ABSTRACT
One of the first lines of host defense against many viruses in vertebrates is the innate immune system, which detects pathogen-associated molecular patterns (PAMPs) using pathogen recognition receptors (PRR). The dynamic interactions between pathogens and hosts create, in some cases, species-specific relationships. Recently, it was shown that murine factor X (mFX)-armored human adenovirus (HAd) stimulated a mFX-Toll-like receptor 4 (TLR4)-associated response in mouse macrophages in vitro and in vivo. Given the importance of studies using animals to better understand host-pathogen interactions, we asked if human FX (hFX)-armored HAd type 5 (HAd5) was capable of activating innate immune sensors in primary human mononuclear phagocytes. To this end, we assayed human mononuclear phagocytes for their ability to be stimulated by hFX-armored HAd5 via a TLR/NF-κB pathway, in particular, a TLR4 pathway. In our hands, we found no significant interaction, activation, or maturation of human mononuclear phagocytes caused by the presence of hFX-armored HAd5.
IMPORTANCE Animals, and mice in particular, are often used as informative and powerful surrogates for how pathogens interact with natural host systems. When possible, extended and targeted studies in the natural host can then be performed. Our data will help us understand the differences in preclinical testing in mice and clinical use in humans in order to improve treatment for HAd diseases and Ad vector effectiveness.
INTRODUCTION
The innate response uses an array of pathogen recognition receptors (PRRs) to detect pathogen-associated molecular patterns (PAMPs). The best-characterized PRRs are the members of the Toll-like receptor family (TLRs); among them, TLR4 is arguably the most studied. Due to the endemic and occasional epidemic nature of human adenovirus (HAd) infections, and the advent of human and nonhuman Ad-derived vectors for short-term (e.g., vaccines) and long-term (e.g., brain gene transfer) (1, 2) transgene expression, understanding their interaction with the innate immune system is fundamental to better treat HAd diseases and to optimize the efficacy of gene transfer vectors. Studies addressing the interaction of HAds with PRR have demonstrated that these pathogens trigger signals from cytosolic- and/or vesicle-compartmentalized double-stranded DNA sensors (3–6).
Wild-type and transgenic mice are commonly used to assess the risk and efficacy of drugs and treatments and have been valuable models from which many paradigms of immunology have been derived. Although there are differences that affect the transcriptional response and functionality of several leukocyte lineages (7, 8) and TLR4 signaling (9), there are many features conserved between human and murine immune systems. Yet a recent pair of studies described contrasting interpretations—using the same data set—of the fidelity of mouse models with respect to mimicking human inflammatory diseases (59, 60).
When pathogens are concerned, the results generated in mice can influence therapies in humans. The interaction between PRRs and PAMPs has dynamically coevolved to create, in some cases, species-specific interactions. Murine factor X (mFX)-armored HAd5 (mFX-HAd5) stimulated TLR4 signaling in mouse spleen macrophages in vitro and after intravenous virus injection (10). We previously described the response of human monocyte-derived dendritic cells (MoDCs) to replication-defective HAd5 vectors and found that they poorly stimulated DC maturation (3). This response in human mononuclear phagocytes is in contrast to the ability of HAd5 vector to efficiently stimulate the maturation of mouse DCs (11, 12). An example of the differences concerns the half-life of HAd in blood. In contrast to human erythrocytes, mouse erythrocytes do not express the coxsackievirus and adenovirus receptor (CAR) (13). Therefore, CAR-tropic Ads, like HAd5, are poorly (<1%) cell associated in mice (14) and cleared from the systemic circulation within an hour (15). In humans, the retention time for HAd5 in blood, after intravenous vector administration or during acute viremia, can be between 6 and 24 h (16–18). The prolonged blood half-life should increase the likelihood of vector interaction with plasma components like FX and cells expressing TLR4. Human FX (hFX) and mFX bind the HAd5 hexon with nanomolar affinity, and the latter is thought to facilitate liver transduction in mice (19, 20) by preventing neutralization of natural antibodies after intravascular delivery in naive immunocompetent mice (21). TLR4 is expressed at different levels by human monocytes, neutrophils, DCs, platelets, and B and T cells (22–25). Therefore, an interaction between HAd5, human FX, and human TLR4 would be relevant in the systemic inflammatory response.
The juxtaposition of the results described above prompted us to explore, in an exclusively human system, the role of human FX and human Ad in human TLR stimulation, activation, and maturation in human mononuclear phagocytes. Our report complements and extends the novel results reported by Doronin et al. (10) and will help us better understand the innate immune response to HAds and their vectors to treat human diseases.
MATERIALS AND METHODS
Cells and culture conditions.
Human MoDCs and human monocyte-derived macrophages were generated in the presence of 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) and of 20 ng/ml interleukin-4 (IL-4) (Peprotech, Neuilly sur Seine, France) and 25 ng/ml GM-CSF, respectively, as previously described (61, 63). THP-1-ASC-GFP cells (26) were cultured under the same conditions as MoDCs and differentiated into THP-1-derived DCs (27). HepG2 cells, a human hepatocellular carcinoma cell line, were maintained in Dulbecco's modified Eagle's medium (DMEM) (Lonza, Basel, Switzerland)–10% fetal calf serum (FCS) (Sigma-Aldrich, Saint-Quentin Fallavier, France).
Adenovirus vectors.
Adenovirus β-galactosidase (Adβgal) (28) and AdLite (29) are E1/E3-deleted HAd5 vectors and were produced as previously described (28). Alexa 555-labeled AdLite (AdLite-555) was generated as previously described (30).
hFX binding to HAd5, immune-complexed adenovirus (IC-Ad) formation, and cell stimulation.
To 400 μl of RPMI medium–10% FCS (Life Technologies, Villebon, France)–penicillin-streptomycin containing 4 × 105 cells, we added 2 × 104 physical particles (pp) of Adβgal/cell alone preincubated with 4 μg of hFX (Cryoprep, Montpellier, France) or sequentially preincubated with ±4 μg hFX and 2.5 μl of Kiovig (Fig. 1A). Kiovig is a solution containing pooled human IgG, and the corresponding amount of Adβgal was mixed in a volume of 35 μl for 15 min at room temperature. TAK-242 (Invivogen, Toulouse, France), a TLR4 inhibitor, was added to the DCs 1 h before stimulation.
FIG 1.
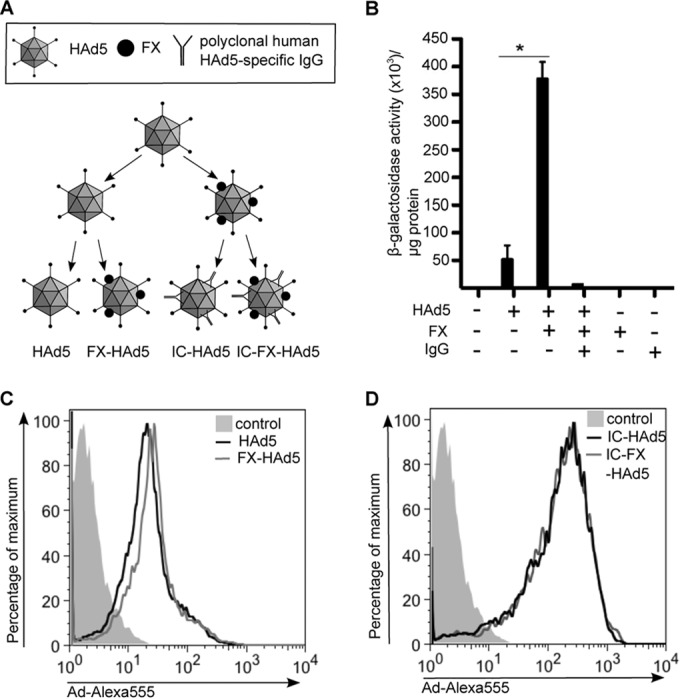
Influence of FX on HAd5 attachment. (A) Scheme of adenovirus complexes used in this study. HAd5 was incubated initially with FX for 15 min to create FX-HAd5, and HAd5 or FX-HAd5 was incubated with Kiovig for another 15 min to create IC-HAd5 and FX-IC-HAd5 and before adding it to the cells. (B) Biological activity and attachment of FX to the HAd5 was assayed by transduction of HepG2 cells. HepG2 cells were incubated with Adβgal with or without FX and with or without IgG (1,000 pp/cell), washed twice with PBS, and cultivated in DMEM–10% FCS. At 2 days postincubation, the cells were lysed and the β-galactosidase activity and protein concentration were determined. This experiment was performed 3 times with similar results. (C and D) The influence of FX on HAd5 or IC-HAd5 attachment to MoDCs was determined using AdLite-555 with or without FX (C) or Adlite-555 with or without FX plus IgG (D), and virus attachment was measured by flow cytometry. Experiments were carried out in 2 individual donors with similar results.
HAd5 transduction efficiency in the presence of hFX.
HepG2 cells were incubated with Adβgal with or without hFX with or without IgG (1,000 pp/cell) as previously described (19, 20). Briefly, 2.5 × 104 HepG2 cells were plated in 24-well dishes in DMEM–10% FCS the day prior to incubation. On the day of infection, cells were washed twice with phosphate-buffered saline (PBS) and 500 μl DMEM was added to each well. Vectors or vectors incubated sequentially with hFX and/or Kiovig were added and incubated for 3 h at 37°C. Medium was removed, and cells were washed once with PBS. Subsequently, medium was replaced with DMEM–10% FCS, and cells were maintained for 72 h at 37°C. At 3 days postinfection, the cells were lysed in lysis buffer (Promega, Charbonnieres, France) and β-galactosidase activity was quantified and normalized to protein content as measured by the bicinchoninic acid (BCA) method (Pierce, St Leon-Rot, Germany).
qRT-PCR.
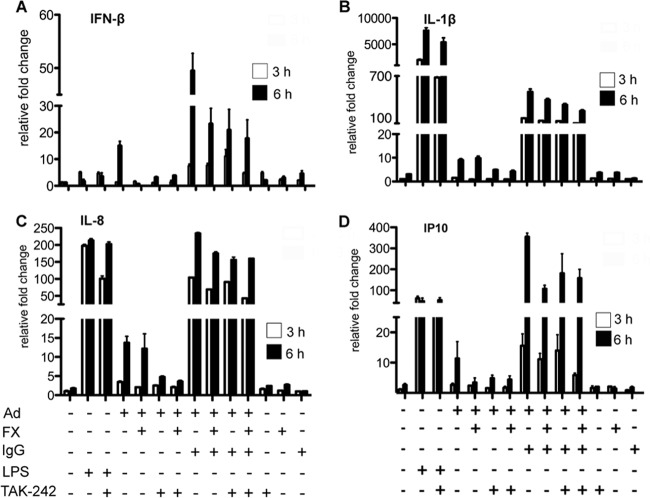
The levels of human IL-8, IL-1β, interferon-inducible protein (IP-10), and beta interferon (IFN-β) mRNAs were analyzed using quantitative reverse transcription-PCR (qRT-PCR). Total RNAs were isolated from THP-1-derived DCs using a High Pure RNA isolation kit (Roche, Meylan, France). Reverse transcription was performed with a Superscript III first-strand synthesis system (Invitrogen, Life Technologies, Villebon, France) using 300 ng of total RNA and random hexamers. The cDNA samples were diluted 1:10 in water and analyzed in triplicate using a LightCycler 480 detection system (Roche, Meylan, France). PCR conditions were 95°C for 5 min and 45 cycles of 95°C for 15 s, 65°C or 70°C for 15 s, and 72°C for 15 s, targeting the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene as a standard. Primer sequences were as follows for IL-1β (5′-AAACAGATGAAGTGCTCCTTCC-3′ [IL-1β forward] and 5′-AAGATGAAGGGAAAGAAGGTGC-3′ [IL-1β reverse]) at 65°C, IP10 (5′-TATTCCTGCAAGCCAATTTTGTC-3′ [IP-10 forward] and 5′-TCTTGATGGCCTTCGATTCTG-3′ [IP-10 reverse]) at 65°C, IL-8 (5′-GTTTTTGAAGAGGGCTGAGAATTC-3′ [IL-8 forward] and 5′-ATGAAGTGTTGAAGTAGATTTGCTTG-3′ [IL-8 reverse]) at 70°C, GAPDH (5′-ACAGTCCATGCCATCACTGCC-3′ [GAPDH forward] and 5′-GCCTGCTTCACCACCTTCTTG-3′ [GAPDH reverse]) at 70°C, and IFN-β (5′-GTCTCCTCCAAATTGCTCTC-3′ [IFN-β forward] and 5′-ACAGGAGCTTCTGACACTGA-3′ [IFN-β reverse]) at 65°C. Relative gene expression levels of each respective gene were generated using the threshold cycle (2-ΔΔCT) method as described previously (31), where the fold change in gene expression was normalized to the level for the GAPDH housekeeping gene in each cell type and gene expression in the THP-1-derived cells was set to 1.
Expression of costimulatory molecules by flow cytometry.
The cell surface levels of CD83 and CD86 were assessed by flow cytometry on DCs exposed to Adβgal or IC-Ads. Cells were collected 18 h postinfection and resuspended in PBS–10% FCS and subsequently stained with CD86-allophycocyanin (APC) or CD83-fluorescein isothiocyanate (FITC) (clone FUN-1 and clone HB15e, respectively; Becton-Dickinson, Le Pont-de-Claix, France) for 40 min at 4°C. Cells were washed twice, and propidium iodide (PI) (Sigma-Aldrich, Saint-Quentin Fallavier, France) or 7-amino-actinomycin (7-AAD) (Becton-Dickinson, Le Pont-de-Claix, France) was added to the cells prior to analyses. Data were analyzed by FlowJo software.
Capsid binding and TLR4 internalization.
MoDCs were incubated with Adβgal or AdLite-555 with or without hFX with or without Kiovig for 3 h. Cells were harvested, and the cells treated with Adβgal were stained for TLR4 expression with anti-human CD284 (TLR4)-Alexa 488 (clone HTA125) (eBioscience, Paris, France), while cells treated with AdLite-555 were washed and resuspended in PBS–10% FCS and kept on ice. After staining and washing were performed, all cells were incubated with 7-AAD to identify dead cells. TLR4 internalization was measured as a decrease of the mean fluorescent intensity (MFI) compared to the results seen with mock-treated cells. Virus attachment was measured as an increase in the MFI and compared to the results seen with mock-treated cells.
Quantification of cytokine secretion.
Tumor necrosis factor alpha (TNF-α) was quantified from supernatant by enzyme-linked immunosorbent assay (ELISA) (OptEIA; Becton-Dickinson, Le Pont-de-Claix, France).
Statistical analysis.
All experiments were performed a minimum of three independent times, and the results are expressed as mean values ± standard deviations (SD). Comparisons of groups for determinations of statistical differences were done using Student's t test. A P value of <0.05 is denoted as significant.
RESULTS
Several studies have shown that the use of hFX-armored HAd5 vectors results in increased gene transfer in vitro. We therefore preincubated Adβgal with recombinant hFX and added these complexes to HepG2 cells. Consistent with study results of others, hFX increased the transduction efficacy by approximately 7-fold (19) (Fig. 1B). These data demonstrated that the HAd5 vectors and recombinant hFX were biologically active and functioned as previously described.
Using in vitro cultures of primary mouse macrophages derived from wild-type mice, Doronin et al. compared HAd5 to mFX-HAd5 and found significantly greater production of proinflammatory cytokines and chemokines (IL-1β, IL-2, TNF-α, CXCL1, and CXCL9) (10). In vitro comparison of primary macrophages of wild-type and TLR4-deficient mice also showed a significant increase in addition to the aforementioned results from cytokines and chemokines. However, whether a xenogenic mix of HAd5 and hFX could stimulate secretion of proinflammatory cytokines and chemokines by mouse macrophages was not assayed. We therefore incubated hFX-HAd5 complexes, generated under conditions nearly identical to those described previously (10), with murine mononuclear phagocytes. Under these conditions, we found no differences in the levels of secretion of proinflammatory cytokines (data not shown). These data support the conclusion that it was the combination of mFX with HAd5 that induced a proinflammatory response in murine mononuclear phagocytes. These results prompted us to extend our finding in an exclusively human setting.
hFX-HAd5 did not increase binding to MoDCs.
A priori, one would not expect coagulation factors to bind cell surface PRRs under healthy conditions. During tissue damage, however, TLR4 may bind endogenous molecules such as high-mobility group box (HMGB) 1 protein or heat shock proteins (32–34). Using surface plasmon resonance, it was shown that mFX-HAd5 binds to murine TLR4, while hFX-HAd5 also bound to murine TLR4, but binding was less efficient (10). It is possible that the configuration of mFX bound to the HAd5 facilitates murine TLR4 binding. One would therefore expect that, compared to HAd5 alone, hFX-HAd5 binding to the cell would be higher on the surface of cells that express TLR4. To address this issue, we labeled a HAd5 vector with Alexa-555 (AdLite-555), preincubated it with hFX, and compared the levels of binding to MoDCs by flow cytometry. We found that preincubation of hFX with AdLite-555 did not significantly increase attachment to MoDCs (Fig. 1C). Because HAd5 vectors interact with the ubiquitous anti-HAd5 humoral response in human blood, we also incubated AdLite-555 with Kiovig and assayed MoDC binding. When Adβgal was preincubated with Kiovig, Adβgal transduction was completely abrogated (Fig. 1B). These data demonstrated that Kiovig contained neutralizing HAd5 antibodies. As expected, IC-HAd5 attachment was higher than that seen with HAd5 alone (Fig. 1C and D). These data were consistent with the interaction of the IgGs with FcRγ receptors expressed by MoDCs. However, when the IC-hFX-HAd5 complex was assayed, we did not find a further increase in MoDC binding (Fig. 1D).
hFX-HAd5 did not induce TLR4 internalization.
Although we could not detect a significant increase in hFX-induced HAd5 binding, this could have been due to transient hFX-TLR4 interactions and/or hFX-HAd5 release prior to TLR4 internalization. To address this possibility, we incubated MoDCs with HAd5 with or without hFX and assayed for the loss of TLR4 from the cell surface. Upon engagement with lipopolysaccharide (LPS), the quintessential TLR4 agonist, TLR4 is internalized to initiate signaling (35). When MoDCs were incubated with LPS, we found a clear reduction of TLR4 from the cell surface (Fig. 2A). In contrast, surface TLR4 levels on MoDCs were not significantly modified by either hFX-HAd5 or hFX-armored IC-HAd5 (Fig. 2B and C).
FIG 2.

TLR4 internalization. Internalization of TLR4 was measured by receptor removal from the cell surface. MoDCs were stimulated with LPS (A), HAd5 with or without FX (B), and HAd5 with or without FX plus IgG (C) or LPS as a positive control for 3 h and subsequently stained for TLR4. This experiment was performed using 3 individual donors with similar results. NT, nontreated; NS, nonstained; LPS, lipopolysaccharide; IC, immune-complexed adenovirus; Ad, adenovirus; FX, coagulation factor X.
FX-HAd5 did not induce proinflammatory cytokine transcription or secretion in human mononuclear phagocytes.
Collectively, the above data indicated that hFX binding to HAd5 poorly augmented cell surface attachment to MoDCs and did not stimulate TLR4 internalization. Additionally, the data suggested that hFX binding to HAd5 did not interfere with polyclonal anti-Ad IgG binding. In human blood, elevated levels of proinflammatory cytokines can be detected within hours ex vivo (36) and in vivo (37) after systemic administration. While the TLR4 internalization is thought to be a requirement for the induction of interferon-β (IFN-β) (35, 38) through a TRIF/TRAF3/IRF3 pathway, the possibility of TLR4 signaling occurring directly from the cell surface and induction of proinflammatory cytokines via MyD88-NF-κB cannot be formally excluded. Furthermore, the possibility could not be excluded that TLR4 activation could occur differently for a large agonist such as the 150-MDa HAd5 or that hFX-HAd5 interacts with an intracellular TLR4 (or other TLR) fraction and induces proinflammatory and/or IFN-1 responses.
To determine if hFX-HAd5 can induce proinflammatory cytokine secretion via TLR/MyD88/NF-κB from the cell surface, we asked if there were TNF-α production and/or secretion. In these assays, we also included THP-1 cells, primary human monocytes, and macrophages because they express higher levels of TLR4 and CD14, a partner in TLR4 signaling, than DCs. As demonstrated by many others, LPS induced a significant increase in TNF-α secretion in MoDCs, monocytes, and macrophages which was abrogated by the TLR4 inhibitor TAK-242 (Fig. 3). When the cells described above were treated with HAd5 vector alone, secreted TNF-α levels were comparable to those seen with the mock-treated cells, as previously found (3) (Fig. 3). When MoDCs, monocytes, and macrophages were preincubated with hFX-HAd5, we did not detect an increase in TNF-α secretion compared to HAd5-treated or mock-treated cells (Fig. 3). Accordingly, pretreating the hFX-HAd5-stimulated cells with TAK-242 did not change TNF-α levels.
FIG 3.
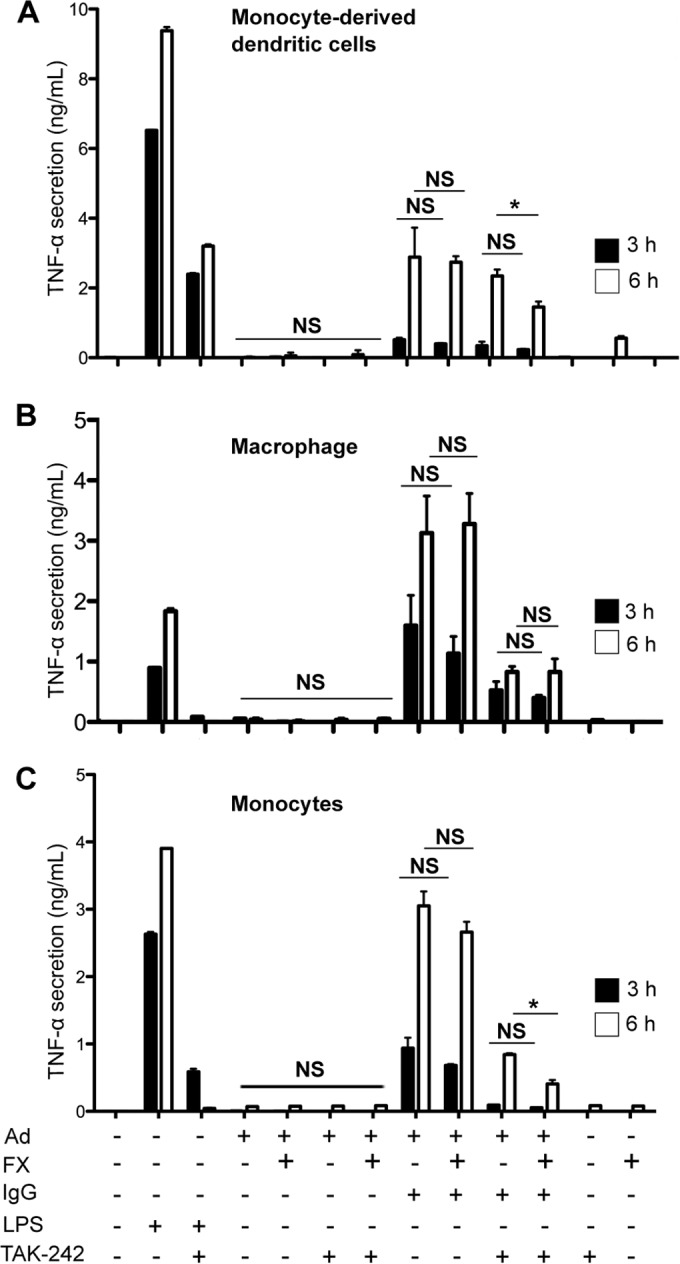
The proinflammatory response in human phagocytes incubated with FX-HAd5. TNF-α secretion of different phagocytes in the presence of a potential TLR4 agonist was analyzed. Cytokine secretion of MoDCs (A), macrophages (B), or monocytes (C) in the presence of 20,000 pp/cell HAd5, FX-HAd5, IC-HAd5, or IC-FX-HAd5 was assessed by ELISA at 3 and 6 h. LPS (10 ng/ml) was used as a positive control and was abrogated by the TLR4 inhibitor TAK-242. TAK-242 was also used to assess involvement of TLR4 in response to HAd5. Experiments were carried out in eight (A), five (B), and six (C) individual donors.
IC-HAd5 induces a significant proinflammatory response in DCs (3). Here we found that IC-HAd5 also induced proinflammatory cytokine secretion in human macrophages and monocytes (Fig. 3) and in THP-1 cells (data not shown). We therefore used hFX-armored IC-HAd5 prepared by preincubating HAd5 vector with hFX and then Kiovig. Under these conditions, we found that IC-FX-HAd5 did not significantly change the amount of TNF-α secreted by monocytes, macrophages, or MoDCs (Fig. 3) or by THP-1-derived DCs (data not shown). To test if hFX is partially involved in the proinflammatory response to IC-HAd5 through TLR4, we also measured TNF-α secretion in TAK-242-treated cells. IC-HAd5-stimulated and TAK-242-treated cells secreted less TNF-α than mock-treated cells. But again, the presence of hFX in the IC-HAd5 did not increase the amount of secreted TNF-α.
To test if an interaction between hFX, HAd5, and TLR affected transcript levels, we measured the levels of selected TRIF/TRAF3/IRF3- and MyD88-NF-κB-dependent proinflammatory cytokine mRNAs by qRT-PCR. Due to the innate differences in mRNA levels found routinely in blood bank donors, we used THP-1-derived DCs. To determine if hFX-HAd5 can induce a type 1 IFN response in human mononuclear phagocytes, we measured IFN-β mRNA changes. In these assays, we did not find an upregulation of IFN-β expression upon challenge with hFX-HAd5 (Fig. 4A). In addition, we found no significant modification in IL-1β, IL-8, or IP-10 transcription upon challenge with hFX-HAd5 (Fig. 4B to D). These data also suggested that hFX-HAd5 did not act as a primer in the two-hit model of inflammasome activation.
FIG 4.
Expression levels of proinflammatory cytokines in response to LPS or HAd5. mRNA expression levels of IFN-β (A), IL-1β (B), IL-8 (C), and IP-10 (D) of THP-1-derived DCs challenged with 10 ng/ml LPS or 20,000 pp/cell HAd5, FX-HAd5, IC-HAd5, or FX-IC-HAd5 were measured at 3 and 6 h. TAK-242 was used to inhibit TLR4 signaling where indicated.
Together, these data showed that the TLR4-induced MyD88-NF-κB and TRIF/TRAF3/IRF3 pathways were functional. hFX-HAd5 did not stimulate a proinflammatory cytokine or IFN-β response from the cell membrane or intracellular TLR4 and therefore did not contribute to the response induced by HAd5 or IC-HAd5 in human mononuclear phagocytes.
FX-HAd5 or hFX-IC-HAd5 did not induce greater costimulatory molecule expression on DC surface.
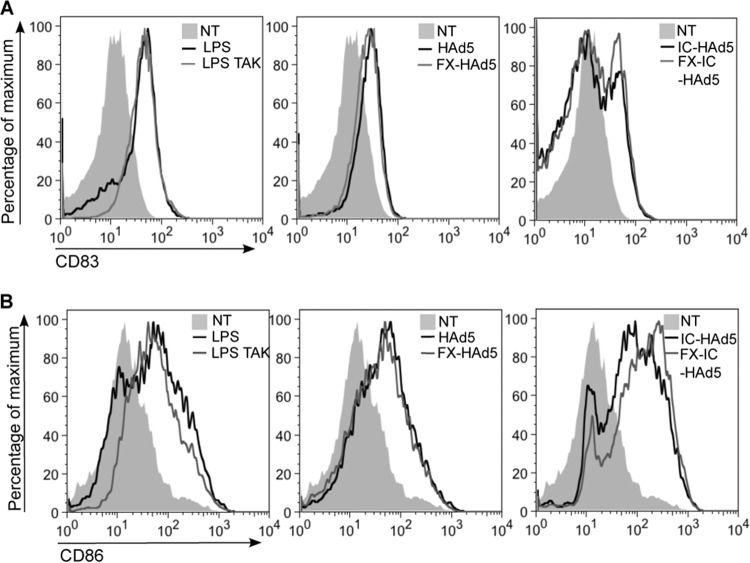
Given that we did not find an involvement of hFX or TLR4 in the inflammatory response to HAd5 or IC-HAd5, we asked if hFX binding to HAd5 had an effect on the phenotypic maturation of MoDCs. DCs are the major antigen-presenting cells and upon maturation upregulate costimulatory surface molecules. To test the effect of hFX binding to HAd5 on DC maturation, we measured by flow cytometry cell surface expression of the maturation marker CD83 and costimulatory molecule CD86 18 h poststimulation. The TLR4-agonist LPS stimulated an upregulation of CD83 and CD86, showing again the presence of the TLR4 pathway in MoDCs (Fig. 5). Incubation of MoDCs with HAd5 led to a modest upregulation of CD83 and CD86, but addition of hFX did not increase the expression levels of either marker. Incubation of DC IC-HAd5 with or without hFX induced a profound response compared to HAd5, but the addition of hFX did not lead to substantial differences in DC maturation as measured by CD83 and CD86 expression (Fig. 5).
FIG 5.
Influence of FX on DC maturation markers CD83 and CD86 in response to HAd5 or IC. MoDCs were stimulated with 20,000 pp/cell HAd5, FX-HAd5, IC-HAd5, FX-IC-HAd5, or LPS, and surface expression of CD83 (A) and CD86 (B) was assessed by flow cytometry 18 h postincubation. Experiments were performed with cells from five individual donors.
In summary, we concluded that human hFX, combined with human adenovirus type 5, does not induce a TLR4 pathway, or likely any other innate immune pathway, in human mononuclear phagocytes.
DISCUSSION
The host-pathogen arms race is characterized by molecular changes to pathogens and hosts. Clearly, the host has been best served by a defense that combats many pathogens simultaneously by using PRRs that recognize common pathogen features. The aim of this study was to investigate, using exclusively human components, the interaction of Ad, FX, TLR4, and mononuclear phagocytes. Better understanding human PRR-HAd interactions will enable us to develop responses to disseminated HAd disease and the use of Ad vectors for gene transfer in the clinic. The collection of primary cells (monocytes, macrophages, or DCs) used here was chosen because they are critical effectors and regulators of inflammation and the innate immune response and because of their characteristic patterns of cell surface TLR4 expression (39, 40). Of the cells used, monocytes produce the highest levels of TLR4 and CD14, which are needed to form a functional LPS receptor (40). We found that hFX-HAd5 did not induce TLR4 internalization, which is indicative of TLR4 engagement. Compared to HAd5, hFX-HAd5 caused no significant increase in the transcription or secretion of proinflammatory cytokine in human monocytes, macrophages, or DCs. Levels of costimulatory markers indicative of DC maturation were also not increased by hFX-HAd5. Additionally, hFX-armored IC-HAd5 caused no significant change in activation or maturation of the human mononuclear phagocytes. Collectively, these data demonstrated that when hFX is bound to HAd5, the complex does not stimulate a TLR/NF-κB-associated innate immune response in human mononuclear phagocytes. Our data complement and extend the in vitro and in vivo results of Doronin et al. (10) in mice.
Mice are the most commonly used animals for preclinical pharmacology and gene transfer studies and can be informative models for human biology. In spite of the practicality of mouse models, the consensus is that it is imperative to take into account their inherent strengths and weaknesses. The ancestors of humans and mice separated approximately 60 million years ago and since then have evolved with their specific pathogens. Species of Adenoviridae propagate in many mammals (including mice), fish, reptiles, birds, and amphibians (41). Differences that distinguish the human immune system from the mouse immune system include the antigen-presenting-cell physiology and phenotypes (42). Notable differences between the responses of mice and humans to HAds (as well as other pathogens) have been known for decades.
Although TLR4 basic biological function and signaling appear well conserved, its agonists vary due to the divergence of the ligand-binding domain. TLR4 forms a complex with MD-2 for efficient binding on the cell surface (43) and with CD14 for the internalization of TLR4 (35). MD-2 acts as a coreceptor for recognition of both exogenous ligands (43, 44) and endogenous ligands (45, 46). In addition, TLR4 can bind bacterial and viral PAMPs and, under inflammatory conditions, also endogenous damage-associated molecular pattern molecules (9). Importantly, the amino acid variability in the extracellular TLR4 ligand recognition domain changes the affinity for different agonists. The extracellular domains of mouse and human TLR4 show only 62% sequence similarity, while the hypervariable ligand binding regions have only 48% sequence similarity (9, 47). Moreover, species differences for MD-2, the coreceptor for TLR4, also affect the ligand recognition (48, 49) and the structural basis for these species differences has been resolved (50), which highlights the differences in PAMP recognition between mouse TLR4 and human TLR4 (51–54).
Added to this are the TLR4 cellular expression patterns and tissue distributions. Pertinent examples of this are plasmacytoid DCs (pDCs), which respond to viral infections with a strong type I IFN response. We did not include human pDCs in this study because, in contrast to murine pDCs, they do not express TLR4 (40, 55). Likewise, there are also differences—albeit small—in the sequence identity and similarity of mouse FX and human FX, which are 77% and 86%, respectively. Interestingly, species differences in the levels of FX-mediated transduction efficacy of HAd5 mediated via heparan sulfates have been also reported for mouse and human FX (56, 57). Furthermore, HAd5 and mouse adenovirus type 1 (MAd1) differ in their FX binding-dependent tropisms in the mouse model (58). While these differences do not exclude the possibility of TLR4-dependent recognition of mFX-complexed MAd1, they imply different steric orientations of mouse FX and human FX on different capsids.
In summary, we found that hFX-armored HAd5 did not induce a TLR/NF-κB-related innate immune response and did not synergize with the response induced by IC-HAd5 in human mononuclear phagocytes. There are other plasma components and proteins in the mucus matrix that can interact with the HAd5 capsid and also, to some extent, interact with FX. We did not include other host blood factors in this study. Among these, there are lactoferrin, α-defensin, complement factor C1 and C3, coagulation FVII, and protein C (61, 62). Combining these factors in an exclusively human assay will contribute to a better understanding of the host-pathogen interaction with HAds.
ACKNOWLEDGMENTS
This work was supported by the European Commission Initial Training Network (FP7; ADVance no. 290002), the Agence Nationale de la Recherche (ANR; project IC-DC), and IGMM.
We thank EKL members for constructive comments, Matt Cotton for AdLite, Teresa Fernandes-Alnemri for THP-1-ASC-GFP cells, Claire Daien for Kiovig, and Phuong Tran for help with the vector preparations.
REFERENCES
- 1.Soudais C, Skender N, Kremer EJ. 2004. Long-term in vivo transduction of neurons throughout the rat central nervous system using novel helper-dependent CAV-2 vectors. FASEB J 18:391–393. doi: 10.1096/fj.03-0438fje. [DOI] [PubMed] [Google Scholar]
- 2.Cubizolle A, Serratrice N, Skander N, Colle M-A, Ibanes S, Gennetier A, Bayo-Puxan N, Mazouni K, Mennechet F, Joussemet B, Cherel Y, Lajat Y, Vite C, Bernex F, Kalatzis V, Haskins ME, Kremer EJ. 2014. Corrective GUSB transfer to the canine mucopolysaccharidosis VII brain. Mol Ther 22:762–773. doi: 10.1038/mt.2013.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perreau M, Pantaleo G, Kremer EJ. 2008. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J Exp Med 205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlan A, Griffin TM, McGuire KA, Wiethoff CM. 2011. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol 85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlan A, Danthi P, Wiethoff CM. 2011. Lysosomal localization and mechanism of membrane penetration influence nonenveloped virus activation of the NLRP3 inflammasome. Virology 412:306–314. doi: 10.1016/j.virol.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulte M, Sorkin M, Al-Benna S, Stupka J, Hirsch T, Daigeler A, Kesting MR, Steinau H-U, Jacobsen F, Steinstraesser L. 2013. Innate immune response after adenoviral gene delivery into skin is mediated by AIM2, NALP3, DAI and MDA5. Springerplus 2:234–240. doi: 10.1186/2193-1801-2-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, Wakamatsu E, Benoist C, Koller D, Regev A. 2013. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci U S A 110:2946–2951. doi: 10.1073/pnas.1222738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mestas J, Hughes CCW. 2004. Of mice and not men: differences between mouse and human immunology. J Immunol 172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 9.Vaure C, Liu Y. 2014. A comparative review of Toll-like receptor 4 expression and functionality in different animal species. Front Immunol 5:316. doi: 10.3389/fimmu.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S, Miyake K, MacDonald JW, Bammler TK, Beyer RP, Farin FM, Stewart PL, Shayakhmetov DM. 2012. Coagulation factor X activates innate immunity to human species C adenovirus. Science 338:795–798. doi: 10.1126/science.1226625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morelli AE, Larregina AT, Raymond W, Zahorchak AF, Plowey JM, Logar AJ, Robbins PD, Falo LD, Thomson AW, Ganster RW. 2000. Recombinant adenovirus induces maturation of dendritic cells via an NF-κB-dependent pathway. J Virol 74:9617–9628. doi: 10.1128/JVI.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philpott NJ, Nociari M, Elkon KB, Falck-Pedersen E. 2004. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc Natl Acad Sci U S A 101:6200–6205. doi: 10.1073/pnas.0308368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seiradake E, Henaff D, Wodrich H, Billet O, Perreau M, Hippert C, Mennechet F, Schoehn G, Lortat-Jacob H, Dreja H, Ibanes S, Kalatzis V, Wang JP, Finberg RW, Cusack S, Kremer EJ. 2009. The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog 5:e1000277. doi: 10.1371/journal.ppat.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, Bazan-Peregrino M, Phipps S, Hale S, Mautner V, Seymour LW, Fisher KD. 2006. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol Ther 14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Alemany R, Suzuki K, Curiel DT. 2000. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol 81(Pt 11):2605–2609. [DOI] [PubMed] [Google Scholar]
- 16.Reid T, Warren R, Kirn D. 2002. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 9:979–986. doi: 10.1038/sj.cgt.7700539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, Randlev B, Heise C, Uprichard M, Hatfield M, Rome L, Rubin J, Kirn D. 2002. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl 1520): phase II viral, immunologic, and clinical endpoints. Cancer Res 62:6070–6079. [PubMed] [Google Scholar]
- 18.Hamid O, Varterasian ML, Wadler S, Hecht JR, Benson A, Galanis E, Uprichard M, Omer C, Bycott P, Hackman RC, Shields AF. 2003. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol 21:1498–1504. doi: 10.1200/JCO.2003.09.114. [DOI] [PubMed] [Google Scholar]
- 19.Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, Kemball-Cook G, Ni S, Lieber A, McVey JH, Nicklin SA, Baker AH. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- 20.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SMK, Greig JA, Denby L, Custers J, Morita T, Francischetti IMB, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJE, Nicklin SA, Baker AH. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Qiu Q, Tian J, Smith JS, Conenello GM, Morita T, Byrnes AP. 2013. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat Med 19:452–457. doi: 10.1038/nm.3107. [DOI] [PubMed] [Google Scholar]
- 22.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't Veer C, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. 2000. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells 1. J Immunol 164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. 2009. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol 182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. 2005. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol 83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 25.Dunzendorfer S, Lee H-K, Soldau K, Tobias PS. 2004. Toll-like receptor 4 functions intracellularly in human coronary artery endothelial cells: roles of LBP and sCD14 in mediating LPS responses. FASEB J 18:1117–1119. doi: 10.1096/fj.03-1263fje. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes-Alnemri T, Wu J, Yu J-W, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri E. 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berges C, Naujokat C, Tinapp S, Wieczorek H, Höh A, Sadeghi M, Opelz G, Daniel V. 2005. A cell line model for the differentiation of human dendritic cells. Biochem Biophys Res Commun 333:896–907. doi: 10.1016/j.bbrc.2005.05.171. [DOI] [PubMed] [Google Scholar]
- 28.Kremer EJ, Boutin S, Chillon M. 2000. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J Virol 74:505–512. doi: 10.1128/JVI.74.1.505-512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glotzer JB, Michou A, Baker A, Saltik M, Cotten M. 2001. Microtubule-Independent motility and nuclear targeting of adenoviruses with fluorescently labeled genomes. J Virol 75:2421–2434. doi: 10.1128/JVI.75.5.2421-2434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soudais C, Boutin S, Hong SS, Danos O, Bergelson JM, Kremer EJ, Hong S, Chillon M, Boulanger P. 2000. Canine adenovirus type 2 attachment and receptor, alternative receptors, and an RGD-independent pathway. J Virol 74:10639–10649. doi: 10.1128/JVI.74.22.10639-10649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi K, Burkart V, Flohe S, Kolb H. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol 164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 33.Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz BS, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TRDJ. 2006. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 176:7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim J-Y, Strassheim D, Sohn J-W, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. 2006. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 35.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. 2011. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147:868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danielsson A, Elgue G, Nilsson BM, Nilsson B, Lambris JD, Tötterman TH, Kochanek S, Kreppel F, Essand M. 2010. An ex vivo loop system models the toxicity and efficacy of PEGylated and unmodified adenovirus serotype 5 in whole human blood. Gene Ther 17:752–762. doi: 10.1038/gt.2010.18. [DOI] [PubMed] [Google Scholar]
- 37.Kirn D. 2001. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther 8:89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 38.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. 2008. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol 166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 40.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 41.Davison AJ, Benko M, Harrach B. 2003. Genetic content and evolution of adenoviruses. J Gen Virol 84:2895–2908. doi: 10.1099/vir.0.19497-0. [DOI] [PubMed] [Google Scholar]
- 42.Shortman K, Liu Y-J. 2002. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 43.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rallabhandi P, Phillips RL, Marina S. 2012. Respiratory syncytial virus fusion protein-induced Toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. mBio 3:e00218-12. doi: 10.1128/mBio.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deguchi A, Tomita T, Omori T, Komatsu A, Ohto U, Takahashi S, Tanimura N, Akashi-Takamura S, Miyake K, Maru Y. 2013. Serum amyloid A3 binds MD-2 to activate p38 and NF-κB pathways in a MyD88-dependent manner. J Immunol 191:1856–1864. doi: 10.4049/jimmunol.1201996. [DOI] [PubMed] [Google Scholar]
- 46.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. 2008. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol 10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 47.Smirnova I, Poltorak A, Chan EK, McBride C, Beutler B. 2000. Phylogenetic variation and polymorphism at the toll-like receptor 4 locus (TLR4). Genome Biol 1:research002.1–research002.10. doi: 10.1186/gb-2000-1-1-research002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasl J, Oblak A, Gioannini TL, Weiss JP, Jerala R. 2009. Novel roles of lysines 122, 125, and 58 in functional differences between human and murine MD-2. J Immunol 183:5138–5145. doi: 10.4049/jimmunol.0901544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. 2000. Mouse Toll-like receptor 4 MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem 275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 50.Ohto U, Fukase K, Miyake K, Shimizu T. 2012. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci U S A 109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steeghs L, Keestra A, van Mourik MA, Uronen-Hansson H, van der Ley P, Callard R, Klein N, van Putten JPM. 2008. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitidis. Infect Immun 76:3801–3807. doi: 10.1128/IAI.00005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroder K, Irvine KM, Taylor MS, Bokil NJ, Le Cao K-A, Masterman K-A, Labzin LI, Semple CA, Kapetanovic R, Fairbairn L, Akalin A, Faulkner GJ, Baillie JK, Gongora M, Daub CO, Kawaji H, McLachlan GJ, Goldman N, Grimmond SM, Carninci P, Suzuki H, Hayashizaki Y, Lenhard B, Hume DA, Sweet MJ. 2012. Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc Natl Acad Sci U S A 109:E944–E953. doi: 10.1073/pnas.1110156109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D, Inflammation and the Host Response to Injury Investigators . 2005. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol 3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 55.Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, Ruxrungtham K. 2008. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol 125:18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Zaiss AK, Lawrence R, Elashoff D, Esko JD, Herschman HR. 2011. Differential effects of murine and human factor X on adenovirus transduction via cell-surface heparan sulfate. J Biol Chem 286:24535–24543. doi: 10.1074/jbc.M111.241562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenaerts L, van Dam W, Persoons L, Naesens L. 2012. Interaction between mouse adenovirus type 1 and cell surface heparan sulfate proteoglycans. PLoS One 7:e31454. doi: 10.1371/journal.pone.0031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenaerts L, Mcvey JH, Baker AH, Denby L, Nicklin S, Verbeken E, Naesens L. 2009. Mouse adenovirus type 1 and human adenovirus type 5 differ in endothelial cell tropism and liver targeting. J Gene Med 11:119–127. doi: 10.1002/jgm.1283. [DOI] [PubMed] [Google Scholar]
- 59.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation and Host Response to Injury, Large Scale Collaborative Research Program . 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takao K, Miyakawa T. 3August2014. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tserel L, Runnel T, Kisand K, Pihlap M, Bakhoff L, Kolde R, Peterson H, Vilo J, Peterson P, Rebane A. 2011. MicroRNA expression profiles of human blood monocyte-derived dendritic cells and macrophages reveal miR-511 as putative positive regulator of Toll-like receptor 4. J Biol Chem 286:26487–26495. doi: 10.1074/jbc.M110.213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perreau M, Guérin M-C, Drouet C, Kremer EJ. 2007. Interactions between human plasma components and a xenogenic adenovirus vector: reduced immunogenicity during gene transfer. Mol Ther 15:1998–2007. doi: 10.1038/sj.mt.6300289. [DOI] [PubMed] [Google Scholar]
- 63.Perreau M, Kremer EJ. 2005. Frequency, proliferation, and activation of human memory T cells induced by a nonhuman adenovirus. J Virol 79:14595–14605. doi: 10.1128/JVI.79.23.14595-14605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]