% lipids = 15 mL which provide 15 × 2.2 = 33 Kcal which equal 44.5% of total daily non protein calorie. |
Essential fatty acid deficiency in VLBW infants
The ELBW infant is particularly vulnerable to insufficient lipid supply because significant in utero fat accretion does not occur until the third trimester.27 At 70% of gestation, there is little fetal lipid uptake. Fetal energy metabolism is not dependent on fat until early in the third trimester, and it then increases only gradually toward term. The accretion of adipose tissue begins at a gestational age of 25 weeks and continues at 1–3 g/kg/day.7 The body composition of a premature infant of 1,000 g is approximately 0.5% glycogen, 8.5% protein, and 1% fat, which equals 10 g of fat44,45 or 20 g in ELBW infants at birth.17 If the infants are not on supplemental lipids, they will lose 1.2 g/day of stored fat.
To prevent fatty acid deficiency, an intake of linoleic acid is recommended at 4%–5% of total calories46,47 and linolenic acid at 1% of total calories.
ELBW infants, with deficient dietary intake, must mobilize fatty acids early for caloric needs.
Essential fatty acid deficiency may be present at birth in premature infants11,48 but mostly can develop over a 72-hour period of deprivation. The clinically apparent manifestations of fatty acid deficiency in premature infants are mostly due to omega-6 fatty acid deficiency (scaly dermatomes, inflamed skin, decreased skin pigmentation, decreased growth, hepatic steatosis, hematologic disturbances, and diminished immune system status) because signs of omega-3 fatty acid deficiency (reduced visual acuity, increased stereotyped behavior and altered learning, and exploratory and aggressive behavior) are difficult to be detected in premature infants clinically.
Lipids and indirect hyperbilirubinemia
Most infants with ELBW develop clinically significant hyperbilirubinemia (jaundice) that requires treatment. ELBW infants are at a higher risk of kernicterus at levels of bilirubin far below those in more mature infants, although specific serum bilirubin levels that are safe versus toxic have never been elucidated. It was suggested that FFAs released after liposis of the parenteral lipids may displace bilirubin from albumin binding sites, especially in premature infants <30 weeks GA and ELBW infants, resulting in increased unbound bilirubin49 and an increased risk of kernicterus. Although it is usually recommended to keep the FFA–albumin molar ratio below 6 to limit the risks of toxicity,50 the ratios above which displacement of bilirubin might occur are differentiated by different authors: 4, 5, 6, and even 10. Starinsky in 197051 was the only one who showed the occurrence of free bilirubin when the ratio exceeded 6. The displacement of bilirubin from binding sites on serum albumin may occur even with adequate metabolism of infused lipids. In vitro, displacement of albumin (ALB)-bound bilirubin by FFAs depends on the relative concentrations of all three compounds. An in vivo study has shown no generation of free bilirubin if the molar FFA–ALB ratio is less than 6.52–54 There is clear evidence that intravenous lipid emulsion does not have a significant effect on indirect hyperbilirubinemia in VLBW and ELBW infants.6,52–55 There is in vitro evidence that a few of the MCFAs may improve the binding of bilirubin to albumin and that this effect varies with the length of the chain.57 Studies have indicated that intermittent lipid infusion is associated with considerable fluctuations in FFA–albumin molar ratios, which did not occur with the continuous infusion.24,52–54 Administering multivitamins containing vitamins C and E with the lipid emulsions via glass bottles and dark delivery tubing (protecting it from phototherapy and ambient lights) provides the most effective way of preventing peroxidation of the lipid. Studies using a higher starting dose of 3–4 g/kg/day of intravenous lipids in newly born (VLBW and ELBW) infants6,52–54,58 showed that high dose (3–4 g/kg/day) immediately after birth can be tolerated in VLBW and ELBW infants, with no adverse effect on bilirubin concentration. Since hyperbilirubinemia and serum lipids appear to be clinically independent factors, the infusion of lipids should not be withheld from jaundice infants on total parenteral nutrition.56
Lipids and hypoglycemia
When the maternal source of glucose has been lost, the ELBW newborn must rapidly develop its own endogenous capacity for producing glucose, which will be from the hepatic glycogen. Due to the high specific requirements of cerebral metabolism (90% of total glucose utilization is by the brain in infants), glucose utilization is very high in ELBW newborns, so with a high glycogenolytic rate, it will deplete its glycogen stores. Sunehag et al.59 demonstrated that very premature infants receiving glucose at half their normal basal glucose turnover rates, in conjunction with appropriate amounts of lipids and amino acids, maintain normoglycemia by the production of glucose primarily via gluconeogenesis. Data from the study by Sunehag60 demonstrated that whereas withdrawal of intralipids from parenteral nutrition resulted in a significant decrease in rates of gluconeogenesis, withdrawal of trophamine did not affect gluconeogenesis, but the rate of glycogenolysis increased. They suggested that intralipid plays a primary role in supporting gluconeogenesis. In a study by Sunehag et al.61, very premature infants were given glycerol by vein, and they found that the rates of gluconeogenesis observed in infants receiving only glycerol were comparable to those observed in infants receiving the fat emulsion containing both glycerol and fatty acids, and conclude that glycerol is the most important component of the lipid emulsion in supporting gluconeogenesis in very premature infants. Ferre et al.62 showed that the intrahepatic oxidation of fatty acids was necessary to provide the energy required to sustain a high rate of gluconeogenesis and proposed that glucose production was enhanced as a result of increased fatty acid oxidation and the generation of ATP, NADH, and acetyl-CoA. From the above discussion we conclude that intralipid is a very important concentrated source of energy, providing nearly three times the energy of glucose on a per gram basis, and early introduction of intravenous lipid emulsion in ELBW infants is very important for both prevention and treatment of hypoglycemia in ELBW infants in the first few days of life.
Lipids and hyperglycemia
Between day 2 and day 7 of life, hyperglycemia (blood glucose concentrations of >150 mg/dL) occurs very frequently among parenterally fed ELBW infants. ELBW infants receiving 3–4 mg/kg/min glucose infusion and 0.25–0.5 g/kg/h intravenous lipids (these exceedingly high lipid infusion rates are not used in preterm or term infant) developed hyperglycemia, which could be due to an increase in FFAs, which can be through decreasing peripheral glucose utilization (peripheral glucose intolerance) and/or inhibiting the effect of insulin to suppress hepatic glucose production (central [hepatic] insulin resistance). Again not only is the intravenous lipid emulsion the offender in the etiology of hyperglycemia of ELBW infants but many other factors are also associated with hyperglycemia of ELBW infants: the initial rates of IV glucose administration, stress (painful procedures such as venipuncture and cut downs, endotracheal tube irritation during ventilator treatment, and catecholamine infusions), medications (theophylline and dexamethasone), the severity of clinical problems in the neonate, the fractional concentration of inspired oxygen, and the respiratory distress score.63 Mitanchez-Mokhtari et al.64 reported that extremely preterm infants with hyperglycemia during the first week of life had a very high concentration of proinsulin, although insulin concentration did not differ significantly from that noted in normoglycemic controls. Mitanchez-Mokhtari et al.64 also found that hyperglycemic neonates responded to exogenous insulin infusion, but needed higher doses to achieve normoglycemia. These data suggest the possibility that insulin resistance is of physiological/biochemical importance in the VLBW neonate. Sunehag et al.59 showed that when the glucose infusion rate is reduced, the VLBW neonate can use part of the energy supplied by noncarbohydrate sources, including glycerol and amino acids, to sustain the blood glucose concentration. It is possible that a reduction in the glucose infusion rate, even if partially offset by an increase in the administration of lipid and amino acids, might allow some reduction in blood glucose concentrations while reducing the risk of energy deficiency. Murdock et al.65 showed that early introduction of amino acids lowers serum blood glucose, and suggested that this was due to the stimulatory effect of amino acids on insulin secretion. Adamkin66 suggested that early parenteral nutrition with amino acids prevents hyperglycemia. Thus, providing glucose at a rate equivalent to the basal glucose turnover rate of these infants (6–8 mg/kg/min or 33–44 μmol/kg/min), in addition to the currently recommended rates of lipid (∼3 g/kg/day on days 3–4 of life) and amino acid substrate (important for protein synthesis) (∼3 g/kg/day), may be a potential strategy to reduce the risk of hyperglycemia, without increasing the risk of either energy insufficiency67,68 or hypoglycemia. Yunis et al.69 showed that in combination with nutrients usually given, such as glucose and amino acids, glucose production is not enhanced by MCFA administration. Williamson et al.70, Stein et al.71, Alstrup et al.72, and Clore et al.73 showed that saturated fatty acids have more potent effects on insulin release, glucose oxidation, glucose production, and gluconeogenesis than unsaturated fatty acids. While, on the other hand, Summers et al.74 showed that monounsaturated fatty acids have a greater effect on glucose-stimulated insulin secretion than saturated fatty acids. Anne et al.75 showed that infants with hyperglycemia could benefit from the administration of parenteral nutrition containing a lipid emulsion with less effect on glucose production, such as linoleic, to combine sufficient energy supply with minimum effects on glucose metabolism.
Lipids and chronic lung disease
Bronchopulmonary dysplasia (BPD) is common in very preterm infants (born at <32-week gestational age), with respiratory distress syndrome. BPD is a form of chronic lung disease (CLD) that develops in preterm neonates treated with oxygen and positive-pressure ventilation.
Concerns about the early use of intravenous lipids in VLBW premature infants have centered on mortality and morbidity associated with their use in the development of CLD due to increased membrane oxidant damage or infiltration of pulmonary tissue by lipid particles. The study by Cooke76 determined, among 659 infants 2:30 weeks from the years 1983–1989, that intravenous lipid during the first 21 days was significantly associated with the development of CLD.
Hammerman and Aramburo77 hypothesized a deleterious effect of early intralipid infusion on pulmonary function and showed an increased incidence and severity of CLD.
Sosenko et al.78 in a study designed to evaluate the effect of early intralipid infusion on CLD, even though there was no difference in the mortality rate or incidence of BPD between the two groups (early lipid vs no lipid), showed that the most concerning data about the potential adverse effect of early lipid is that infants with birth weight 600–800 g receiving early lipids were more likely to have pulmonary hemorrhage and had a higher mortality rate than the control group.
Many studies investigated the effect of early infusion of lipid emulsion, but found no effect on the incidence of death or CLD.6,55,79,80
And the concerns about the association of increase in mortality and higher incidence of CLD completely have been refuted by meta-analysis of six randomized controlled trials (RCTs) published only in abstract by Fox et al.81
Because of the vulnerability of the VLBW premature to the effects of undernutrition, the early withholding of nutrition in general, and lipid nutrition specifically, from these critically ill neonates could potentially increase their risk of developing CLD and damage.13
Lipids and diffusion of oxygen in the lungs, blood pH, and pulmonary hypertension
The use of the conservative approach of intravenous lipid emulsion infusion for VLBW infants is due to the possible pulmonary deposition associated with a decrease in pulmonary oxygen diffusion, decrease in arterial oxygen tension (pO2), as suggested by Greene et al.82 and Pereira et al.83, and increase pulmonary vasomotor tone and arterial pressure.84–86
Cashore in 198287 showed that fat emulsion administered at a rate of 0.2 g/kg/h had no effect on oxygenation of neonates weighing less than 1500 g. Branes et al in 1986,25 studying fat emulsion tolerance in VLBW neonates (820–1510 g and 27–34 weeks of gestation) and its effect on diffusion of oxygen in the lungs and on blood pH using three regimens of administration of fat emulsion for a period of 8 days, found that even starting with a high infusion of 4 g lipids/kg/day (0.167 mg/kg/h) had no deleterious effect on blood pH and alveolar–arteriolar oxygen diffusion gradient.
Giovannini et al.88 and Zeigler et al.4 suggested that the production of CO2 is 40% lower per unit of energy during intravenous administration of fats, an important consideration in case of pulmonary insufficiency.
In their study, Prasertsom et al.89 used two-dimensional echocardiography and estimated pulmonary vascular resistance from right ventricular pre-ejection period to ejection time (RVPEP/ET) in 11 preterm infants with respiratory distress to test the effect of different doses of a continuous lipid infusion (1.5 and 3 g/kg/day of intravenous lipid). They found that continuous 24-h infusion of lipid caused a significant dose- and time-dependent increase in pulmonary vascular resistance. Intravenous lipid may aggravate pulmonary hypertension. They mentioned that pulmonary arterial pressure returned to baseline 24 hours after the intravenous lipid had been discontinued, and all the infants in their study improved clinically despite the increases in RVPEP/ET. Many authors focused on the role of eicosanoid metabolism, rather than a mechanical obstructive effect on the development of pulmonary hypertension during intravenous lipid infusion.
Lipid and immunity
Some authors had been focusing only on the adverse effect of intravenous lipid infusion on the immune system. Colomb et al.90, Sweeney et al.91, and Okada et al.92 reported that in vitro studies showed adverse effect of lipids on the survival of monocytes and binding of interleukin 2 to its receptors. On the other hand, Strunk et al.93, Usmani et al.94, and Wheeler et al.95 reported that in vivo studies did not reveal adverse effects of lipid emulsions on complement factors or polymorphonuclear leukocyte function. Dahlstrom et al.96 did not find an impairment of monocyte activation and complement factors in children on long-term parenteral nutrition.
Weirnik et al.97 studied the effect of intralipid on the function of mononuclear monocytes in vivo and in vitro and found that in vivo after intralipid administration, the NBT reduction of monocytes on stimulation with Escherichia coli was significantly higher, there was no difference in the mean monocyte count, there was no significant difference in the mean number of ingested yeast particles, and the granulocyte counts were significantly higher than before. While in vitro the NBT reduction of monocytes did not change significantly after incubation with intralipid, a significant decrease in the NBT- activity of the granulocytes at rest, significant increase in the chemotactic motility of monocytes, and the spontaneous migration of monocytes were not statistically significant, mean while at the same time regarding to the granulocytes, both the chemotactic and spontaneous migrations were significantly decreased after incubation with lipid. In both in vivo and in vitro after incubation in intralipid, the monocytes ingested a higher number of yeast particles per cell, but the number of attached particles decreased, and as for the granulocytes, the number of ingested particles per cell was decreased, while the number of attached particles per cell increased after interlipid exposure.
Lipids and sepsis
There are conflicting data about the use of intravenous lipids in VLBW and ELBW infants and sepsis, whether intravenous lipid infusion can increase the incidence of sepsis in VLBW and ELBW infants, or the presence of sepsis will increase the incidence of unwanted side effects of intravenous lipid in VLBW and ELBW infants.
A case–control study among 882 infants reported that infants with coagulase-negative staphylococcus bacteremia were 5.8 times as likely as controls to have received lipid emulsion before the onset of bacteremia. Freeman et al.98 reported that 56% of all the cases of nosocomal bacteremia could be attributed to lipid administration. In contrast, other investigators described sick VLBW infants with a lower incidence of coagulase negative staphylococcal bacteremia, although they received significantly larger amounts of lipids.99 In their studies Hammerman and Aramburo,77 Gilberston et al.55, and Sosenko et al.78 reported that there was no clinical evidence of increased susceptibility to bacterial infection between early and no early lipid group.
Park et al.100,101 and Dahlström et al.96 showed that in septic premature infants, triglyceride levels tend to be higher and fatty acid oxidation and lipid clearance were lower than in nonseptic patients, but we should mention that in those studies high doses of heparin were applied and 10% lipid emulsion was used. Toce et al.102 showed that there is no association between hypertriglyceridemia and infection. On the other hand, several reports have shown strong correlations between low plasma cholesterol and mortality in critically ill or infected patients.103–105
Hypertriglyceridemia is generally thought to benefit cells involved in the immune response and tissue repair by neutralizing certain microorganisms and their toxins.106,107 Sammalkorpi K et al.109, suggested that, the host response in inflammation is accompanied by profound alterations in lipid metabolism, and hence the distribution and composition of lipoprotein subclasses especially high density lipoproteins (HDL), which mainly consist of apolipoproteins and phospholipids. In patients with bacterial focus and systemic inflammatory response and severe sepsis, a decrease in total HDL could be demonstrated, with a loss of mainly large apolipoprotein A-I (apoA-I) HDL particles, a total loss of apoC-I, and an increase in apoE HDL and phospholipid transfer protein (PLTP) activity was increased paralleled by a redistribution of PLTP into a population of small (120- to 200-kDa) particles, probably representing PLTP homodimers or lipid-complexed PLTP.110
HDLs mediate reverse cholesterol transport and the clearance of inflammatory mediators such as bacterial lipopolysaccharide (LPS)110 and scavenging radicals and inhibit oxidation to protect the cell membranes (HDL carries proteins such as paraoxonase, clusterin [apoJ, S40:40], protectin [CD59], platelet-activating factor acetylhydrolase, and ceruloplasmin transferring).111 HDLs are also capable of binding mediators of the inflammatory response, such as LPS, a membrane lipid of gram-negative bacteria.110,112 Elevated HDL concentrations protect against the lethal effects of endotoxic shock. ApoE-containing HDLs were found to accumulate during inflammation, which may contribute to the increase in PLTP activity and to an improved supply of fatty acid molecules for energy supply and phospholipids to various peripheral tissues, which must maintain cellular membrane homeostasis under conditions of inflammatory stress.108 Lipoproteins bind the bioactive lipid, a portion of the molecule, and prevent it from stimulating monocytes and macrophages and other LPS-responsive cells. Lipoprotein-bound LPS is cleared from the circulation principally by the liver,113 where the LPS may then be excreted into the bile. However, the use of lipid emulsion and nutritional support are very important in septic VLBW and ELBW infants, to avoid excessive carbohydrate intakes and to prevent the skin manifestation (dermatitis) caused by essential fatty acid deficiency, and thereby decrease the incidence of bacterial and fungal infection.
Lipids and platelets
Concerns have been raised regarding the possible adverse effects of intravenous lipid infusion on platelets count and function. Goulet et al.114 reported that long-term administration of PN with lipid emulsion induced hyperactivation of the monocyte–macrophage system with hematologic abnormalities, including recurrent thrombocytopenia due to the reduced platelet lifespan and hemophagocytosis in bone marrow. Herson et al.115 reported thrombocytopenia associated with intravenous lipid emulsion infusion in children who were on very long-term home Total parenteral nutrition (TPN) for months to years. On the other hand, Jarrnvig et al.116, Porta et al.117, and Planas et al.118 showed that intravenous lipid emulsion infusion does not affect either platelet number or platelet function. In their studies on VLBW and ELBW infants, Hammerman and Aramburo55 and Gilbertson et al.77 reported that there was no significant difference between early and no early lipid infusion. However, delay in intravenous lipid emulsion infusion to VLBW and ELBW infant caused essential fatty acid deficiency, especially linoleic acid deficiency, which is associated with low platelet count. We think that the dogma about intravenous lipid infusion–associated thrombocytopenia might be either related to the effects of α-tocopherol (vitamin E) on membrane properties of platelets or heparin-induced platelet dysfunction, or both, but not caused by lipid emulsion per se. Studies of prolonged vitamin E deficiencies in rat have indeed demonstrated a variety of hematological effects, ie, elevated platelet and reticulocyte counts, normocytic anemia, increased in vitro platelet aggregation, and peroxide-induced erythrocyte hemolysis.119,120 On the other hand, supplementation of vitamin E to vitamin E–deficient patients caused a return of the high platelet count to its normal level. So the initially appearing high platelet count in fatty acid and vitamin E–deficient VLBW and ELBW infants will return to its actual low level after the induction of intravenous lipid emulsion and the fat-soluble vitamins, especially vitamin E. Platelet aggregation is a three-phase response: shape change, microaggregation (primary aggregation), and macroaggregation (secondary aggregation).121 Heparin impairs primary hemostasis, at least in part, by impairing platelet function.122 Intravenous heparinization inhibits platelet macroaggregation markedly.123,124 The inhibition of platelet macroaggregation is not dose related when heparin doses of 30 U/kg, or more are administered, and the VLBW and ELBW infants usually receive 0.5 to 1 unit heparin/1 ml parenteral nutrition infusion and for relatively long periods which could be the cause behind the thrombocytopenia. One should always not forget the many other factors causing thrombocytopenia in VLBW and ELBW infants.
Lipids and retinopathy of prematurity
In the outer segments of the retina, more than 50% of their fatty acid content of the photoreceptors of the eye is DHA. It is the special properties of permeability and perhaps fluidity of the DHA that probably account for this high concentration.125 In addition to its important role in brain development, DHA plays a vital function in developing vision sharpness or acuity. DHA is found in high concentrations in the photoreceptors of the retina and supplies lipids to the retinal membrane. During the first 6 months of life, a baby’s retinocortical system – which enables it to distinguish between light and dark – matures rapidly. Healthy full-term infants have shown an average 20% improvement each month, between the ages of 2 and 7 months. Later on, this ability to see fine light and dark contrasts will help babies recognize facial features and expression.126–128 It has been noted that, when levels of DHA are too low, abnormal visual functioning and peripheral neuropathy occur.129 Several RCTs have reported that preterm infants fed LCP-UFA-enriched formulas have enhanced visual development, including improved retinal sensitivity and visual acuity, compared with those fed unsupplemented formulas.126–128,130–132 Many authors studied the effect of early introduction of intravenous lipid emulsion to the VLBW and ELBW infants on retinopathy of prematurity (ROP). Hammerman and Aramburo77 reported an increase in the incidence of ROP in the early lipid group to no early lipid group. Gilbertson et al.55, Sosenko et al.78, and Ibrahim et al.6 did not find any significant difference in the incidence of ROP, between early and no early lipid groups. Douglas D et al.10 showed that there was a significant increase in the incidence of ROP in no early lipid group, compared with the early lipid group. Salama et al.133 showed that early aggressive introduction of intravenous fat emulsion is associated with better retinal development in preterm infants, and thus decreases the incidence of ROP. The researchers found that increasing omega-3 fatty acids and decreasing omega-6 fatty acids in the diet reduced the area of vessel loss that ultimately causes the growth of the abnormal vessels and blindness. Omega-6 fatty acids contribute to the growth of abnormal blood vessels in the retina. Omega-3 fatty acids create chemical compounds known as bioactive mediators, which protect against the growth of abnormal blood vessels, a condition that characterizes some forms of retinopathy. In part, this occurs because these mediators suppress a type of inflammatory protein called tumor necrosis factor alpha (TNF-alpha). TNF-alpha is found in one type of cell, called microglia, which can be closely associated with retinal blood vessels.134
Lipids and necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is the most common severe neonatal gastrointestinal emergency that predominantly affects premature infants.135
No single study mentioned an increase in the incidence of NEC in early intravenous lipid infusion to VLBW and ELBW infants. Hammerman and Aramburo,77 Gilbertson et al.55, Sosenko et al.78, and Ibrahim et al.6 showed that there was no statistically significant difference between early and no early lipid groups. Douglas D et al.10 showed that there was a significant increase in the incidence of NEC in the no early lipid group (14%), compared with early lipid group (0%). Caplan and Tilling136 showed that LCPUFA reduced intestinal inflammation and decreased the incidence of NEC. Carlson and Ziegler128 showed that infants fed the phospholipid-containing formula had a lower incidence of NEC, compared with infants in the nonsupplemented group.
Lipids and PDA
No single study mentioned an increase in the incidence of PDA in early intravenous lipid infusion to VLBW and ELBW infants. Brownlee et al.80 and Ibrahim et al.6 reported no statistical difference in the incidence of PDA between early and no early lipid groups. Gilbertson et al.55, Hammerman and Aramburo,77 and Sosenko et al.78 showed no significant trend to decrease the incidence of PDA between early and no early lipid groups. Further studies to evaluate the incidence of spontaneous closure of PDA and re-opening of a PDA with the use of early intravenous lipid emulsion infusion to the VLBW and ELBW infant are needed.
Lipids and intraventricular hemorrhage
ELBW infants (birth weights of <1,000 g) are already at risk of cerebral bleeding, particularly during their first week of life, as a result of immature blood vessels in the germinal matrix, and the nonfully developed autoregulation of cerebral blood pressure.
One of the dogmas is that intravenous lipid infusion to VLBW and ELBW infant increases the incidence of intraventricular hemorrhage (IVH), but actually to our knowledge no single study showed that the use of intravenous lipid infusion per se was the cause of IVH. We think that there is a misunderstanding because many authors reported increased incidence of IVH with the use of PN in general. Many authors reported that hyperglycemia in VLBW and ELBW infants on PN is associated with IVH. On the other hand, because the brain is the principal glucose consumer, accounting for 90% of glucose utilization in newborn infants, a large cerebral bleeding will disturb brain metabolism and reduce its glucose consumption, leading to hyperglycemia.137 Previously the dogma was that the intravenous lipid emulsion causes hyperglycemia in VLBW and ELBW infants. But as we have shown previously, the use of intravenous lipid in combination with amino acids decreases the incidence of hyperglycemia in the VLBW and ELBW infants. So we expect, decrease or at least no increase in the incidence of IVH with the use of intravenous lipid infusion in VLBW and ELBW infants. Hammerman and Aramburo,77 Gillbertson et al.55, Sosenko et al.78, and Ibrahim et al.6, showed that there was no significant difference in the incidence of IVH (all grades) in the early and no early lipid groups.
Lipids and growth and neurodevelopment
Fatty acids are contained in the membranes of every cell in our body, particularly concentrated in the membranes of brain cells, heart cells, retinal cells, and immune system cells. Because brain activity depends greatly on the functions provided by lipid membranes, most of the dry weight of the brain is a lipid (fat). DHA is particularly concentrated in membranes that are functionally active, namely in synapses and in the retina. A fatty acid in phosphatidylethanolamine of human gray matter cell membrane is roughly 25% DHA, 25% stearic acid, 14% AA, and 12% oleic acid.125 The greatest dependence on DHA occurs in the fetus during the last trimester of pregnancy, and (to a lesser extent) in the infant during the first 3 months after birth. It is during this period that brain synapses are forming most rapidly, and an infant’s demand for DHA exceeds the capacity of the enzymes to synthesize. The DHA content of the brain increases three to five times during the last trimester and again during the first 12 weeks after birth. Research has shown that when infants receive reduced amounts of DHA, they have a smaller brain development. Researchers have noticed that DHA is required for the development of the cerebral cortex and also for the numerous functions the brain is responsible for, including that of signal transmission and cognitive functions.
A treatment of intravenous infusion of glucose, amino acids, and emulsion enriched with essential fatty acids, linoleic acid, and linolenic acid was given to 30 pregnant women with intrauterine growth retardation and 28 nonessential fatty acids treated cases as controls. There was a marked gain in fetal biparietal diameter, and on the estimated weight of the treated group over the control group. The mean birth weight was significantly different in the two groups. The fetal biparietal diameter increases much more in fetuses of malnutritioned mothers if she treated with essential fatty acids at 28–34 gestational weeks, than those treated at 34–37 weeks, which indicates that early initiation complements of n-3 and n-6 fatty acids to the mother, may correct pregnancy-induced essential fatty acids deficiency and maternal-fetal malnutrition, which demonstrates a fetal catch-up of growth in the brain and the whole body. Ehrenkranz et al.138 reported that motor development at 8 years of age correlated most strongly with HC growth from birth to discharge. Franz et al.139 showed that poor early neonatal HC growth was associated with abnormal neurological examination and abnormal mobility at the age of 5.4 years, and poor early neonatal weight gain was associated with abnormal neurological examination with lower mental processing composite scores in multiple regression models, accounting for perinatal risk factors and socioeconomic status. Ehrenkranz et al.138 reported that, a higher rate of HC growth and weight gain from birth to discharge was associated with a lower incidence of cerebral palsy, subnormal mental developmental index, and neurodevelopmental impairment. As we said previously the most common cause of poor growth in VLBW and ELBW infant is the inadequate nutrition. Many authors studied the effect of early aggressive use of parenteral nutrition on growth and neurodevelopment in general (Table 2).
Table 2.
Effect of early aggressive use of parenteral nutrition on growth and neurodevelopment.
| STUDY BY | CONCLUSION |
|---|---|
| Wilson DC et al., 2005140 | Intensive early neonatal nutritional support, improved growth and weight gain in very low birth weight (VLBW) infants. |
| Brandt I et al., 2003141 | Increased energy supply during the first 10 days of life, was associated not only with improved HC growth, but also with improved neurodevelopment until 6 years of life in a cohort study of more mature VLBW infants. |
| Tan MJ and Cooke RW in 2008142 | Intravenous supplementation of 4 g protein/kg/d and 4 g/kg/d lipids, to ELBW infants reduced days to regain birth weight, and improved cumulative energy and protein intake. |
| Ibrahim et al., 20046 | With the use of 4 g/kg/d from the first day of life, in ELBW infants does not cause significant difference in the mean weight gain. |
| Douglas D et al., 200810 | There were no significant differences in the anthropometric measures at discharge, between the premature received 2 g lipids/kg/d from the first day and the control group, but infants how received 2 g/kg/d/lipid emulsion from the first day were discharged an average 6.9 days earlier than infants in the control group and at discharge, more infants in the group who started on 2 g lipids/kg/d were more or equal 10th percentile for weight for age, compared with infants who started on 0.5 g/kg/d of lipids. |
Summary
Here, we would like to summarize this review using the words of our great teacher professor Josef Neu:
Most if not all the dogmas that have prevented the early use of intravenous lipids have either been disproved, not based on fact, or weak. There are compelling reasons for early use of lipids, which include prevention of EFA deficiency, provision of energy, and provision of substrates for LCPUFA synthesis all of which are important for the growth and development of VLBW and ELBW infants.
No treatment is without risk. Clinicians must balance the benefits versus the risks when using 2–3 g/kg/day intravenous lipid infusion from day 1 of life in VLBW and ELBW infants. We found that the benefits are much more than the risks, if any. So we strongly recommend giving the VLBW and ELBW infants 2–3 g/kg/day intravenous lipid as a continuous infusion over 24 hours at a rate not exceeding 0.15 g/kg/h within the first 24 hours of life.
Footnotes
Author Contributions
GS is responsible for the concept of the review. GS and MK wrote and edited the manuscript. MA and M. Alquran collected and reviewed the references. GS and M. Alquran made critical revisions and approved final version. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Praveen Kumar, Associate Editor
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Adamkin D, Gelke K, Andrews B. Fat emulsions and hypertriglyceridemia. JPEN J Parenter Enteral Nutr. 1984;8(5):563–7. doi: 10.1177/0148607184008005563. [DOI] [PubMed] [Google Scholar]
- 2.Van Aerde JE, Sauer PJ, Pencharz PB, Smith JM, Swyer PR. Effect of replacing glucose with lipid on the energy metabolism of newborn infants. Clin Sci. 1989;76(6):581–8. doi: 10.1042/cs0760581. [DOI] [PubMed] [Google Scholar]
- 3.Van Aerde JE, Sauer PJ, Pencharz PB, Smith JM, Heim T, Swyer P. Metabolic consequences of increasing energy intake by adding lipid to parenteral nutrition in full-term infants. Am J Clin Nutr. 1994;59(3):659–62. doi: 10.1093/ajcn/59.3.659. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birthweight infant. Clin Perinatol. 2002;29:225–44. doi: 10.1016/s0095-5108(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 5.Innis SM, Adamkin DH, Hall RT, et al. Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. J Pediatr. 2002;140(5):547–53. doi: 10.1067/mpd.2002.123282. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop R. Aggressive early total parenteral nutrition in low-birthweight infants. J Perinatol. 2004;24(8):482–6. doi: 10.1038/sj.jp.7211114. [DOI] [PubMed] [Google Scholar]
- 7.Uauy R, Tsang R, Koletzko B, Zlotkin S. Concepts, definitions and approaches to define the nutritional needs of LBW infants. In: Tsang R, Uauy R, Koletzko B, Zlotkin S, editors. Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines. 2nd ed. Cincinnati, OH: Digital Education Publishing; 2005. pp. 1–21. [Google Scholar]
- 8.Koletzko B, Innis S. Lipids. In: Tsang R, Uauy R, Koletzko B, Zlotkin S, editors. Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines. 2nd ed. Cincinnati, OH: Digital Education Publishing; 2005. pp. 97–137. [Google Scholar]
- 9.Thureen PJ. Early aggressive nutrition in very preterm infants. Nestle Nutr Workshop Ser Pediatr Program. 2007;59:193–204. doi: 10.1159/000098536. [DOI] [PubMed] [Google Scholar]
- 10.Douglas D, Connie M, Shirley G, Matt N, Kamlesh S. Randomized trial of very low birth weight infants receiving higher rates of infusion of intravenous fat emulsions during the first week of life. Pediatrics. 2008;122:743–51. doi: 10.1542/peds.2007-2282. [DOI] [PubMed] [Google Scholar]
- 11.Tomsits E, Rischak K, Szollar L. Effects of early nutrition on free radical formation in VLBW infants with respiratory distress. J Am Coll Nutr. 2000;19:237–41. doi: 10.1080/07315724.2000.10718922. [DOI] [PubMed] [Google Scholar]
- 12.Pitkanen O, Hallman M, Anderson S. Generation of free radicals in lipid emulsion used in parenteral nutrition. Pediatr Res. 1990;29:56–9. doi: 10.1203/00006450-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Sosenko IR. Do polyunsaturated fatty acids protect against oxidant-induced lung damage? J Nutr. 1995;125(6 suppl):1652S–6. doi: 10.1093/jn/125.suppl_6.1652S. [DOI] [PubMed] [Google Scholar]
- 14.Putet G. Lipid metabolism of the micropremie. Clin Perinatol. 2000;27:57–69. doi: 10.1016/s0095-5108(05)70006-3. [DOI] [PubMed] [Google Scholar]
- 15.Silvers KM, Sluis KB, Darlow BA, McGill F, Stocker R, Winterbourn CC. Limiting light-induced lipid peroxidation and vitamin loss in infant parenteral nutrition by adding multivitamin preparations to intralipid. Acta Paediatr. 2001;90(3):242–9. [PubMed] [Google Scholar]
- 16.Picaud JC, Steghens JP, Auxenfans C, Barbieux A, Laborie S, Claris O. Lipid peroxidation assessment by malondialdehyde measurement in parenteral nutrition solutions for newborn infants: a pilot study. Acta Paediatr. 2004;93(2):241–5. [PubMed] [Google Scholar]
- 17.Neu J. Aggressive nutritional support and nutritional adjuncts for premature and critically ill neonates. Acta Pharmacol Sin. 2002;23(suppl):19–21. [Google Scholar]
- 18.Wardle S, Drury J, Garr R, Weindling A. Effect of blood transfusion on lipid peroxidation in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;86:46–8. doi: 10.1136/fn.86.1.F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu R, Muller DP, Papp E, et al. Free radical formation in infants: the effect of critical illness, parenteral nutrition, and enteral feeding. J Pediatr Surg. 1999;34(7):1091–5. doi: 10.1016/s0022-3468(99)90573-0. [DOI] [PubMed] [Google Scholar]
- 20.Heird WC, Kashyap S, Gomez MR. Protein intake and energy requirements of the infant. Semin Perinatol. 1991;15:438–48. [PubMed] [Google Scholar]
- 21.Gustafson A, Kjellmer I, Olegard R, Victorin L. Nutrition in low birth-weight infants. I. Intravenous injection of fat emulsion. Acta Paediatr Scand. 1972;61:149. doi: 10.1111/j.1651-2227.1972.tb15919.x. [DOI] [PubMed] [Google Scholar]
- 22.Snezhana P, Ljiljana K. Associative tolerance of intravenously administered lipid and gestational age in preterm infants receiving total parenteral nutrition. Macedonian J Med Sci. 2009;2(1):63–8. [Google Scholar]
- 23.Vileisis RA, Cowett RM, Oh W. Glycemic response to lipid infusion in the premature neonate. J Pediatr. 1982;100(1):108–12. doi: 10.1016/s0022-3476(82)80248-5. [DOI] [PubMed] [Google Scholar]
- 24.Kao LC, Cheng MH, Warburton D. Triglycerides, free fatty acids, free fatty acids/albumin molar ratio, and cholesterol levels in serum of neonates receiving long-term lipid infusions: controlled trial of continuous and intermittent regimens. J Pediatr. 1984;104:429–35. doi: 10.1016/s0022-3476(84)81111-7. [DOI] [PubMed] [Google Scholar]
- 25.Brans YW, Dutton EB, Andrew DS, Menchaca EM, West DL. Fat emulsion tolerance in very-low-birth-weight neonates: effect on diffusion of oxygen in the lungs and on blood pH. Pediatrics. 1986;78:79–84. [PubMed] [Google Scholar]
- 26.Thureen PJ. Early aggressive nutrition in the neonate. Pediatr Rev. 1999;20:e45–55. doi: 10.1542/pir.20-9-e45. [DOI] [PubMed] [Google Scholar]
- 27.Brunzell JD, Hazzard WR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973;52:1578–85. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haumont D, Deckelbaum RJ, Richelle M, et al. Plasma lipid and plasma lipoprotein concentrations in low-birth-weight infants given parenteral nutrition with 20% compared to 10% intralipid. J Pediatr. 1989;115:787–93. doi: 10.1016/s0022-3476(89)80663-8. [DOI] [PubMed] [Google Scholar]
- 29.Haumont D, Richelle M, Deckelbaum RJ, Coussaert E, Carpentier YA. Effect of liposomal content of lipid emulsions on plasma lipid concentrations in low birth weight infants receiving parenteral nutrition. J Pediatr. 1992;121:759–63. doi: 10.1016/s0022-3476(05)81912-2. [DOI] [PubMed] [Google Scholar]
- 30.Hultin M, Müllertz A, Zundel MA, et al. Metabolism of emulsions containing medium- and long-chain triglycerides or interesterified triglycerides. J Lipid Res. 1994;35(10):1850–60. [PubMed] [Google Scholar]
- 31.Weissman C, Chiolero R, Askanazi J, Gil KM, Kinney JM. Intravenous infusion of a medium-chain triglyceride-enriched lipid emulsion. Crit Care Med. 1988;16:1183–90. doi: 10.1097/00003246-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Josef N. Myths and dogmas in neonatal gastroenterology and nutrition. NeoReviews. 2007;8:e485–90. [Google Scholar]
- 33.Spear ML, Stahl GE, Paul MH, Egler JM, Pereira GR, Polin RA. The effect of 15-hour fat infusions of varying dosage on bilirubin binding to albumin. JPEN J Parenter Enteral Nutr. 1985;9(2):144–7. doi: 10.1177/0148607185009002144. [DOI] [PubMed] [Google Scholar]
- 34.Berkow SE, Spear ML, Stahl GE, et al. Total parenteral nutrition with intralipid in premature infants receiving TPN with heparin: effect on plasma lipolytic enzymes, lipids, and glucose. J Pediatr Gastroenterol Nutr. 1987;6(4):581–8. doi: 10.1097/00005176-198707000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Peterson J, Bihain BE, Bengtsson-Olivecrona G, Deckelbaum RJ, Carpentier YA, Olivecrona T. Fatty acid control of lipoprotein lipase: a link between energy metabolism and lipid transport. Proc Natl Acad Sci U S A. 1990;87(3):909–13. doi: 10.1073/pnas.87.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puntis JWL. Nutritional support in neonatology. In: Sobotka L, editor. Basics in Clinical Nutrition. Prague: Galen; 2004. pp. 425–39. [Google Scholar]
- 37.Cairns PA, Wilson DC, Jenkins J, McMaster D, McClure BG. Tolerance of mixed lipid emulsion in neonates: effect of concentration. Arch Dis Child Fetal Neonatal Ed. 1996;75(2):F113–6. doi: 10.1136/fn.75.2.f113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institutes of Health . Fat Tolerance from Lipid Emulsion Infusion Packaged in Glass or Plastic (study ID: 2005-P-00275; identifier: NCT00278928) Atlanta, GA: National Institutes of Health; 2006. [Google Scholar]
- 39.Satish S, Manoj M, Avneet K, et al. Growth of very low birth-weight Indian infants during hospital stay. Indian Pediatr. 2010;47:851–6. doi: 10.1007/s13312-010-0146-7. [DOI] [PubMed] [Google Scholar]
- 40.Hertz D, Kam CA, Liu YM, Liechty EA, Denne SC. Intravenous glucose suppresses glucose production but not proteolysis in extremely premature newborns. J Clin Invest. 1993;92(4):1752–8. doi: 10.1172/JCI116763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffy B, Gunn J, Collinge J, Pencharz P. The effect of varying protein quality and energy intake on the nitrogen metabolism of parenterally fed very low birth-weight (<1600 g) infants. Pediatr Res. 1981;15:1040–4. doi: 10.1203/00006450-198107000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Zlotkin SH, Bryan MH, Anderson GH. Intravenous nitrogen and energy intakes required to duplicate in utero nitrogen accretion in prematurely born human infants. J Pediatr. 1981;99:115–20. doi: 10.1016/s0022-3476(81)80975-4. [DOI] [PubMed] [Google Scholar]
- 43.Piedboeuf B, Chessex P, Hazan J, Pineault M, Lavoie JC. Total parenteral nutrition in the newborn infant: energy substrates and respiratory gas exchange. J Pediatr. 1991;118:97–102. doi: 10.1016/s0022-3476(05)81857-8. [DOI] [PubMed] [Google Scholar]
- 44.Widdowson EM. Growth and composition of the fetus and newborn. In: Assali NS, editor. Biology of Gestation. II. New York: Academic Prees Inc; 1968. [Google Scholar]
- 45.Sinclair JC, Driscoll JM, Heird WC, et al. Supportive management of the sick neonate. Pediatr Clin North Am. 1970;17:863. doi: 10.1016/s0031-3955(16)32485-3. [DOI] [PubMed] [Google Scholar]
- 46.Hansen AE, Wiese HF, Boelsche AN, et al. Role of linoleic acid in infant nutrition: clinical and chemical study of 428 infants fed on milk mixtures varying in kind and amount of fat. Pediatrics. 1963;31:171. [Google Scholar]
- 47.Holman RT. Essential fatty acid deficiency. In: Holman RT, editor. Progress in the Chemistry of Fats and Other Lipids. Vol. 9. Oxford: Pergamon Press; 1968. pp. 275–348. [Google Scholar]
- 48.Gutcher G, Farrell P. Intravenous infusion of lipid for the prevention of essential fatty acid deficiency in premature infants. Am J Clin Nutr. 1991;54(6):1024. doi: 10.1093/ajcn/54.6.1024. [DOI] [PubMed] [Google Scholar]
- 49.Odell GB, Cukier JO, Ostrea EM, Jr, Maglalang AC, Poland RL. The influence of fatty acids on the binding of bilirubin to albumin. J Lab Clin Med. 1977;89(2):295–307. [PubMed] [Google Scholar]
- 50.Andrew G, Chan G, Schiff C. Lipid metabolism in the neonate II. The effect of intralipid on bilirubin binding in vitro and in vivo. J Pediatr. 1976;88:279–84. [PubMed] [Google Scholar]
- 51.Starinsky R, Shafrir E. Displacement of albumin-bound bilirubin by free fatty acids. Implications for neonatal hyperbilirubinemia. Clin Chim Acta. 1970;29(2):311–8. doi: 10.1016/0009-8981(70)90052-5. [DOI] [PubMed] [Google Scholar]
- 52.Brans YW, Ritter DA, Kenny JD, Andrew DS, Dutton EB, Carrillo DW. Influence of intravenous fat emulsion on serum bilirubin in very low birthweight neonates. Arch Dis Child. 1987;62:156–60. doi: 10.1136/adc.62.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adamkin DH, Radmacher PG, Klingbeil RL. Use of intravenous lipid and hyper-bilirubinemia in the first week. J Pediatr Gastroenterol Nutr. 1992;14:135–9. doi: 10.1097/00005176-199202000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Adamkin DH. Early total parenteral nutrition in very low birthweight infants: is it safe? Is it worth it? J Pediatr. 2013;163(3):622–4. doi: 10.1016/j.jpeds.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 55.Gilbertson N, Kovar IZ, Cox DJ, Crowe L, Palmer NT. Introduction of intravenous lipid administration on the first day of life in the very low birth weight neonate. J Pediatr. 1991;119:615–23. doi: 10.1016/s0022-3476(05)82416-3. [DOI] [PubMed] [Google Scholar]
- 56.Rubin M, Naor N, Sirota L, et al. Are bilirubin and plasma lipid profiles of premature infant’s dependent on the lipid emulsions infused? J Pediatr Gastroenterol Nutr. 1995;21(1):25–30. doi: 10.1097/00005176-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Brodersen R. Binding of bilirubin to albumin. CRC Crit Rev Clin Lab Sci. 1980;11(4):305–99. [PubMed] [Google Scholar]
- 58.Salama G, Obeidat M, Alhadidi A, Frehat M, Khlefat A. Randomized clinical trial of early aggressive versus late and slow intravenous lipid infusion in preterm infants and bilirubin and lipid profiles. JRMS. 2013;20(4):50–6. [Google Scholar]
- 59.Sunehag AL, Haymond MW, Schanler RJ, Reeds PJ, Bier DM. Gluconeogenesis in very low birth weight infants receiving total parenteral nutrition. Diabetes. 1999;48:791. doi: 10.2337/diabetes.48.4.791. [DOI] [PubMed] [Google Scholar]
- 60.Sunehag AL. The role of parenteral lipids in supporting gluconeogenesis in very premature infants. Pediatr Res. 2003;54:480–6. doi: 10.1203/01.PDR.0000081298.06751.76. [DOI] [PubMed] [Google Scholar]
- 61.Sunehag AL. Parenteral glycerol enhances gluconeogenesiss in very premature infants. Pediatr Res. 2003;53:635–41. doi: 10.1203/01.PDR.0000054774.90893.0F. [DOI] [PubMed] [Google Scholar]
- 62.Ferré P, Pegorier JP, Marliss EB, Girard JR. Influence of exogenous fat and gluconeogenic substrates on glucose homeostasis in the newborn rat. Am J Physiol. 1978;234(2):E129–36. doi: 10.1152/ajpendo.1978.234.2.E129. [DOI] [PubMed] [Google Scholar]
- 63.Anusha H, Richard M. Neonatal hyperglycaemia. Pediatr Rev. 1999;20:16. [Google Scholar]
- 64.Mitanchez-Mokhtari D, Lahlou N, Kieffer F, Magny JF, Roger M, Voyer M. Both relative insulin resistance and defective islet beta-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics. 2004;113:537–41. doi: 10.1542/peds.113.3.537. [DOI] [PubMed] [Google Scholar]
- 65.Murdock N, Crighton A, Nelson LM, Forsyth JS. Low birthweight infants and total parenteral nutrition immediately after birth. II. Randomised study of biochemical tolerance of intravenous glucose, amino acids, and lipid. Arch Dis Child Fetal Neonatal Ed. 1995;73:F8–12. doi: 10.1136/fn.73.1.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adamkin H. Nutrition management of the very low-birthweight infant: I. Total parenteral nutrition and minimal enteral nutrition. NeoReviews. 2006;7:e602–7. [Google Scholar]
- 67.Sauer PJ, Carnielli VP, Sulkers EJ, van Goudoever JB. Substrate utilization during the first week of life. Acta Paediatr Suppl. 1994;405:49–53. doi: 10.1111/j.1651-2227.1994.tb13398.x. [DOI] [PubMed] [Google Scholar]
- 68.Micheli JL, Pfister R, Junod S, Laubscher B, Tolsa JF, Schutz Y. Calame. Water, energy and early postnatal growth in preterm infants. Acta Paediatr Suppl. 1994;405:35–42. doi: 10.1111/j.1651-2227.1994.tb13396.x. [DOI] [PubMed] [Google Scholar]
- 69.Yunis K, Oh W, Kalhan S, Cowett R. Glucose kinetics following administration of an intravenous fat emulsion to low-birth-weight neonates. Am J Physiol. 1992;263:E844–9. doi: 10.1152/ajpendo.1992.263.5.E844. [DOI] [PubMed] [Google Scholar]
- 70.Williamson J, Browning E, Scholz R. Control mechanisms of gluconeogenesis and ketogenesis. J Biol Chem. 1969;224:4607–16. [PubMed] [Google Scholar]
- 71.Stein D, Stevenson B, Chester M, et al. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J Clin Invest. 1997;100:398–403. doi: 10.1172/JCI119546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alstrup K, Gregersen S, Jensen H, Thomsen J, Hermansenn K. Differential effects of cis and trans fatty acids on insulin release from isolated mouse islets. Metabolism. 1999;48:22–9. doi: 10.1016/s0026-0495(99)90005-7. [DOI] [PubMed] [Google Scholar]
- 73.Clore JN, Allred J, White D, Li J, Stillman J. The role of plasma fatty acid composition in endogenous glucose production in patients with type 2 diabetess mellitus. Metabolism. 2002;51:1471–7. doi: 10.1053/meta.2002.35202. [DOI] [PubMed] [Google Scholar]
- 74.Summers LK, Fielding BA, Bradshaw HA, et al. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia. 2002;45(3):369–77. doi: 10.1007/s00125-001-0768-3. [DOI] [PubMed] [Google Scholar]
- 75.van Kempen Anne AMW, van der Crabben Saskia N, Ackermans Mariëtte T, et al. Stimulation of gluconeogenesis by intravenous lipids in preterm infants: response depends on fatty acid profile. Am J PhysiolEndocrinolMetab. 2006;2006;290:E723–30. doi: 10.1152/ajpendo.00303.2005. [DOI] [PubMed] [Google Scholar]
- 76.Cooke RW. Factors associated with chronic lung disease in preterm infants. Arch Dis Child. 1991;66:776–9. doi: 10.1136/adc.66.7_spec_no.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hammerman C, Aramburo MJ. Decreased lipid intake reduces morbidity in sick premature neonates. J Pediatr. 1988;113:1083–8. doi: 10.1016/s0022-3476(88)80587-0. [DOI] [PubMed] [Google Scholar]
- 78.Sosenko IR, Rodriguez-Pierce M, Bancalari E. Effect of early initiation of intravenous lipid administration on the incidence and severity of chronic lung disease in premature infants. J Pediatr. 1993;123:975–82. doi: 10.1016/s0022-3476(05)80397-x. [DOI] [PubMed] [Google Scholar]
- 79.Alwaidh MH, Bowden L, Shaw B, Ryan SW. Randomised trial of effect of delayed intravenous lipid administration on chronic lung disease in preterm neonates. J Pediatr Gastroenterol Nutr. 1996;22:303–6. doi: 10.1097/00005176-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 80.Brownlee KG, Kelly EJ, Ng PC, Kendall-Smith SC, Dear PR. Early or late parenteral nutrition for the sick preterm infant? Arch Dis Child. 1993;69:281–3. doi: 10.1136/adc.69.3_spec_no.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fox GF, Wilson DC, Ohlsson A. Effect of early vs. late introduction of intravenous lipid to preterm infants on death and chronic lung disease (CLD) – results of meta-analyses. Ped Res. 1998;43(suppl 2):214A. [Google Scholar]
- 82.Greene HL, Hazlett D, Demaree R. Relationship between intralipid-induced hyperlipemia and pulmonary function. Am J Clin Nutr. 1976;29:127–35. doi: 10.1093/ajcn/29.2.127. [DOI] [PubMed] [Google Scholar]
- 83.Pereira GR, Fox WW, Stanley CA, Baker L, Schwartz JG. Decreased oxygenation and hyperlipemia during intravenous fat infusions in premature infants. Pediatrics. 1980;66:26–30. [PubMed] [Google Scholar]
- 84.McKeen CR, Brigham KL, Bowers RE. Pulmonary vascular effects of fat emulsion infusion in unanesthetised sheep: prevention by indomethacin. J Clin Invest. 1978;61:1291–7. doi: 10.1172/JCI109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hageman JR, McCulloch K, Gora P, Olsen EK, Pachman L, Hunt CE. Intralipid alterations in pulmonary prostaglandin metabolism and gas exchange. Cric Care Med. 1983;11:794–8. doi: 10.1097/00003246-198310000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Hageman JR, Hunt CE. Fat emulsions and lung function. Clin Chest Med. 1986;7:69–77. [PubMed] [Google Scholar]
- 87.Cashore WJ. Growth and transcutaneous oxygen transport in very low birthweight infants receiving intravenous fat emulsion. Acta Chir Scand. 1982;517:123–34. [PubMed] [Google Scholar]
- 88.Giovannini I, Chiarla C, Boldrini G, Castagneto M. Impact of fat and glucose administration on metabolic and respiratory interactions in sepsis. JPEN J Parenter Enteral Nutr. 1989;13(2):141–6. doi: 10.1177/0148607189013002141. [DOI] [PubMed] [Google Scholar]
- 89.Prasertsom W, Phillipos EZ, Van Aerde JE, Robertson M. Pulmonary vascular resistance during lipid infusion in neonates. Arch Dis Child Fetal Neonatal Ed. 1996;74(2):F95–8. doi: 10.1136/fn.74.2.f95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Colomb V, Jobert-Giraud A, Lacaille F, Goulet O, Fournet JC, Ricour C. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J Parenter Enteral Nutr. 2000;24:345–50. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 91.Sweeney B, Puri P, Reen DJ. Polyunsaturated fatty acids influence neonatal monocyte survival. Pediatr Surg Int. 2001;17:254–8. doi: 10.1007/s003830100589. [DOI] [PubMed] [Google Scholar]
- 92.Okada Y, Klein NJ, van Saene HK, Webb G, Holzel H, Pierro A. Bactericidal activity against coagulase-negative staphylococci is impaired in infants receiving long-term parenteral nutrition. Ann Surg. 2000;231:276–81. doi: 10.1097/00000658-200002000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strunk RC, Murrow BW, Thilo E, Kunke KS, Johnson EG. Normal macrophage function in infants receiving intralipid by low-dose intermittent administration. J Pediatr. 1985;106:640–5. doi: 10.1016/s0022-3476(85)80094-9. [DOI] [PubMed] [Google Scholar]
- 94.Usmani SS, Harper RG, Usmani SF. Effect of a lipid emulsion (Intralipid) on polymorphonuclear leukocyte functions in the neonate. J Pediatr. 1988;113:132–6. doi: 10.1016/s0022-3476(88)80547-x. [DOI] [PubMed] [Google Scholar]
- 95.Wheeler JG, Boyle RJ, Abramson JS. Intralipid infusion in neonates: effects on polymorphonuclear leukocyte function. J Pediatr Gastroenterol Nutr. 1985;4:453–6. doi: 10.1097/00005176-198506000-00022. [DOI] [PubMed] [Google Scholar]
- 96.Dahlström KA, Goulet OJ, Roberts RL, Ricour C, Ament ME. Lipid tolerance in children receiving long-term parenteral nutrition: a biochemical and immunologic study. J Pediatr. 1988;113:985–90. doi: 10.1016/s0022-3476(88)80568-7. [DOI] [PubMed] [Google Scholar]
- 97.Wiernik A, Jarstand C, Julander I. The effect of intralipid on mononuclear and polymorphonuclear phagocytes. Am J Clin Nutr. 1983;37:256–61. doi: 10.1093/ajcn/37.2.256. [DOI] [PubMed] [Google Scholar]
- 98.Freeman J, Goldmann DA, Smith NE, Sidebottom DG, Epstein MF, Platt R. Association of intravenous lipid emulsion and coagulase-negative staphylococcal bacteremia in neonatal intensive care units. N Engl J Med. 1990;323:301–8. doi: 10.1056/NEJM199008023230504. [DOI] [PubMed] [Google Scholar]
- 99.Elizabeth B, Judith A. Total parenteral nutrition for premature infants. Newborn Infant Nurs Rev. 2004;4:133–55. [Google Scholar]
- 100.Park W, Paust H, Schroder H. Lipid infusion in premature infants suffering from sepsis. JPEN J Parenter Enteral Nutr. 1984;8:290–2. doi: 10.1177/0148607184008003290. [DOI] [PubMed] [Google Scholar]
- 101.Park W, Paust H, Brösicke H, Knoblach G, Helge H. Impaired fat utilization in parenterally fed low-birth-weight infants suffering from sepsis. JPEN J Parenter Enteral Nutr. 1986;10:627–30. doi: 10.1177/0148607186010006627. [DOI] [PubMed] [Google Scholar]
- 102.Toce SS, Keenan WJ. Lipid intolerance in newborns is associated with hepatic dysfunction but not infection. Arch Pediatr Adolesc Med. 1995;149:1249–53. doi: 10.1001/archpedi.1995.02170240067010. [DOI] [PubMed] [Google Scholar]
- 103.Fraunberger P, Nagel D, Walli AK, Seidel D. Serum cholesterol and mortality in patients with multiple organ failure. Crit Care Med. 2000;28:3574–5. doi: 10.1097/00003246-200010000-00047. [DOI] [PubMed] [Google Scholar]
- 104.Windler E, Ewers-Grabow U, Thiery J, Walli A, Seidel D, Greten H. The prognostic value of hypocholesterolemia in hospitalized patients. Clin Investig. 1994;72:939–43. doi: 10.1007/BF00577732. [DOI] [PubMed] [Google Scholar]
- 105.Gordon BR, Parker TS, Levine DM, et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med. 2001;29:1563–8. doi: 10.1097/00003246-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 106.Feingold KR, Grunfeld C. Lipids: a key player in the battle between the host and microorganisms. J Lipid Res. 2012;53(12):2487–9. doi: 10.1194/jlr.E033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feingold KR, Grunfeld C. The role of HDL in innate immunity. J Lipid Res. 2011;52(1):1–3. doi: 10.1194/jlr.E012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sammalkorpi K, Valtonen V, Kerttula Y, Nikkila E, Taskinen M. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 1988;37:859–65. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 109.Barlage S, Fröhlich D, Böttcher A, et al. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. J Lipid Res. 2001;42(2):281–90. [PubMed] [Google Scholar]
- 110.Levine D, Parker T, Donnelly T, Walsh A, Rubin A. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1993;90:12040–4. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mackness MI, Durrington PN. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis. 1995;115(2):243–53. doi: 10.1016/0021-9150(94)05524-m. [DOI] [PubMed] [Google Scholar]
- 112.Parker T, Levine D, Chang C, Laxer J, Coffin C, Rubin L. Neutralizes gram-negative bacterial Reconstituted high-density lipoprotein lipopolysaccharides in human whole blood. Infect Immun. 1995;63(1):253. doi: 10.1128/iai.63.1.253-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Munford R, Anderson M, Dietschy M. Sites of tissue binding and uptake in vivoof bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981;68:1503–13. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goulet O, Girot R, Maier-Redelsperger M, Bougle D, Virelizier JL, Ricour C. Hematologic disorders following prolonged use of intravenous fat emulsions in children. JPEN J Parenter Enteral Nutr. 1986;10:284–8. doi: 10.1177/0148607186010003284. [DOI] [PubMed] [Google Scholar]
- 115.Herson VC, Block C, Eisenfeld L, Maderazo EG, Krause PJ. Effects of intravenous fat infusion on neonatal neutrophil and platelet function. J Parenter Enteral Nutr. 1989;13:620–2. doi: 10.1177/0148607189013006620. [DOI] [PubMed] [Google Scholar]
- 116.Jarnvig IL, Naesh O, Hindberg I, Behnke O, Bregengaad C, Wilhjelm B. Platelet responses to intravenous infusion of intralipid in healthy volunteers. Am J Clin Nutr. 1990;52:628–31. doi: 10.1093/ajcn/52.4.628. [DOI] [PubMed] [Google Scholar]
- 117.Porta I, Planas M, Padró JB, Picó M, Valls M, Schwartz S. Effect of two lipid emulsions on platelet function. Infusionsther Transfusionsmed. 1994;21:316–21. doi: 10.1159/000223001. [DOI] [PubMed] [Google Scholar]
- 118.Planas M, Porta I, Sagristá ML, Mora M, Padró JB, Picó M. Fatty acid composition of platelet membrane lipids after administration of two different fat emulsions in critically ill patients. Intensive Care Med. 1999;25:395–8. doi: 10.1007/s001340050864. [DOI] [PubMed] [Google Scholar]
- 119.Machlin LJ, Filipski R, Willis AL, Kuhn DC, Brin M. Influence of vitamin E on platelet aggregation and thrombocythemia in the rat. Proc Soc Exp Biol Med. 1975;149(1):275–7. doi: 10.3181/00379727-149-38787. [DOI] [PubMed] [Google Scholar]
- 120.Machlin LJ, Filipski R, Nelson J, Horn LR, Brin M. A further study of the specificity of the vitamin E requirement for reproduction. J Nutr. 1977;84:395–400. [Google Scholar]
- 121.Pedvis L, Wong T, Frojmovic M. Differential inhibition of the platelet activation sequence: shape change, micro- and macro-aggregation, by a stable prostacyclin analogue (Iloprost) Thomb Haemost. 1988;59:323–8. [PubMed] [Google Scholar]
- 122.Heiden D, Mielke CHJ, Rodvien R. Impairment by heparin of primary haemostasis and platelet [14C] 5-hydroxytryptamine release. Br J Haematol. 1977;36:427–36. doi: 10.1111/j.1365-2141.1977.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 123.Besterman EM, Gillett MP. Heparin effects on plasma lysolecithin formation and platelet aggregation. Atherosclerosis. 1973;17:503–13. doi: 10.1016/0021-9150(73)90040-3. [DOI] [PubMed] [Google Scholar]
- 124.Belcher PR, Muriithi EW, Milne EM, Wanikiat P, Wheatley DJ, Armstrong RA. Heparin, platelet aggregation, neutrophils and cardiopulmonary bypass. Thromb Res In Press. 2000 doi: 10.1016/s0049-3848(99)00243-1. [DOI] [PubMed] [Google Scholar]
- 125.Ehringer W, Belcher D, Wassall SR, Stillwell W. A comparison of the effects of linoleic (18:3ω3) and docosahexaenoic(22:6ω3) acids on phospholipid bilayers. Chem Phys Lipids. 1990;54:79–88. doi: 10.1016/0009-3084(90)90063-w. [DOI] [PubMed] [Google Scholar]
- 126.Carlson SE, Werkman SH, Rhodes PG, Tolley EA. Visual acuity development in healthy preterm infants: effect of marine oil supplementation. Am J Clin Nutr. 1993;58:35–42. doi: 10.1093/ajcn/58.1.35. [DOI] [PubMed] [Google Scholar]
- 127.Carlson SE, Werkman SH. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until 2 months. Lipids. 1996;31:85–90. doi: 10.1007/BF02522416. [DOI] [PubMed] [Google Scholar]
- 128.Carlson SJ, Ziegler EE. Nutrient intakes and growth of very low birth weight infants. J Perinatol. 1998;18(4):252–8. [PubMed] [Google Scholar]
- 129.Hoffman DR, Birch EE, Birch DG, Uauy RD. Effects of supplementation with omega 3 long-chain polyunsaturated fatty acids on retinal and cortical development in premature infants. Am J Clin Nutr. 1993;57(suppl):807S–12. doi: 10.1093/ajcn/57.5.807S. [DOI] [PubMed] [Google Scholar]
- 130.Birch EE, Castaneda YS, Wheaton DH, Birch DG, Uauy RD, Hoffman DR. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 months. Am J Clin Nutr. 2005;81:871–9. doi: 10.1093/ajcn/81.4.871. [DOI] [PubMed] [Google Scholar]
- 131.Birch D, Birch E, Hoffman DR, Uauy R. Retinal development of very low birth-weight infants fed diets differing in n-3 fatty acids. Investig Ophthalmol Vis Sci. 1992;33:2365–76. [PubMed] [Google Scholar]
- 132.O’Connor DL, Hall R, Adamkin D, et al. Ross Preterm Lipid Study. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective randomized controlled trial. Pediatrics. 2001;108:359–71. doi: 10.1542/peds.108.2.359. [DOI] [PubMed] [Google Scholar]
- 133.Salama G, Sunna N, Alhadidi A, Khlefat A, Ayyash F. Early aggressive intravenous fat emulsion decreases the incidence of retinopathy of prematurity. N Iraq J Med. 2013;9(1):13–8. [Google Scholar]
- 134.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of ω-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neu J. Necrotizing enterocolitis. World Rev Nutr Diet. 2014;110:253–63. doi: 10.1159/000358474. [DOI] [PubMed] [Google Scholar]
- 136.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13(2):111–5. doi: 10.1097/00008480-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 137.Bier DM, Leake RD, Haymond MW, et al. Measurement of “true” glucose production rates in infancy and childhood with 6, 6-dideuteroglucose. Diabetes. 1977;26:1016–23. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- 138.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 139.Franz AR, Pohlandt F, Bode H, et al. Intrauterine, early neonatal, and post-discharge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123(1):e101–9. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 140.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115(4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 141.Brandt I, Sticker EJ, Lentze MJ. Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. J Pediatr. 2003;142(5):463–8. doi: 10.1067/mpd.2003.149. [DOI] [PubMed] [Google Scholar]
- 142.Tan MJ, Cooke RW. Improving head growth in very preterm infants – a randomised controlled trial I: neonatal outcomes. Arch Dis Child Fetal Neonatal Ed. 2008;93:F337–41. doi: 10.1136/adc.2007.124230. [DOI] [PubMed] [Google Scholar]