ABSTRACT
Specific types of human papillomavirus (HPV) are strongly associated with the development of cervical carcinoma. The HPV E6 oncoprotein from HPV degrades p53 and abrogates cell cycle checkpoints. Nonetheless, functional p53 has been observed in cervical cancer. We have previously identified a p53-independent function of E6 in attenuating the postmitotic G1-like checkpoint that can lead to polyploidy, an early event during cervical carcinogenesis that predisposes cells to aneuploidy. How E6 promotes cell cycle progression in the presence of p53 and its target, p21, remains a mystery. In this study, we examined the expression of cell cycle-related genes in cells expressing wild-type E6 and the mutant that is defective in p53 degradation but competent in abrogating the postmitotic checkpoint. Our results demonstrated an increase in the steady-state levels of G1- and G2-related cyclins/Cdks in E6-expressing keratinocytes. Interestingly, only Cdk1 remained active in E6 mutant-expressing cells while bypassing the postmitotic checkpoint. Furthermore, the downregulation of Cdk1 impaired the ability of both wild-type and mutant E6 to induce polyploidy. Our study thus demonstrated an important role for Cdk1, which binds p21 with lower affinity than Cdk2, in abrogating the postmitotic checkpoint in E6-expressing cells. We further show that E2F1 is important for E6 to upregulate Cdk1. Moreover, reduced nuclear p21 localization was observed in the E6 mutant-expressing cells. These findings shed light on the mechanisms by which HPV induces genomic instability and hold promise for the identification of drug targets.
IMPORTANCE HPV infection is strongly associated with the development of cervical carcinoma. HPV encodes an E6 oncoprotein that degrades the tumor suppressor p53 and abrogates cell cycle checkpoints. Nonetheless, functional p53 has been observed in cervical cancer. We have recently demonstrated a p53-independent abrogation of the postmitotic checkpoint by HPV E6 that induces polyploidy. However, the mechanism is not known. In this study, we provide evidence that Cdk1 plays an important role in this process. Previously, Cdk2 was thought to be essential for the G1/S transition, while Cdk1 only compensated its function in the absence of Cdk2. Our studies have demonstrated a novel role of Cdk1 at the postmitotic G1-like checkpoint in the presence of Cdk2. These findings shed light on the mechanisms by which HPV induces genomic instability and hold promise for the identification of drug targets.
INTRODUCTION
Genomic instability in the form of polyploidy, the state in which cells have more than two sets of chromosomes, has been suggested to play a causal role in tumorigenesis (1). Polyploidy can lead to numerical and structural chromosome abnormalities by increasing the rate of DNA breakage and damage (2). Tetrasomy in basal keratinocytes has been found in low-grade, squamous intraepithelial lesions of the cervix infected with high-risk but not low-risk human papillomavirus (HPV) types (3). Significantly, tetraploidy occurred as an early event during cervical carcinogenesis and predisposed cells to aneuploidy (4). Polyploidy can be induced by the abrogation of cell cycle checkpoints (5).
Cell proliferation is regulated at several checkpoints, whose defects contribute to polyploidy and genomic instability (6). The checkpoints in eukaryotic cells include the G1, G2/M, spindle, and postmitotic G1 checkpoints (5). The G1 checkpoint is mainly regulated through phosphorylation of the retinoblastoma protein (pRb) by cyclin D/Cdk4-Cdk6 in early G1, followed by the phosphorylation of cyclin E1-cyclin A/Cdk2 complexes (7). Cdk1 (Cdc2) can substitute for G1 Cdks in their absence and functions in the G1/S-phase transition (8, 9). Hyperphosphorylation of pRb results in its dissociation from members of the E2F family of transcription factors. Free E2F mediates the transcription of genes required for DNA synthesis and promotes the S-phase transition (10). Upon exposure to genotoxic agents, p53 is activated and turns on transcription of the Cdk inhibitor p21, which binds to and inactivates the cyclin E1/Cdk2 and cyclin A2/Cdk2 complexes, resulting in pRb hypophosphorylation and cell cycle arrest at the G1-S transition (11, 12).
After cells with intact spindle checkpoint activity arrest in metaphase for prolonged periods of time, they eventually adapt to the checkpoint and progress into a G1-like state with tetraploid genomes (5). The replication of DNA in these cells is usually blocked by p53- and pRb-dependent cell cycle arrest, which is referred to as the postmitotic checkpoint (5). It appears that the structural integrity and dynamics of microtubules, rather than tetraploidy per se, are key to induce cell cycle arrest at this checkpoint (see reference 13 and references therein). The postmitotic checkpoint shares many features with the G1 checkpoint: cell cycle arrest coincides with high concentrations of p21 and hypophosphorylated pRb (14, 15). p53 appears to play a key role in mediating the postmitotic checkpoint (5, 16, 17), and p21 is responsible for at least part of this p53-mediated, postmitotic arrest response (5, 18, 19).
Infection by high-risk HPV types (such as HPV-16) is strongly associated with the development of cervical carcinoma (20). The oncogenic properties of high-risk HPVs primarily reside in the E6 and E7 genes. The ability of high-risk HPV E6 to promote the degradation of tumor suppressor p53 and thereby reduce the level of the p53 target p21 has been suggested as a mechanism by which HPV induces cellular transformation (reviewed in reference 21). However, p21 is expressed in a subset of HPV-positive cervical intraepithelial neoplasia (CIN) and cervical tumor tissues (22, 23). We have previously identified a p53-independent function of E6 in attenuating the postmitotic checkpoint (24). This observation is biologically relevant. In addition to the environmental factors, E6-expressing cells contain a high percentage of metaphase-lagging (misaligned) chromosomes that could potentially trigger the spindle checkpoint (25, 26). However, how E6 promotes cell cycle progression in the presence of p53 remains a mystery. The key question is how Cdks remain active in the presence of elevated p21. In this study, we explored the mechanism by which HPV E6 abrogates the postmitotic checkpoint. Our results demonstrate an important role for Cdk1 in abrogating the postmitotic checkpoint in E6-expressing cells.
MATERIALS AND METHODS
Cell culture.
Primary human keratinocytes (PHKs) were derived from neonatal human foreskin epithelium obtained from the University of Massachusetts Hospital, as described previously (24). Spontaneously immortalized human keratinocyte NIKS cells were described previously (24). Both PHKs and NIKS were maintained on mitomycin C-treated J2-3T3 feeder cells in F medium as described previously (27). Human retinal pigment epithelial cells (RPE1 cells) (28) were maintained in a 1:1 blend of Dulbecco's modified Eagle's medium and Ham's nutrient mixture F-12 medium plus 10% fetal bovine serum. All cells were cultured in medium with the addition of penicillin and streptomycin at 37°C with 5% CO2.
PHK, NIKS, and RPE1 cells expressing HPV-16 E6, HPV-16 E6 mutant F2V (bearing a mutation of Phe-2 to Val), or vector were established by retrovirus-mediated infection using the pBabe-puro-based retroviral vector as described previously (24). The cells were maintained in puromycin and used within 8 passages.
RNA extraction and reverse transcription (RT)-PCR.
Total cellular RNA was extracted from cells using the RNeasy minikit (Qiagen, Alameda, CA) and then reverse transcribed to cDNA as described in the protocol of the Improm-II reverse transcriptase system kit (Promega, Madison, WI). The PCR primers were as follows: HPV-16 E6 forward, 5′-CTGCAATGTTTCAGGACCCA-3′; HPV-16 E6 reverse, 5′-CCTAATTAACAAATC-3′; β-actin forward, 5′-TGGCATTGCCGACAGGATGCAGAA-3′; and β-actin reverse, 5′-CTCGTCATACTCCTGCTTGCTGAT-3′. β-Actin was used as a control for RNA loading and reverse transcription efficiency.
Western blotting.
Total cellular proteins were extracted with radioimmunoprecipitation assay (RIPA) lysis buffer, and a Western blot assay was performed with specific antibodies against Cdk1 (610038; BD Biosciences), Cdk2 (sc-6248; Santa Cruz), Cdk4 (sc-260; Santa Cruz), cyclin A2 (sc-751; Santa Cruz), cyclin B1 (sc-752; Santa Cruz), cyclin D1 (sc-718; Santa Cruz), cyclin E1 (sc-198; Santa Cruz), E2F1 (sc-193; Santa Cruz), p21 (610233; BD Biosciences), p53 (sc-126; Santa Cruz), Sp1 (9389S; CST), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-25778; Santa Cruz), or β-tubulin (T4026; Sigma).
Kinase assay.
A kinase assay was performed as described previously (29). Briefly, total cellular proteins were lysed in lysis buffer for 1 h. One hundred micrograms of protein extract was immunoprecipitated with appropriate antibodies and protein A/G plus-agarose (Santa Cruz) and incubated for 4 h at 4°C. The beads were first washed three times with lysis buffer without glycerol and then twice with kinase buffer (50 mM HEPES, [pH 7.5], 10 mM MgCl2,1 mM dithiothreitol, 2.5 mM EGTA, 0.1 mM sodium orthovanadate, 1 mM sodium fluoride, and protease inhibitor). Kinase assays were performed in the presence of 10 μCi of [γ-32P]ATP (3,000 Ci/mmol; PerkinElmer Life Sciences), 20 μM ATP, and 1 μg of full-length pRb (QED Bioscience, Inc.) as the substrate for 30 min at 30°C. Following the kinase reaction, samples were boiled in loading buffer and separated by 8% SDS-PAGE. Phosphorylated proteins were visualized by autoradiography. In parallel, Western blot assays for Cdk1 were performed to assess the amounts of Cdk1 that were precipitated by immunoprecipitation.
Flow cytometry.
Asynchronous cultures of cells were treated with dimethyl sulfoxide (DMSO) (Sigma) or 50 ng/ml nocodazole (Sigma) for 48 h. The cells were harvested, fixed in 70% ethanol overnight, and resuspended in a phosphate-buffered saline–propidium iodide (PI) (50 μg/ml; Sigma)–RNase A (70 μg/ml; Sigma) solution. The PI-stained cells were analyzed by flow cytometry. Cell cycle analysis was performed using FlowJo software (Becton Dickinson).
siRNA.
Chemically modified Stealth small interfering RNA (siRNA) targeting Cdk1 or Cdk2 and control siRNA were purchased from Invitrogen. The sequences of the siRNAs were as follows: Cdk1-sp2 siRNA, 5′-GATCAACTCTTCAGGATTT-3′, described in reference 30; Cdk1-beck siRNA, 5′-GATGTAGCTTTCTGACAAAAA-3′, described in reference 31; Cdk2-tetsu siRNA, 5′-GCCAGAAACAAGTTGACGG-3′, described in reference 30; Cdk2-beck siRNA, 5′-GTTTCAGTATTAGATGCAC-3′, described in reference 31; and E2F1 siRNA, 5′-GUCACGCUAUGAGACCUCA-3′.
Cells (1.2 × 105) were seeded onto a 60-mm dish the day before transfection and transfected with 20 nM siRNA per target gene using Lipofectamine RNAiMAX transfection reagent according to the manufacturer's instructions (Invitrogen). Thirty-six hours after transfection, the cells were treated with DMSO or 50 ng/ml nocodazole and incubated for an additional 48 h. Cells were harvested for protein knockdown analysis by Western blotting or for cell cycle analysis by flow cytometry.
Cell fractionation.
The preparation of cytoplasmic and nuclear extracts was performed using a nuclear extract kit (Active Motif) according to the manufacturer's protocol, with minor modifications. Briefly, cells were first collected by centrifugation at 1,000 × g for 5 min, and the pellets were then lysed in 500 μl hypotonic buffer. After incubation for 15 min on ice, 25 μl of detergent was added and the lysate was vortexed for 10 s, followed by centrifugation at 14,000 × g for 30 s at 4°C. Supernatants were harvested as cytoplasmic fractions, while the pellets were resuspended in 50 μl complete lysis buffer and centrifuged at 14,000 × g for 10 min at 4°C. The supernatants were saved as the nuclear fractions.
Statistical analysis.
Data are presented as the means ± standard deviations. Data sets were graphed and analyzed using the two-tailed Student's t test. A P value of ≤0.05 was considered statistically significant.
RESULTS
Expression of postmitotic checkpoint-related proteins in HPV E6-expressing cells.
We have previously identified several HPV-16 E6 mutants that are defective for p53 degradation and yet competent for attenuating the postmitotic checkpoint (24). The ability of E6 mutants to abrogate the postmitotic checkpoint was demonstrated in PHKs. To alleviate the concern that vector-control PHKs do not proliferate efficiently and therefore are not comparable to PHKs expressing E6, we also used immortalized keratinocytes (NIKS cells) to demonstrate the ability of the E6 mutant to bypass the postmitotic checkpoint (24). NIKS cells contain a wild-type p53 sequence and exhibit many characteristics of early-passage keratinocytes, including the ability to stratify, differentiate, and sustain the HPV life cycle (32). As a first step toward understanding the mechanism by which E6 abrogates the postmitotic checkpoint without degrading p53, we examined the expression of genes known to be involved in this checkpoint in NIKS cells expressing the p53 degradation-defective E6 mutant F2V (bearing a mutation of Phe-2 to Val), wild-type E6, and the retroviral vector.
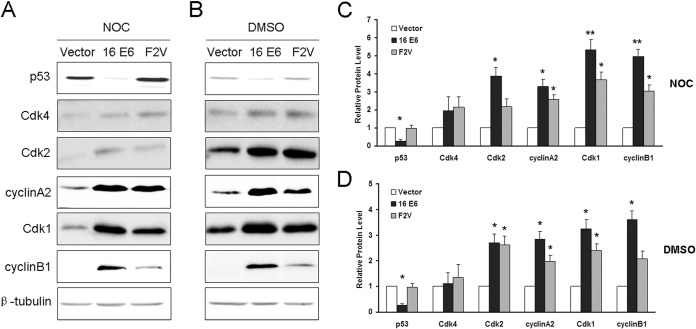
As shown by the results in Fig. 1A, the steady-state level of p53 in cells expressing wild-type E6 was lower, while its steady-state level in cells expressing F2V was similar to that in the vector control cells. These results indicate that p53 is degraded in cells expressing wild-type E6, while the E6 mutant F2V is defective for p53 degradation. Both early-G1 Cdk (Cdk4) and late-G1 Cdk (Cdk2), as well as cyclin A2, were expressed at modestly increased levels (1.5-fold or more) in NIKS cells expressing wild-type E6 and mutant F2V that were treated with nocodazole for 48 h compared with their levels in the vector control cells. In our previous study, by employing combined approaches of flow cytometry, cell cycle markers, and mitotic shake-off, we demonstrated that under this condition, both vector control and E6-expressing cells exited from mitosis. While the vector control cells arrested at a G1-like stage with 4C DNA content, E6 and E6 mutant F2V-expressing cells entered into S phase, replicated their DNA, and became polyploid (24). While the steady-state levels of p53 and its target p21 were low in the wild-type E6-expressing cells, they were maintained in F2V-expressing cells (24). Notably, the results presented in Fig. 1A showed that the steady-state level of mitotic Cdk1 was significantly increased in wild-type E6- and mutant F2V-expressing NIKS cells (5- and 4-fold, respectively). The level of the Cdk1 partner cyclin B1 was also significantly increased (∼3-fold) in F2V-expressing cells, and the increase was even greater (>5-fold) in NIKS cells expressing wild-type E6. The changes in the steady-state levels of cell cycle-related proteins were not simply a result of nocodazole treatment, as the steady-state levels of Cdk4, Cdk2, Cdk1, cyclin A2, and cyclin B1 were also increased in vehicle DMSO-treated, wild-type E6-, and F2V-expressing cells (Fig. 1B). Notably, the overexpression of Cdk1 and Cdk2 was consistent with what was observed in cervical intraepithelial neoplasia, cervical carcinoma, and HPV-positive or HPV E6/E7-expressing cells (33, 34).
FIG 1.
Expression of postmitotic checkpoint-related proteins in cells expressing HPV E6 and E6 mutant F2V. Total protein extracts from NIKS cells expressing vector, HPV-16 E6, or F2V that were treated with nocodazole (50 ng/ml for 48 h) (A) or DMSO (B) were resolved by SDS-PAGE and blotted with antibodies against p53, Cdk1, Cdk2, Cdk4, cyclin A2, and cyclin B1. β-Tubulin was used as the loading control. Data from a representative experiment of at least three independent samples are shown. Not all experiments were performed at the same time. (C) Quantification of the protein expression levels from the experiment whose results are shown in panel A. (D) Quantification of the protein expression levels from the experiment whose results are shown in panel B. Error bars reflect the standard deviations of the means. NOC, nocodazole. *, P < 0.05; **, P < 0.01.
In contrast, the levels of cyclin E1, Cdc25A, c-Myc, p14, p16, and p27 did not increase in wild-type E6- or mutant F2V-expressing NIKS cells (not shown). Interestingly, the cyclin D1 levels increased in F2V-expressing cells but not in wild-type E6-expressing cells (not shown). Our results indicate that, in HPV E6- and E6 mutant F2V-expressing cells, both G1 and mitotic Cdks, along with cyclin A2 and cyclin B1, are upregulated. However, because cyclin A2 and cyclin B1 are able to bind both Cdk1 and Cdk2 and the cyclins/Cdks have the potential to form complexes with each other (8, 35, 36), these data could not distinguish which Cdk is functionally active when E6-expressing cells bypass the postmitotic checkpoint.
Cdk1 remains active while E6 mutant-expressing cells bypass the postmitotic checkpoint.
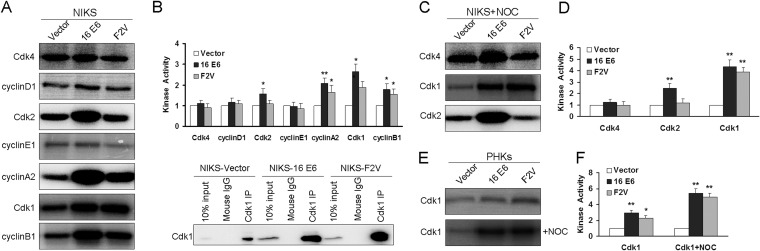
To identify Cdks that are responsible for promoting E6-expressing cells to abrogate the postmitotic checkpoint, we performed in vitro kinase assays to measure the activities of relevant Cdks and cyclins using pRb as the substrate. Notably, the activities of early G1 Cdk4 and cyclin D1 in E6- and E6 mutant F2V-expressing cells were similar to their activities in the vector control cells (Fig. 2A), suggesting that they are not important for these cells to proliferate. Surprisingly, the activity of the late G1 Cdk2 partner cyclin E1 in E6 mutant F2V-expressing cells was also similar to its activity in the vector control cells, although the Cdk2 activity was higher (Fig. 2A and B). These results suggest that the G1 cyclins and Cdks are not essential for E6 mutant F2V-expressing cells to proliferate.
FIG 2.
Kinase activities of postmitotic checkpoint-related proteins. Total protein extracts of NIKS cells or PHKs expressing HPV-16 E6, mutant F2V, or vector were immunoprecipitated with anti-Cdk1, anti-Cdk2, anti-Cdk4, anti-cyclin A2, anti-cyclin B1, anti-cyclin D1, and anti-cyclin E1 antibodies. In vitro kinase assays were performed with the full-length pRb as a substrate. Autoradiograms showing levels of phosphorylated pRb from experiments that are representative of at least three experiments are provided. (A) Kinase activities of cyclins/Cdks in asynchronous NIKS cells. (B) Top, quantification of the kinase activities from the experiment whose results are shown in panel A; bottom, Western blot assay of the immunoprecipitates showing the levels of Cdk1. (C) Kinase activities of Cdk1, Cdk2, and Cdk4 in NIKS cells treated with nocodazole for 48 h. (D) Quantification of kinase activities from the experiment whose results are shown in panel C. (E) Kinase activities of Cdk1 in PHKs with or without nocodazole treatment. (F) Quantification of kinase activities from the experiment whose results are shown in panel E. Error bars reflect the standard deviations of the means. NOC, nocodazole. *, P < 0.05; **, P < 0.01.
Interestingly, and in contrast to the results for the G1 cyclins and Cdks, the mitotic Cdk (Cdk1) was more active in both F2V- and wild-type E6-expressing cells than in vector control cells (Fig. 2A and B). Consistent with its steady-state level, the kinase activity of the Cdk1 partner cyclin B1 in F2V-expressing cells was higher than in the vector control cells but lower than in the wild-type E6-expressing cells (Fig. 2A and B). Cyclin A2, which normally partners with both Cdk1 and Cdk2, also remained more active in wild-type and F2V-expressing cells than in vector control cells (Fig. 2A and B). These results point to a critical role for Cdk1 in E6-expressing cells for them to proliferate. Notably, as the amounts of Cdk1 immunoprecipitates from E6- and F2V-expressing cells were greater than the amount from vector control cells (Fig. 2B, bottom), the increased kinase activity in E6 and F2V cells may simply be a result of more Cdk1 protein rather than elevated enzymatic activity.
We then examined the kinase activities of selected Cdks when cells were at the postmitotic checkpoint transition. Notably, the activity of Cdk1 but not that of Cdk2 was much higher in F2V- as well as wild-type E6-expressing cells than in the vector control cells 48 h after cells were treated with nocodazole, which is a condition where these cells enter S phase with a tetraploid genome (Fig. 2C and D) (24). There were no significant differences in Cdk4 activities among the cells expressing E6, F2V, or vector. We then confirmed this observation in PHKs. Importantly, the activities of Cdk1 were also higher in PHKs expressing F2V and in cells expressing wild-type E6 than in vector control cells after nocodazole treatment (Fig. 2E and F). These results suggest that Cdk1 plays an important role in abrogating the postmitotic checkpoint in HPV E6-expressing cells in the presence of p53.
HPV-16 E6 is capable of inducing polyploidy in the presence of p53 and p21 in hTERT-immortalized epithelial cells.
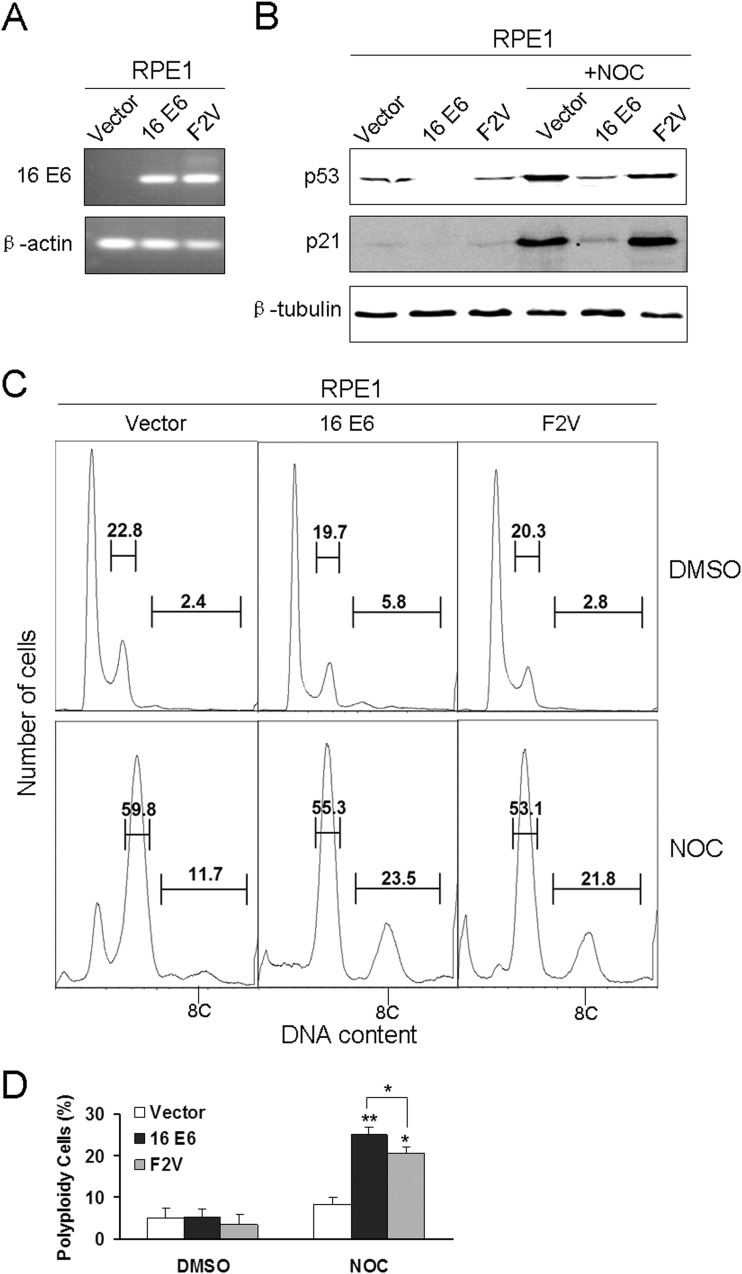
As the transfection efficiencies in PHKs and NIKS cells are not satisfactory, we employed RPE1 cells (28). RPE1 cells expressing the wild-type E6 (RPE1-E6) and vector control (RPE1-vector) were described previously, and we have demonstrated that the wild-type HPV-16 E6 abrogates the postmitotic checkpoint in RPE1 cells (24). Accordingly, we established E6 mutant F2V-expressing RPE1 cells (RPE1-F2V) by retrovirally mediated infection. The expression of E6 was determined by RT-PCR (Fig. 3A). To check the status of p53 in F2V-expressing RPE1 cells, we examined the steady-state levels of p53 and its transcriptional target p21 by Western blotting. As shown in Fig. 3B, p53 was detectable in RPE1-F2V and RPE1-vector cells but not in RPE1-E6 cells. Moreover, the steady-state levels of p21 were comparable in RPE1-F2V and RPE1-vector cells but undetectable in RPE1-E6 cells. Upon nocodazole treatment, which activates p53 and triggers the postmitotic checkpoint (5), the steady-state levels of both p53 and p21 went up in RPE1-F2V and RPE1-vector cells. Although p53 was also upregulated in RPE1-E6 cells, its level was much lower than the levels in RPE1-F2V and RPE1-vector cells. These results indicate that p53 functions in RPE1-F2V cells.
FIG 3.
Expressions and activities of HPV-16 E6 and F2V in RPE1 cells. (A) Total RNA isolated from RPE1 cells expressing HPV-16 E6, F2V, or vector was subjected to RT-PCR using HPV-16 E6 primers. β-Actin was used as a control. (B) Total protein extracts from RPE1 cells expressing HPV-16 E6, F2V, or vector treated with or without nocodazole were collected, resolved by SDS-PAGE, and blotted with antibodies against p53, p21, and β-tubulin. (C) RPE1-E6, F2V, and vector control cells were treated with DMSO or nocodazole, and the DNA content was detected by flow cytometry. Polyploid cells are indicated as 8C. Results representative of three experiments are shown. (D) Percentages of polyploid cells from three experiments are summarized in a histogram format. Error bars reflect the standard deviations of the means. NOC, nocodazole. *, P < 0.05; **, P < 0.01.
The integrity of the postmitotic checkpoint in RPE1-F2V cells was then studied by examining polyploidy formation. Upon nocodazole treatment, significantly more polyploidy was formed in RPE1-F2V and RPE1-E6 cells than in vector control cells (Fig. 3C), indicating that, similar to what was observed in PHKs and NIKS cells, F2V can abrogate the postmitotic checkpoint and induce polyploidy in RPE1 cells. However, consistent with what was observed in PHKs and NIKS cells (24), the level of F2V-induced polyploidy was lower than the level in wild-type E6-expressing RPE1 cells (P < 0.05) (Fig. 3D), indicating that although p53 degradation by E6 is not required, it does play a role in E6-mediated abrogation of the postmitotic checkpoint.
Cdk1 is important for E6 to induce polyploidy.
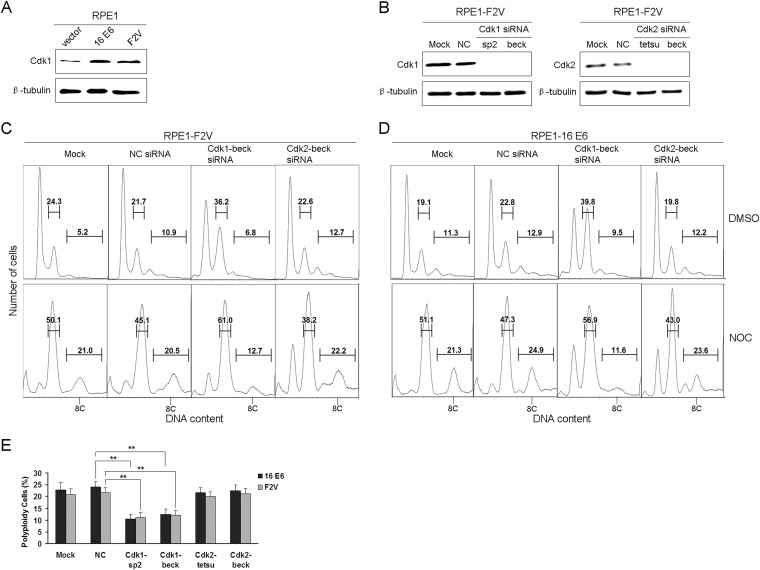
Next, we examined the expression of Cdk1 in RPE1 cells expressing E6 and F2V. As shown by the results in Fig. 4A, consistent with what was observed in keratinocytes, the steady-state level of Cdk1 was increased in both E6- and F2V-expressing RPE1 cells (2.8- and 2.3-fold, respectively). We then examined the role of Cdk1 in polyploidy formation in RPE1-F2V cells. For this, we used the siRNA strategy to knock down Cdk1. Cdk2 siRNAs were used as a control. Two siRNAs for each Cdk were employed. These siRNAs were previously demonstrated to specifically downregulate Cdk1 and Cdk2, respectively (30, 31). Consistent with these results, both siRNAs efficiently knocked down the steady-state levels of their target proteins (Fig. 4B). Next, we examined the effect of Cdk1 knockdown on polyploidy formation in F2V-expressing RPE1 cells. Significantly, downregulation of Cdk1 but not of Cdk2 by siRNAs reduced polyploidy formation in F2V-expressing cells treated with nocodazole. The results for one of the siRNAs (beck) are shown in Fig. 4C, and those for both siRNAs are summarized in Fig. 4E. Notably, downregulation of Cdk1 but not of Cdk2 also reduced polyploidy formation in RPE1-E6 cells (Fig. 4D and E), suggesting that Cdk1 is also important for wild-type E6-expressing cells to bypass the postmitotic checkpoint. These results suggest an important role for Cdk1 in polyploidy formation and postmitotic checkpoint abrogation in E6-expressing cells. As p21 binds Cdk1 with lower affinity than Cdk2 (37), our observation that Cdk1 instead of Cdk2 plays an important role in polyploidy formation in E6-expressing cells implicates a mechanism by which E6 abrogates the postmitotic checkpoint in the presence of p21.
FIG 4.
Downregulation of Cdk1 impairs the ability of E6 mutant F2V to induce polyploidy. (A) Total protein extracts from RPE1-vector, RPE1-E6, and RPE1-F2V cells were analyzed by Western blotting for Cdk1. (B) siRNAs efficiently knock down Cdk1 and Cdk2. RPE1-F2V cells were transfected with two siRNAs specific to Cdk1 and Cdk2, respectively, for 36 h, and the levels of Cdk1 and Cdk2 were measured by Western blotting. β-Tubulin was used as a loading control in the experiments whose results are shown in panels A and B. Data from one representative experiment of three are shown. (C, D) Downregulation of Cdk1 impairs the ability of F2V and E6 to induce polyploidy. RPE1-F2V (C) and RPE1-E6 cells (D) were transfected with siRNAs targeting Cdk1 (Cdk1-beck siRNA) or Cdk2 (Cdk2-beck siRNA), and 36 h later, the cells were treated with nocodazole at 50 ng/ml for an additional 48 h and analyzed by flow cytometry. Polyploid cells are indicated as 8C. Results representative of three experiments are shown. (E) Quantification of the percentages of polyploid cells from the experiments whose results are shown in C and D, as well as results from experiments using Cdk1-sp2 siRNA and Cdk2-tetsu siRNA. Error bars reflect the standard deviations of the means. NOC, nocodazole. **, P < 0.01.
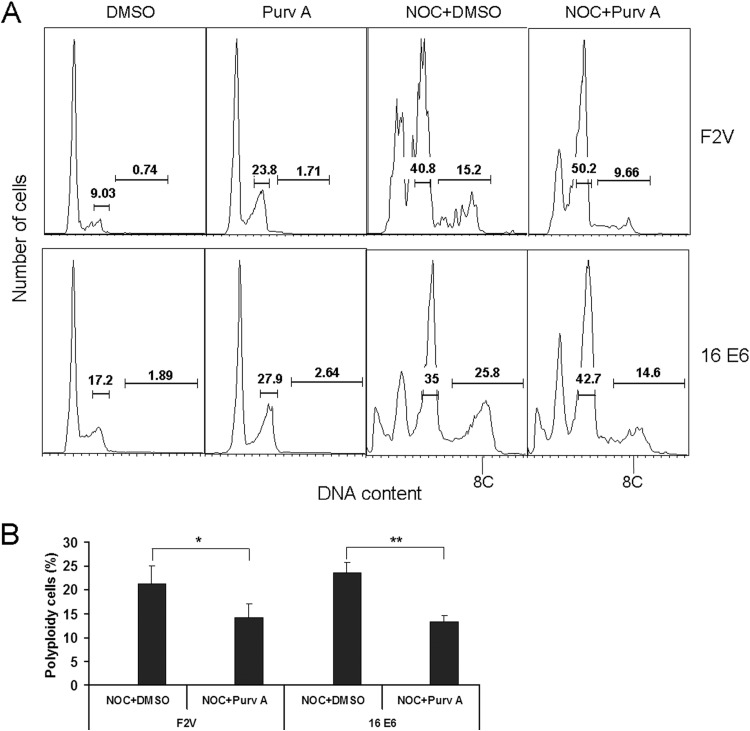
There is a concern that reduced polyploidy by Cdk1 siRNAs is a result of cell arrest at G2 phase, which would lead to a reduction in the number of cells entering into mitosis, as well as the postmitotic stage. To alleviate this concern, we used a small-molecule inhibitor of Cdk1 that could be applied after nocodazole treatment, when cells have already passed the G2 and spindle checkpoints. The small-molecule Cdk1 inhibitor purvalanol A selectively inhibits Cdk1, although at higher concentrations, it may also inhibit Cdk2 (38). At 3 μM, purvalanol A modestly increased the G2 but not the G1 populations of F2V- and E6-expressing NIKS cells (Fig. 5A). We therefore added purvalanol A to E6-expressing NIKS cells already treated with nocodazole for 24 h, a point at which NIKS control cells and E6-expressing NIKS cells have largely exited from mitosis, as we showed previously (24). Significantly, the treatment of cells with purvalanol A significantly reduced F2V- and E6-induced polyploidy (P = 0.003) (Fig. 5B). These results provide additional evidence for the role of Cdk1 in polyploidy formation in E6-expressing cells.
FIG 5.
Pharmacological inhibition of Cdk1 reduces E6-induced polyploidy. (A) F2V- and E6-expressing NIKS cells were treated with DMSO or 3 μM purvalanol A for 24 h. In parallel experiments, cells were treated with nocodazole for 24 h, followed by treatment with purvalanol A or DMSO for an additional 24 h in the presence of nocodazole before being analyzed by flow cytometry. Polyploid cells are indicated as 8C. Data from one representative experiment of four are shown. (B) Percentages of polyploid cells from the experiment whose results are shown in panel A are summarized in a histogram format. Error bars reflect the standard deviations of the means. Purv A, purvalanol A; NOC, nocodazole. *, P < 0.05; **, P < 0.01.
Role of E2F1 in upregulation of Cdk1 in E6-expressing cells.
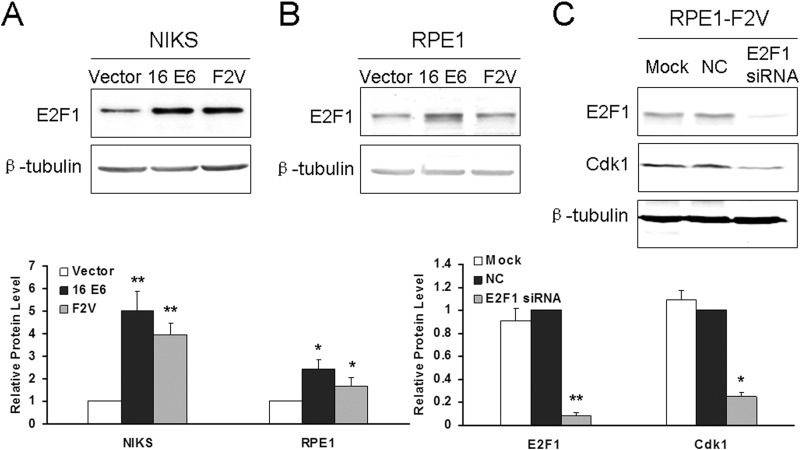
It was reported that E2F1 regulates Cdk1 expression (39). On the other hand, E6 turns on E2F1 (40). We therefore examined the possibility that E6 and F2V increase Cdk1 expression by turning on E2F1. As shown by the results in Fig. 6A, E2F1 is significantly upregulated in both E6- and F2V-expressing cells. Furthermore, downregulation of E2F1 by siRNA significantly reduced the steady-state levels of Cdk1 (Fig. 6B). These results demonstrate that E2F1 plays an important role in the upregulation of Cdk1 by E6. Since the p53 degradation-defective E6 mutant F2V is competent in upregulation of E2F1, E6 can turn on E2F1 in a p53 degradation-independent manner.
FIG 6.
Role of E2F1 in regulation of Cdk1 in E6-expressing cells. (A and B) Total protein extracts from NIKS (A) and RPE1 (B) cells expressing vector, E6, and F2V were analyzed for E2F1 by Western blotting. β-Tubulin was used as a loading control. (C) RPE1-F2V cells were transfected with siRNA specific to E2F1 for 36 h, and the levels of E2F1 and Cdk1 were measured by Western blotting. β-Tubulin was used as a loading control. Bottom, quantification of the relative protein levels in the experiments whose results are shown in panels A to C.
Reduced nuclear p21 localization in E6 mutant F2V-expressing cells.
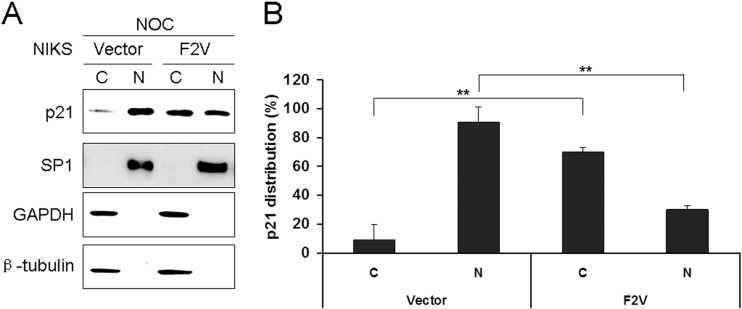
To further explore whether E6 has additional mechanisms to overcome the inhibitory effect of p21 in bypassing the postmitotic checkpoint, we examined its cellular localization. We performed Western blot analysis of p21 expression following subcellular fractionation to determine and quantify the intracellular localization of p21 in NIKS cells expressing F2V and control, nocodazole-treated cells. Only nuclear and cytoplasmic proteins were prepared and analyzed. Successful fractionation was demonstrated by the expected subcellular localization of nuclear (SP1) and cytoplasmic (β-tubulin and GAPDH) protein markers (Fig. 7A). While the majority of p21 proteins (∼90%) were localized in the nucleus in the vector control cells, only less than half (∼30%) of the p21 remained in the nucleus in F2V-expressing cells (Fig. 7B). Most of the p21 (∼70%) was found in the cytoplasm in F2V-expressing cells. These results demonstrate an ability of E6 to affect the cellular localization of p21. As most Cdk1 substrates related to the postmitotic checkpoint are expected to be in the nucleus when cells bypass the checkpoint, this observation provides another mechanism for how Cdk1 remains active in the presence of p21 within the same cell. By translocating p21 from the nucleus to the cytoplasm, less p21 will be available to bind and inhibit nuclear Cdk1.
FIG 7.
Cellular localization of the p21 protein in E6 mutant-expressing cells. (A) NIKS cells expressing F2V or vector were treated with nocodazole, and cytoplasmic (C) and nuclear (N) fractions were prepared and immunoblotted with antibodies specific for p21, β-tubulin, GAPDH (cytoplasmic protein marker), or SP1 (nuclear marker). Fifty micrograms of the 800 μg of cytoplasmic proteins and 10 μg of the 80 μg of nuclear proteins were loaded. Data from one representative experiment of three are shown. (B) Quantification of steady-state levels of cytoplasmic and nuclear p21 in F2V or vector cells in the experiment whose results are shown in panel A. Error bars reflect the standard deviations of the means. NOC, nocodazole. **, P < 0.01.
DISCUSSION
Previously, Cdk2 was thought to be essential for the G1/S transition, while Cdk1 was thought to function only at the G2/M progression. A role for Cdk1 at the G1 checkpoint in the absence of Cdk2 has been demonstrated during the past decade (8, 9). The essential role of G1 Cdks has been challenged by observations indicating that they are not necessary for the mitotic cell cycle in mammalian cells (8, 9, 41–43). In the absence of all three G1 Cdks, Cdk1 compensates for their functions (9). Because the postmitotic checkpoint shares many features with the G1 checkpoint (14, 15), it is not surprising that Cdk1 activity is important for bypassing the postmitotic checkpoint in E6-expressing cells. Nonetheless, in the presence of G1 Cdks, a role for Cdk1 in S-phase progression has not been shown. The data presented in this study indicate a role for Cdk1 at the postmitotic G1 checkpoint in the presence of functional Cdk2 and other G1 Cdks. In this regard, these results call for a novel concept of Cdk1 functions at cell cycle checkpoints.
p21 is generally believed to bind Cdk1 with lower affinity than for its association with Cdk2 (37). Theoretically, p21 expression should be decreased during carcinogenesis, as it normally functions as an inhibitor of cell proliferation. In cervical cancer, which is frequently associated with HPV infection, the p21 protein level is expected to be low due to E6-mediated degradation of p53. Interestingly, increased p21 expression was significantly correlated with advanced cervical cancer in several studies (22, 23). How the growth-inhibitory function of high levels of p21 is overcome in cervical cancer is not fully understood. It is therefore medically relevant and important to study the p53-independent functions of E6 that bypass the inhibitory effect of p21. Because p21 binds Cdk1 with lower affinity than Cdk2 (37), our observation that Cdk1 instead of Cdk2 plays an important role in polyploidy formation in E6-expressing cells implicates a mechanism by which E6 abrogates the postmitotic checkpoint in the presence of p21.
While it was suggested that a single p21 molecule is sufficient for kinase inhibition and that p21-saturated complexes contain only one stably bound inhibitor molecule (44), other studies suggest that kinase complexes containing p21 transit between active and inactive states and that Cdk2 complexes associated with one p21 molecule remain active until they associate with additional p21 molecules (37, 45). In this study, we demonstrated that a significant amount of p21 localizes in the cytoplasm of E6 mutant-expressing cells after nocodazole treatment (Fig. 6), a condition where these cells enter S phase with a tetraploid genome (24). As a result, the decreased level of p21 in the nucleus may not be sufficient to bind or inhibit all Cdk1. A reduction in the levels of p21 in the nucleus of E6 mutant-expressing cells should contribute to the activation of Cdks, which could partially explain why Cdk1 remains active and is required for polyploidy formation in E6-expressing cells. Interestingly, immunostaining analysis revealed that p21 was predominantly cytoplasmic in breast cancer (46). While nuclear localization of p21 in low-grade cervical squamous intraepithelial lesions infected with HPV has been observed (47), p21 localization in HPV-positive tissues in response to mitotic stress remains to be examined.
The mechanism by which p21 localizes to the cytoplasm in E6-expressing cells remains to be explored. Multiple protein kinases have been shown to phosphorylate p21, which regulates its subcellular localization (48). For example, Akt catalyzes the phosphorylation of p21 on T145, leading to the accumulation of cytoplasmic p21 (49). It is well known that Akt is activated in E6-expressing cells (50), and thus, it is possible that Akt stimulates the translocation of p21 from nucleus to cytoplasm by phosphorylating it on T145 in E6-expressing cells. Cytoplasmic p21 can bind to and prevent the activation of procaspase 3, hence blocking apoptosis (51). Cytoplasmic p21 may also gain new functions, such as cell motility, which contributes to invasion and metastasis (52). Future studies will explore these possibilities.
Although the increased kinase activity of Cdk1 in E6- and F2V-expressing cells can be explained by a lower binding affinity of p21 and/or cytoplasmic localization of p21, we do not know the mechanism underlying the upregulation of cyclin B1 and cyclin A2, at the protein level. Notably, the cell cycle profiles for regularly cultured E6- and F2V-expressing cells are similar to that of vector control cells (Fig. 3C) (24). It is generally believed that HPV oncogenes abrogate cell cycle checkpoints and alter the expression of cell cycle-related proteins. In doing so, HPV generates a favorable environment for viral DNA replication in otherwise differentiating cells. Future studies should explore the mechanism by which Cdk1-associated cyclins are upregulated in E6- and E6 mutant-expressing cells.
In this study, we examined the expression of postmitotic checkpoint-related genes in cells expressing an HPV-16 E6 mutant that is defective in p53 degradation but competent in abrogating the checkpoint. Our results demonstrate an increase in the steady-state levels of both G1- and G2-related cyclins/Cdks in E6-expressing keratinocytes. Interestingly, only Cdk1 remains active in E6 mutant-expressing cells upon microtubule disruption. Furthermore, downregulation of Cdk1 impairs the ability of E6 to induce polyploidy. Our study thus demonstrated an important role for Cdk1 in E6-induced polyploidy. As p21 binds Cdk1 with lower affinity than Cdk2 (37), our results implicate a mechanism by which E6 abrogates the postmitotic checkpoint in the presence of p21. Furthermore, less p21 protein was found in the nucleus of HPV E6 mutant-expressing cells than in the nucleus of control cells when entering S phase with a tetraploid genome. These studies shed light on mechanisms by which HPV induces polyploidy.
ACKNOWLEDGMENTS
We thank Elliot Androphy for helpful discussions, Xueli Fan for help preparing the PHK-Vector, and Sriramana Kanginakudru for critical readings of the manuscript.
J. J. Chen was supported by grant no. R01CA119134 from the National Cancer Institute. W. Zhang was supported by the National Natural Science Foundation of China (grant no. 81101259) and Doctoral Fund of Shandong Province of China (grant no. 2011BSE27019).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or NIH.
The authors declare no conflict of interest.
REFERENCES
- 1.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. 2005. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 2.Ganem NJ, Storchova Z, Pellman D. 2007. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Giannoudis A, Evans MF, Southern SA, Herrington CS. 2000. Basal keratinocyte tetrasomy in low-grade squamous intra-epithelial lesions of the cervix is restricted to high and intermediate risk HPV infection but is not type-specific. Brit J Cancer 82:424–428. doi: 10.1054/bjoc.1999.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olaharski AJ, Sotelo R, Solorza-Luna G, Gonsebatt ME, Guzman P, Mohar A, Eastmond DA. 2006. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis 27:337–343. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- 5.Lanni JS, Jacks T. 1998. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol 18:1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartwell LH, Kastan MB. 1994. Cell cycle control and cancer. Science 266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 7.Sherr CJ, Roberts JM. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 8.Aleem E, Kiyokawa H, Kaldis P. 2005. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol 7:831–836. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- 9.Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, Caceres JF, Dubus P, Malumbres M, Barbacid M. 2007. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 10.Zheng L, Lee WH. 2001. The retinoblastoma gene: a prototypic and multifunctional tumor suppressor. Exp Cell Res 264:2–18. doi: 10.1006/excr.2000.5129. [DOI] [PubMed] [Google Scholar]
- 11.Stewart ZA, Pietenpol JA. 2001. p53 signaling and cell cycle checkpoints. Chem Res Toxicol 14:243–263. doi: 10.1021/tx000199t. [DOI] [PubMed] [Google Scholar]
- 12.Vogelstein B, Lane D, Levine AJ. 2000. Surfing the p53 network. Nature 408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 13.Wong C, Stearns T. 2005. Mammalian cells lack checkpoints for tetraploidy, aberrant centrosome number, and cytokinesis failure. BMC Cell Biol 6:6. doi: 10.1186/1471-2121-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borel F, Lohez OD, Lacroix FB, Margolis RL. 2002. Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells. Proc Natl Acad Sci U S A 99:9819–9824. doi: 10.1073/pnas.152205299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meraldi P, Honda R, Nigg EA. 2002. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J 21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreassen PR, Lohez OD, Lacroix FB, Margolis RL. 2001. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol Biol Cell 12:1315–1328. doi: 10.1091/mbc.12.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sablina AA, Agapova LS, Chumakov PM, Kopnin BP. 1999. p53 does not control the spindle assembly cell cycle checkpoint but mediates G1 arrest in response to disruption of microtubule system. Cell Biol Int 23:323–334. doi: 10.1006/cbir.1999.0362. [DOI] [PubMed] [Google Scholar]
- 18.Khan SH, Wahl GM. 1998. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res 58:396–401. [PubMed] [Google Scholar]
- 19.Stewart ZA, Leach SD, Pietenpol JA. 1999. p21(Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol Cell Biol 19:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev 2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 21.Fan X, Chen JJ. 2004. Regulation of cell cycle progression and apoptosis by the papillomavirus E6 oncogene. Crit Rev Eukaryot Gene Expr 14:183–202. doi: 10.1615/CritRevEukaryotGeneExpr.v14.i3.30. [DOI] [PubMed] [Google Scholar]
- 22.Bae DS, Cho SB, Kim YJ, Whang JD, Song SY, Park CS, Kim DS, Lee JH. 2001. Aberrant expression of cyclin D1 is associated with poor prognosis in early stage cervical cancer of the uterus. Gynecol Oncol 81:341–347. doi: 10.1006/gyno.2001.6196. [DOI] [PubMed] [Google Scholar]
- 23.Cheung TH, Lo KW, Yu MM, Yim SF, Poon CS, Chung TK, Wong YF. 2001. Aberrant expression of p21(WAF1/CIP1) and p27(KIP1) in cervical carcinoma. Cancer Lett 172:93–98. doi: 10.1016/S0304-3835(01)00624-3. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Heilman SA, Illanes D, Sluder G, Chen JJ. 2007. p53-independent abrogation of a postmitotic checkpoint contributes to HPV E6-induced polyploidy. Cancer Res 67:2603–2610. doi: 10.1158/0008-5472.CAN-06-3436. [DOI] [PubMed] [Google Scholar]
- 25.Duensing S, Munger K. 2002. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res 62:7075–7082. [PubMed] [Google Scholar]
- 26.Plug-Demaggio AW, McDougall JK. 2002. The human papillomavirus type 16 E6 oncogene induces premature mitotic chromosome segregation. Oncogene 21:7507–7513. doi: 10.1038/sj.onc.1205903. [DOI] [PubMed] [Google Scholar]
- 27.Flores ER, Allen-Hoffmann BL, Lee D, Lambert PF. 2000. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol 74:6622–6631. doi: 10.1128/JVI.74.14.6622-6631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uetake Y, Sluder G. 2004. Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a “tetraploidy checkpoint.”. J Cell Biol 165:609–615. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Li J, Kanginakudru S, Zhao W, Yu X, Chen JJ. 2010. The human papillomavirus type 58 E7 oncoprotein modulates cell cycle regulatory proteins and abrogates cell cycle checkpoints. Virology 397:139–144. doi: 10.1016/j.virol.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.L'Italien L, Tanudji M, Russell L, Schebye XM. 2006. Unmasking the redundancy between Cdk1 and Cdk2 at G2 phase in human cancer cell lines. Cell Cycle 5:984–993. doi: 10.4161/cc.5.9.2721. [DOI] [PubMed] [Google Scholar]
- 31.Beck H, Nahse V, Larsen MS, Groth P, Clancy T, Lees M, Jorgensen M, Helleday T, Syljuasen RG, Sorensen CS. 2010. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J Cell Biol 188:629–638. doi: 10.1083/jcb.200905059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores ER, Allen-Hoffmann BL, Lee D, Sattler CA, Lambert PF. 1999. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 262:344–354. doi: 10.1006/viro.1999.9868. [DOI] [PubMed] [Google Scholar]
- 33.Garner-Hamrick PA, Fostel JM, Chien WM, Banerjee NS, Chow LT, Broker TR, Fisher C. 2004. Global effects of human papillomavirus type 18 E6/E7 in an organotypic keratinocyte culture system. J Virol 78:9041–9050. doi: 10.1128/JVI.78.17.9041-9050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanai M, Shiozawa T, Xin L, Nikaido T, Fujii S. 1998. Immunohistochemical detection of sex steroid receptors, cyclins, and cyclin-dependent kinases in the normal and neoplastic squamous epithelia of the uterine cervix. Cancer 82:1709–1719. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Bashir T, Pagano M. 2005. Cdk1: the dominant sibling of Cdk2. Nat Cell Biol 7:779–781. doi: 10.1038/ncb0805-779. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Moscinski LC. 2011. Cdc2: a monopotent or pluripotent CDK? Cell Prolif 44:205–211. doi: 10.1111/j.1365-2184.2011.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, Fox MP, Wei N. 1995. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell 6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L, Kim SH, Lockhart DJ, Schultz PG. 1998. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281:533–538. doi: 10.1126/science.281.5376.533. [DOI] [PubMed] [Google Scholar]
- 39.Konishi Y, Bonni A. 2003. The E2F-Cdc2 cell-cycle pathway specifically mediates activity deprivation-induced apoptosis of postmitotic neurons. J Neurosci 23:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, Takhar PP, Degenkolbe R, Koh CH, Zimmermann H, Yang CM, Guan Sim K, Hsu SI, Bernard HU. 2003. The human papillomavirus type 11 and 16 E6 proteins modulate the cell-cycle regulator and transcription cofactor TRIP-Br1. Virology 317:155–164. doi: 10.1016/j.virol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Berthet C, Klarmann KD, Hilton MB, Suh HC, Keller JR, Kiyokawa H, Kaldis P. 2006. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev Cell 10:563–573. doi: 10.1016/j.devcel.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet 35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 43.Tetsu O, McCormick F. 2003. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 3:233–245. doi: 10.1016/S1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 44.Hengst L, Gopfert U, Lashuel HA, Reed SI. 1998. Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1). Genes Dev 12:3882–3888. doi: 10.1101/gad.12.24.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Hannon GJ, Beach D. 1994. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev 8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 46.Winters ZE, Hunt NC, Bradburn MJ, Royds JA, Turley H, Harris AL, Norbury CJ. 2001. Subcellular localisation of cyclin B, Cdc2 and p21(WAF1/CIP1) in breast cancer. Association with prognosis. Eur J Cancer 37:2405–2412. doi: 10.1016/S0959-8049(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 47.Giannoudis A, Herrington CS. 2000. Differential expression of p53 and p21 in low grade cervical squamous intraepithelial lesions infected with low, intermediate, and high risk human papillomaviruses. Cancer 89:1300–1307. doi:. [DOI] [PubMed] [Google Scholar]
- 48.Child ES, Mann DJ. 2006. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle 5:1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- 49.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825. doi: 10.1016/0092-8674(93)90500-P. [DOI] [PubMed] [Google Scholar]
- 50.Spangle JM, Munger K. 2010. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol 84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. 1998. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene 17:931–939. doi: 10.1038/sj.onc.1202021. [DOI] [PubMed] [Google Scholar]
- 52.Lee S, Helfman DM. 2004. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem 279:1885–1891. doi: 10.1074/jbc.M306968200. [DOI] [PubMed] [Google Scholar]