ABSTRACT
CD4+ T lymphocytes play a central role in the immune system and mediate their function after recognition of their respective antigens presented on major histocompatibility complex II (MHCII) molecules on antigen-presenting cells (APCs). Conventionally, phagocytosed antigens are loaded on MHCII for stimulation of CD4+ T cells. Certain epitopes, however, can be processed directly from intracellular antigens and are presented on MHCII (endogenous MHCII presentation). Here we characterized the MHCII antigen presentation pathways that are possibly involved in the immune response upon vaccination with modified vaccinia virus Ankara (MVA), a promising live viral vaccine vector. We established CD4+ T-cell lines specific for MVA-derived epitopes as tools for in vitro analysis of MHCII antigen processing and presentation in MVA-infected APCs. We provide evidence that infected APCs are able to directly transfer endogenous viral proteins into the MHCII pathway to efficiently activate CD4+ T cells. By using knockout mice and chemical inhibitory compounds, we further elucidated the molecular basis, showing that among the various subcellular pathways investigated, proteasomes and autophagy are key players in the endogenous MHCII presentation during MVA infection. Interestingly, although proteasomal processing plays an important role, neither TAP nor LAMP-2 was found to be involved in the peptide transport. Defining the molecular mechanism of MHCII presentation during MVA infection provides a basis for improving MVA-based vaccination strategies by aiming for enhanced CD4+ T-cell activation by directing antigens into the responsible pathways.
IMPORTANCE This work contributes significantly to our understanding of the immunogenic properties of pathogens by deciphering antigen processing pathways contributing to efficient activation of antigen-specific CD4+ T cells. We identified autophagosome formation, proteasomal activity, and lysosomal integrity as being crucial for endogenous CD4+ T-cell activation. Since poxvirus vectors such as MVA are already used in clinical trials as recombinant vaccines, the data provide important information for the future design of optimized poxviral vaccines for the study of advanced immunotherapy options.
INTRODUCTION
T lymphocytes are major components of the adaptive immune system and mediate their function upon recognition of their respective antigens presented on the surfaces of antigen-presenting cells (APCs) by major histocompatibility complex class I/II (MHCI/II) molecules (1). Cytotoxic CD8+ T cells that mediate killing of infected or tumor cells are activated by antigen presentation on MHCI (2). Also referred to as the “leader of the immunological orchestra,” CD4+ T cells possess more regulatory functions and are induced by antigen presentation on MHCII. There are several subsets of CD4+ T cells with different effector functions, such as Th1 or Th2 cells, which fight intracellular as well as extracellular pathogens by activating macrophages, CD8+ T cells, and B cells. Furthermore, CD4+ subsets are involved in antimicrobial and autoimmune responses (Th17 cells), and they regulate the immune response and maintain self-tolerance (nTreg, iTreg, Tr1, and Th cells) (3, 4).
The proper processing and presentation of antigens by APCs are key steps in the induction of cell-mediated immunity. Conventionally, intracellular cytosolic antigens are presented on MHCI while phagocytosed extracellular antigens are loaded on MHCII to stimulate CD8+ and CD4+ T cells, respectively (1). However, it is now generally accepted that besides these two classical pathways, extracellular antigens are also loaded on MHCI in a process called cross-presentation (5). Conversely, several studies over the past 2 decades have also provided evidence that intracellular antigens can be processed for presentation on MHCII. The first hint that intracellular antigens are loaded on MHCII was obtained by sequence analysis of peptides bound to MHCII, showing that the majority of those ligands were derived from endogenous proteins (6). Since then, endogenous MHCII presentation has been shown to occur not only for self-antigens to mediate tolerance (7, 8) but also for viral antigens (like measles virus matrix and nucleocapsid protein, influenza A hemagglutinin, HCV core protein, and EBV nuclear antigen 1) as well as tumor antigens (such as MUC-1 and mutated CDC27) to broaden the spectrum of immunogenic MHCII ligands (9). Moreover, classical presentation seems to play a relatively minor role, while alternative presentation pathways seem to contribute substantially to MHCII peptide presentation (10).
Different pathways have been suggested to be involved in MHCII presentation of intracellular antigens (9). First, secreted or transmembrane proteins can be translocated by the Sec61 translocon into the endoplasmic reticulum (ER), where they associate with MHCII and are further guided to endosomal compartments (11). Second, similar to the classical MHCI pathway, proteasomally degraded cytosolic peptides can be transported into the ER by TAP (transporter associated with antigen processing) to bind MHCII complexes (12). Third, cytosolic peptides can also be directly imported into endosomal MHCII loading compartments mediated by the peptide transporter LAMP-2 through a process called chaperone-mediated autophagy (13). Finally, macroautophagy has recently attracted more and more attention as an important pathway in the processing of endogenous MHCII presentation (14). Macroautophagy is a homeostatic degradation process that enables the cell to survive in case of stresses like accumulation of misfolded proteins and damaged organelles and starvation and energy deprivation. Cytoplasmic proteins and organelles are engulfed and self-digested within autophagic vacuoles that fuse with lysosomes to catabolize the autophagic cargo. Thus, nutrients for energy metabolism as well as new proteins and membrane components are provided to enable cellular survival (15). The delivery of cytosolic components into the endosomal/lysosomal compartment via autophagy also enables the degradation of intracellular cytosolic antigens making them accessible for MHCII presentation (16–18). In this context, autophagy also plays an important role in immunity and inflammation (19). Irrespective of the pathway used, endogenous MHCII presentation in APCs expands the repertoire of MHCII ligands, thereby increasing the source of antigens that can trigger CD4+ T-cell responses.
Modified vaccinia virus Ankara (MVA) is a highly attenuated poxvirus that was developed by growth selection from its ancestral vaccinia strain (chorioallantois vaccinia virus Ankara [CVA]) in chicken embryo fibroblasts (20). MVA possesses a broad cell tropism, causing self-limiting infections without genomic integration into the host cell genome. Importantly, infections are abortive, since replication is blocked in most mammalian cell types due to deletions and mutations that have been acquired during attenuation (21, 22). Nevertheless, upon infection the full cascade of vaccinia virus gene expression occurs, characterized by three distinct phases orchestrated by viral promoters with early, intermediate, or late expressional activity during infection. Protein synthesis is unimpaired, as the late block in morphogenesis during viral replication allows abundant expression of genes. Given the high capacity for packaging DNA into the genomic deletion sites and the excellent safety profile combined with the high immunogenicity of the virus, MVA is a potent and versatile vector system for development of recombinant vaccines based on expression of heterologous antigens (23).
Since the successful application of MVA as a smallpox vaccine in over 120,000 humans during the eradication of smallpox, it has been further developed as vector system and is now widely used for prophylactic or therapeutic vaccination against infectious diseases and cancer in preclinical and clinical trials (24).
To further elucidate the immunogenic properties of MVA, we were interested in identifying the cellular and molecular pathways leading to efficient antigen presentation in infected APCs. Given the importance of CD4+ T cells in adaptive immune responses we investigated a possible role of endogenous MHCII presentation and the underlying cellular pathways in MVA-infected primary murine dendritic cells, which constitute a major fraction of target cells after MVA application in vivo (25). Using an in vitro coculture system of MVA-infected bone marrow-derived dendritic cells (BMDCs) and antigen-specific CD4+ T-cell lines recognizing viral and recombinant antigens we provide clear evidence that endogenous MHCII presentation occurs and is highly efficient to stimulate CD4+ T cells. By manipulating target cells using chemical inhibitors and BMDCs from ATG7−/−, TAP−/−, and Lamp-2−/− mice, we found that autophagy and proteasomal processing play an important role. Interestingly, neither TAP nor LAMP-2 was involved in these pathways.
MATERIALS AND METHODS
Animals.
C57BL/6 and BALB/c mice were derived via in-house breeding. Genetically modified mice were kindly provided as follows: ATG7flox/flox mice, Thomas Misgeld (Institute of Neuroscience, TUM Munich, Germany); CD11c-Cre mice, Thomas Brocker (Institute for Immunology, LMU Munich, Germany); TAP−/− mice, Cytos Biotechnology AG (Schlieren, Switzerland); Lamp-2−/− mice, Paul Saftig (Department of Biochemistry, University of Kiel, Germany). ATG7flox/flox mice were crossed with CD11c-Cre mice to obtain ATG7flox/flox CD11c-Cre−/− (WT), ATG7+/flox CD11c-Cre+/− (ATG7+/− [heterozygous knockout]) and ATG7flox/flox CD11c-Cre+/− (ATG7−/− [homozygous knockout]) mice. Mouse husbandry was conducted under specific-pathogen-free conditions according to the Federation of European Laboratory Animal Science Associations protocols (FELASA). Experiments were performed with the approval of the responsible animal welfare authority.
Viruses.
Wild-type or recombinant modified vaccinia virus Ankara (MVA) expressing ovalbumin and/or enhanced green fluorescent protein (EGFP) under the control of the early (PK1L) or early/late (P7.5) promoter was used in this study (MVA, MVA-OVAPK1L [26], MVA-EGFPPK1L, and MVA-OVAP7.5/EGFPPK1L). All viruses were propagated and titrated in chicken embryonic fibroblasts (CEFs) according to standard methods (27). All viruses were purified by two consecutive ultracentrifugation steps through a 36% (wt/vol) sucrose cushion.
Antibodies and peptides.
Anti-mouse CD4 conjugated to eFluor450, anti-mouse interleukin 2 (IL-2)–Alexa Fluor647, and anti-mouse MHC class II (I-A/I-E)–eFluor450 were purchased from eBioscience (Frankfurt, Germany). Rat anti-mouse gamma interferon conjugated to fluorescein isothiocyanate (IFN-γ–FITC) was obtained from BD Pharmingen (Heidelberg, Germany). For Western blot analysis, rabbit anti-LC3B antibody and rabbit anti-ovalbumin (anti-OVA) antibody were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany), rabbit anti-H3 antibody from Genesis Biotech Inc. (Taiwan), mouse anti-β-actin antibody from Abcam (Cambridge, United Kingdom), and peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG from Jackson ImmunoResearch Europe (Suffolk, United Kingdom). MVA-specific (A4L66–80, A33R116–130, B2R46–60, B5R46–60, D13L486–500, E9L179–193, F15L55–69, H3L272–286, I1L7–21, I1L21–35, L4R176–190, OVA323–339, and OVA265–280) and control (Flu-NP311–325) peptides were produced by Biosyntan GmbH (Berlin, Germany). Peptides were dissolved in dimethyl sulfoxide (DMSO) in a stock concentration of 1 μg/μl.
Inhibitors.
3-Methyladenine (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), epoxomicin, MDL28170, Z-Leu-Leu-CHO (Enzo Life Sciences GmbH, Lörrach, Germany), bafilomycin A1 (BioViotica, Dransfeld, Germany), PD150,606 (AdipoGen AG, Liestal, Switzerland), and chloroquine diphosphate (BioVision, Milpitas, CA, USA) were purchased and prepared according to the manufacturer's recommendations.
Vaccination of mice.
Female C57BL/6 mice at the age of 8 to 10 weeks were vaccinated by intraperitoneal application of 1 × 108 IU MVA-OVAPK1L in 500 μl phosphate-buffered saline (PBS). Vaccination was performed in a short-term prime-boost regimen with the second vaccination at day 5 (26). Mice were sacrificed at day 6 postvaccination, spleens were harvested, and either immune responses were analyzed by intracellular cytokine staining (ICS) for selection of immunogenic epitopes or splenocytes were cultured for generation of CD4+ T-cell lines as described below.
Selection of immunogenic epitopes.
For selection of immunogenic epitopes, 4 × 106 freshly isolated splenocytes from vaccinated mice were plated at 200 μl per well (96-well F-bottom plates). Cells were incubated with a 2-μg/ml final concentration of MVA-specific or control peptides and 1 μg/ml brefeldin A (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) for 14 h. Thereafter, ICS was performed as described below.
Generation of T-cell lines.
CD4+ T-cell lines were established by stimulation of splenocytes from vaccinated mice with peptides and maintained by periodical restimulation.
Briefly, for the first round of stimulation, lipopolysacharide (LPS)-activated B cells (referred to as LPS-blasts) were generated by cultivation of splenocytes from naive mice with 25 μg/ml LPS and 7 μg/ml dextran sulfate for 3 days at 37°C, 5% CO2, and 90% humidity. LPS-blasts were irradiated with 30 Gy and pulsed with 5 μg/ml of peptides for 30 min at 37°C. Thereafter, 3 × 106 irradiated and peptide-pulsed LPS-blasts were cocultivated with 7 × 106 pooled splenocytes from 2 MVA-OVAPK1L-vaccinated mice per well in 24-well plates with RPMI 1640 (Lonza, Cologne, Germany) containing 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin for 7 days.
For maintenance of T-cell lines, the cultures were restimulated every 7 days for the first 20 weeks and thereafter every 14 days according to the following scheme. Splenocytes from naive mice were irradiated with 30 Gy and pulsed with 2 μg/ml of peptide for 30 min at 37°C. Peptides were washed away, and 6 × 106 irradiated and peptide-pulsed splenocytes were plated per well in 24-well plates. T-cell cultures were divided in half, and to one half of T cells 2 × 106 to 3 × 106 cells were added per well and incubated in RPMI 1640 medium containing 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 5% TCGF (conditioned medium obtained as supernatant from rat splenocytes stimulated with 5 μg/ml concanavalin A as previously described [28]). The remaining half of the T-cell culture was used for experiments.
Preparation of BMDCs.
Bone marrow was collected from femurs and tibias from C57BL/6, BALB/c, ATG7flox/flox CD11c-Cre−/− (WT), ATG7+/flox CD11c-Cre+/− (ATG7+/−), ATG7flox/flox CD11c-Cre+/− (ATG7−/−), TAP−/−, and Lamp-2−/− mice, and cells were incubated with TAC medium (0.144 M NH4Cl and 0.017 M Tris [pH 7.65]) for 2 min at 37°C. Cells were washed, filtered through a 100-μm cell strainer, seeded in 94- by 16-mm petri dishes (5 × 106 cells per dish), and cultivated with 10 ml RPMI 1640 containing 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% granulocyte-macrophage colony-stimulating factor (GM-CSF) (conditioned medium obtained as supernatant from B16 cells expressing GM-CSF; cells were a kind gift from Georg Häcker, Freiburg, Germany). At day 3, 10 ml of fresh medium was added (final volume of 20 ml per petri dish), and at day 6, 10 ml of medium was replaced by 10 ml of fresh medium. BMDCs were used for experiments at day 7.
Cell culture assay.
Cell culture assays were performed with BMDCs as antigen-presenting cells for the stimulation of T cells according to the following protocol.
Briefly, 1.5 × 106 BMDCs were incubated in 500 μl RPMI containing 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin and were left untreated or were treated with inhibitors at various concentrations for 90 min at 37°C. Inhibitors remained in the culture, and cells were infected with MVA at various multiplicities of infection (MOIs). Where indicated, MVA was treated with 0.3 μg/ml psoralen plus UVA (PUVA) for 10 min on ice and then exposed to UVA for 30 min (corresponding to 0.6 J/cm2) on ice prior to infection. Infection was performed for 60 min at 37°C. Thereafter 1 × 105 cells were plated in 96-well F-bottom plates and incubated for 5 h at 37°C. Additionally, uninfected cells were plated at the same density and were pulsed with peptide (2 μg/ml), fed with full-length OVA protein (10 μg/ml) as positive controls for stimulation of T cells, or treated with DMSO as negative controls to assess the background activity of T cells.
T cells were added (3 × 105 per well; effector-to-target ratio, 3:1), including brefeldin A in a final concentration of 1 μg/ml. The cultures were incubated for 14 h at 37°C, and the stimulation of the T cells was analyzed by ICS as described below.
ICS.
Cells were transferred into 96-well V-bottom plates and incubated with blocking buffer (PBS plus 1% bovine serum albumin [BSA]) containing 1 μg/ml ethidium monoazide bromide (Life Technologies GmbH, Darmstadt, Germany) for 20 min under light exposure. Thereafter, ICS was performed with a BD Cytofix/Cytoperm fixation/permeabilization kit according to the manufacturer's protocol (BD Pharmingen, Heidelberg, Germany). Briefly, cells were washed twice with blocking buffer, and surface staining was performed with anti-CD4 antibodies for 30 min at 4°C. Cells were washed, and permeabilization was performed with Cytofix/Cytoperm solution for 15 min at 4°C. Thereafter, cells were washed again and incubated with anti-IFN-γ and anti-IL-2 for 30 min at 4°C. Finally, cells were washed and fixed with 1% paraformaldehyde (PFA). Flow cytometry was performed with BD FACSCanto II (BD Biosciences, Heidelberg, Germany).
Western blotting.
For detection of LC3, BMDCs were left untreated (negative control), treated with 50 μM chloroquine for 2 h (positive control), or infected with MVA-EGFPP7.5 for 8 h. For detection of virus-derived proteins (H3 and OVA) BMDCS were left untreated (positive control) or were treated with indicated inhibitors and then infected with MVA or MVA-OVAPK1L for 14 h. Untreated BMDCs were used as negative controls. Cells were washed with ice-cold PBS and harvested with lysis buffer containing 10% glycerol, 20 mM Tris-HCl (pH 7), 137 mM NaCl, 2 mM EDTA, 0.5% Triton X-100, complete miniprotease inhibitor (Roche Diagnostics GmbH, Mannheim, Germany), and PhosSTOP phosphatase inhibitor (Roche Diagnostics GmbH, Mannheim, Germany) for 15 min on ice. Cell lysates were sonicated on ice for 30 s and centrifuged at 13,000 rpm for 30 min at 4°C to remove cellular debris. Protein concentrations of cell extracts were determined using Bradford assay. Samples (40 μg) were mixed with 2× Laemmli loading buffer (Bio-Rad GmbH, Munich, Germany) and subjected to 12% SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) overnight at a constant 25 V and 4°C with a Mini-Trans blot system (Bio-Rad GmbH, Munich, Germany). Membranes were blocked with 5% BSA in Tris-buffered saline supplemented with 0.1% Tween 20 (TBST) for 30 min at room temperature. Rabbit anti-LC3B, rabbit anti-OVA, rabbit anti-H3, and mouse anti-β-actin antibodies were diluted in TBST and incubated with membranes for 2 h at room temperature. Primary antibodies were intensively washed away and peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG was incubated with membranes for 30 min. Chemiluminescence detection was done with Super Signal West Dura chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA). For determination of LC3, bands of LC3-I, LC3-II, and β-actin were quantified by ImageJ software.
Statistical analysis.
All results are expressed as means and standard errors of the mean (SEM) for a given sample size (n), either pooled or representative of independent experiments. The statistical significance was tested by applying the unpaired t test (two tailed) using GraphPad Prism 5. P values of <0.05 were considered significant.
RESULTS
Establishment of CD4+ T-cell lines.
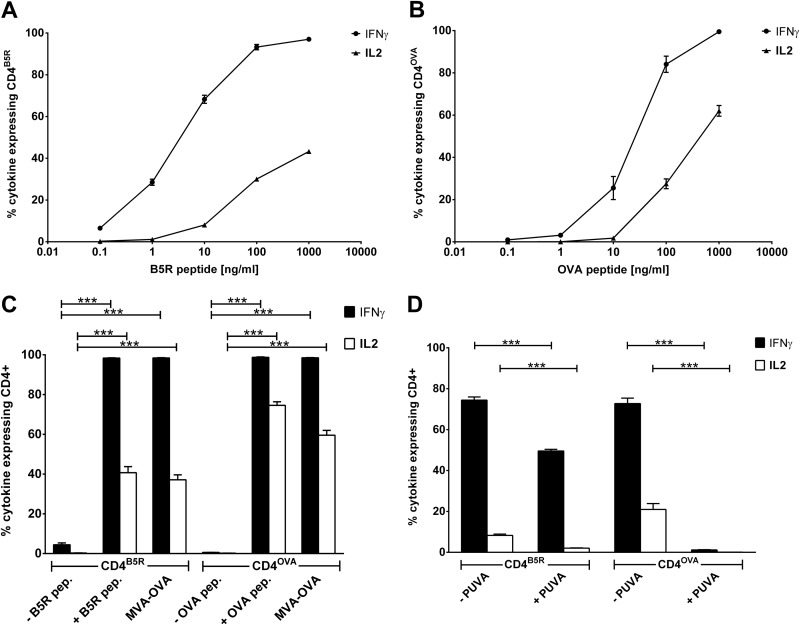
To select appropriate MVA-derived MHCII epitopes suitable for the establishment of antigen-specific CD4+ T-cell lines, we analyzed the CD4+ T-cell response in MVA-OVAPK1L prime-boost-vaccinated C57BL/6 mice (I-Ab) to 13 potential MHCII antigens and found B5R46–60 (extracellular enveloped virus [EEV] type I membrane glycoprotein) and OVA265–280 to be the most immunogenic (data not shown). Based on these findings, we established B5R46–60- and OVA265–280-recognizing CD4+ T-cell lines (referred to here as CD4B5R and CD4OVA) by weekly restimulation of splenocytes from MVA-OVAPK1L prime-boost-vaccinated C57BL/6 mice with the respective antigens. The development and quality of the cell lines were assessed based on the percentage of cells in the culture expressing IFN-γ and IL-2 in response to the corresponding peptide and to stimulation with DMSO as a negative control. After 20 weeks (day 140) of periodical restimulation, the proportion of cells expressing cytokines in response to their cognate antigen was at maximum for both cell lines with almost no background activity in the negative controls (data not shown). To use both T-cell lines as a read-out system for determining strength and quality of antigen presentation in infected APCs, they were further characterized with respect to their cytokine expression profile. Using BMDCs that were pulsed with different amounts of peptide as target cells for the CD4+ T-cell lines, we found a direct correlation between the amount of antigen presented on the surfaces of APCs and the strength of cytokine expression (Fig. 1A and B). The more antigen was present, the higher the number of cells expressing one or more cytokines was. Importantly, a higher antigen density on APCs was needed to induce the production of IL-2 than IFN-γ.
FIG 1.
Simulation of CD4+ T-cell lines and presentation of virion-incorporated versus de novo-synthesized antigens. (A and B) Kinetics of cytokine expression of CD4+ T-cell lines. BMDCs were pulsed with different concentrations of peptide. CD4B5R (A) and CD4OVA (B) were added to the culture for 14 h, and the numbers of IFN-γ- and IL-2-expressing CD4+ T cells were quantified by ICS and FACS analysis. Data are means and SEM (n = 3, pooled from three experiments). (C) Recognition of infected BMDCs by CD4+ T-cell lines. BMDCs were treated with DMSO (− B5R pep. and − OVA pep.), pulsed with peptide (+ B5R pep. and + OVA pep.), or infected with MVA-OVA (MOI, 10). CD4B5R and CD4OVA were added to the culture for 14 h, and the numbers of IFN-γ- and IL-2-expressing CD4+ T cells were determined by ICS and FACS analysis. Data are means and SEM (n = 4, pooled from 3 experiments). (D) Presentation of virion-incorporated versus de novo-synthesized antigens. BMDCs were infected with MVA-OVA (MOI, 10) for 6 h. MVA was either pretreated with psoralen plus UVA (+ PUVA) for 30 min on ice or left untreated (− PUVA). CD4B5R and CD4OVA were added to the culture for 14 h, and the numbers of IFN-γ- and IL-2-expressing CD4+ T cells were quantified by ICS and FACS analysis. Data are means and SEM (n = 5, pooled from 3 experiments). ***, P ≤ 0.001 (unpaired t test, two tailed).
MHCII presentation by MVA-infected BMDCs.
Next we established an in vitro cell culture assay for the investigation of MHCII presentation and related pathways during MVA infection. Cocultivation of MVA-OVA-infected BMDCs as target cells for antigen-specific CD4B5R and CD4OVA revealed that viral infection enables APCs to stimulate both CD4+ T-cell lines at a magnitude that is comparable to that of stimulation with peptide-pulsed BMDCs (positive control) (Fig. 1C). In contrast to OVA, the viral protein B5 is incorporated into the virion and therefore may serve as exogenous antigen. In order to determine whether B5R46–60 was presented on MHCII after de novo synthesis in MVA-infected cells or whether B5 was brought into the cell as part of the virion for exogenous antigen processing, we investigated MHC class II presentation while preventing the de novo synthesis of viral proteins. Inhibition of protein synthesis in infected cells by cycloheximide and anisomycin proved to be impractical, as both inhibitors also completely prevented expression of IFN-γ and IL-2 in CD4+ T cells (data not shown). Therefore, PUVA treatment of MVA prior to infection was applied to prevent viral gene expression and thus de novo viral protein synthesis. Quantitative PCR (qPCR) determining viral gene expression for B5R or OVA confirmed the effectiveness of PUVA treatment with a 210-fold decrease in gene expression after 15 min of treatment (data not shown). We observed a strong difference in the effect between both CD4+ T-cell lines (Fig. 1D). PUVA treatment completely abolished the OVA-specific IFN-γ and IL-2 production by CD4OVA, indicating that the presentation of OVA depends exclusively on the endogenous route. In contrast, there was a significant but far less pronounced effect on B5 presentation (e.g., about a 4-fold reduction of IL-2 by CD4B5R), indicating that endogenous presentation of newly synthesized B5 contributes but that the dominant presentation pathway seems to be the exogenous route for this antigen.
Validation of the cell culture system.
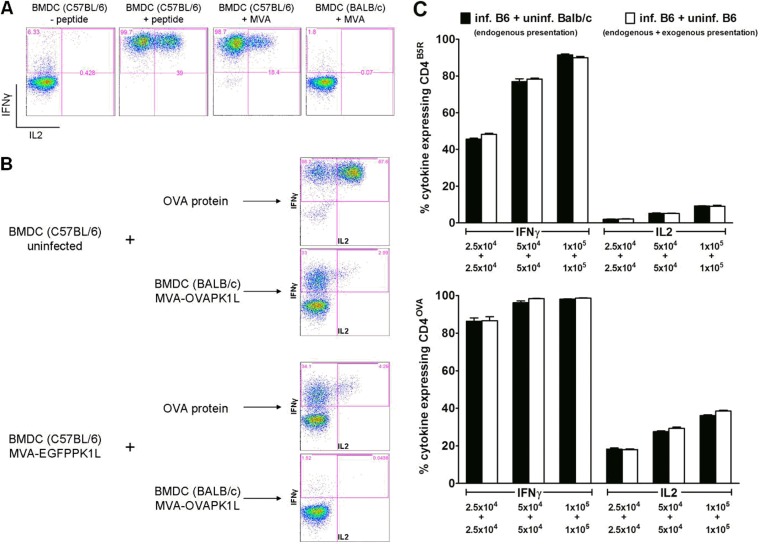
In the in vitro cell culture assay, CD4+ T cells could be stimulated only by infected BMDCs derived from MHC-matched C57BL/6 mice (MHCII: I-Ab), while no cytokine expression was obtained using BMDCs from MHC-mismatched BALB/c mice (MHCII: I-Ad) (Fig. 2A), indicating MHCII restriction of CD4+ stimulation.
FIG 2.
Validation of the cell culture system. (A) MHCII restriction of CD4+ T-cell lines. Peptide-pulsed or MVA-infected BMDCs from C57BL/6 and BALB/c mice were used as APCs for stimulation of CD4B5R. (B) Ability of uninfected and infected BMDCs to phagocytose soluble or cell-associated antigens. Uninfected or MVA-EGFP-infected (and sorted) BMDCs from C57BL/6 mice were incubated 5 h postinfection with full-length ovalbumin or were cocultured with MVA-OVA-infected MHC-mismatched BMDCs from BALB/c mice that have been additionally treated with psoralen plus UVA for 30 min 5 h postinfection to induce apoptosis. The ability to process and present soluble or cell-associated antigen on MHCII was assessed by analyzing the cytokine expression in CD4OVA cells that were added to these cultures for 14 h. (C) Determination of exogenous antigen presentation by uninfected cells in the cell culture system. Cocultures with equally increasing numbers of MVA-OVA/EGFP-infected and sorted BMDCs from C57BL/6 mice with uninfected BMDCs from either BALB/c mice (“inf. B6 + uninf. BALB/c” indicates endogenous presentation) or C57BL/6 mice (“inf. B6 + uninf. B6” indicates endogenous and exogenous presentation) were used for stimulation of CD4B5R and CD4OVA. The activation of both CD4+ T-cell lines after 14 h was analyzed by ICS and FACS analysis for IFN-γ and IL-2. Data are means and SEM (n = 3, pooled from three experiments). No significant differences (unpaired t test, two-tailed) were found between cocultures allowing only endogenous presentation and cultures allowing endogenous as well as exogenous presentation in all cell density-matched groups. All infections were performed with an MOI of 10.
We also compared the abilities of uninfected and infected BMDCs to phagocytose and present exogenous soluble antigen as well as exogenous cell-associated antigen on MHCII. We therefore tested the stimulation of CD4OVA by uninfected or MVA-EGFPPK1L-infected and sorted BMDCs from C57BL/6 mice that had been preincubated with ovalbumin (soluble antigen) or previously cocultured with MVA-OVAPK1L-infected but MHC-mismatched BMDCs from BALB/c mice which had been additionally treated with PUVA to induce apoptosis and to inactivate the virus (cell-associated antigen). As shown in Fig. 2B, uninfected BMDCs were able to stimulate CD4OVA efficiently after incubation with soluble ovalbumin and less efficiently after incubation with cell-associated antigen. In contrast, there was only marginal stimulation of CD4OVA by MVA-infected BMDCs preincubated with soluble ovalbumin and no stimulation after cocultivation with cell-associated antigen.
When an MOI of 10 was used for infection of BMDCs, about 20 to 30% of cells were uninfected, as determined by fluorescence-activated cell sorting (FACS) analysis of MVA-EGFPPK1L infected BMDCs (data not shown). Given that uninfected BMDCs were able to stimulate CD4+ by uptake of soluble and—although quite inefficiently—cell-associated antigens, we determined the actual contribution of exogenous antigen presentation by uninfected cells within the coculture assay (Fig. 2C). We therefore compared the capacity of target cell cultures consisting of MVA-OVAP7.5/EGFPPK1L-infected and sorted BMDCs from C57BL/6 mice with uninfected BMDCs from either BALB/c (not allowing exogenous presentation due to MHC mismatch) or C57BL/6 (allowing exogenous presentation) mice to stimulate CD4+ T cells. As depicted in Fig. 2C, there was comparable CD4+ T-cell activation in both target cell cultures, indicating no significant role for exogenous presentation by uninfected cells. Taken together, these data show that (i) the antigen presentation in the culture system is MHCII restricted, (ii) infected cells are unable to present exogenous cell-associated antigens, and (iii) uninfected cells do not contribute to the stimulation of CD4+ T cells within the assay system.
Autophagy mediates endogenous MHCII presentation in MVA-infected BMDCs.
To identify the intracellular pathways that might be involved in endogenous MHCII presentation during MVA infection, we modified the assay system. MVA-infected BMDCs pretreated with chemical inhibitors or derived from knockout mice to block candidate pathways were used to stimulate CD4+ T cells.
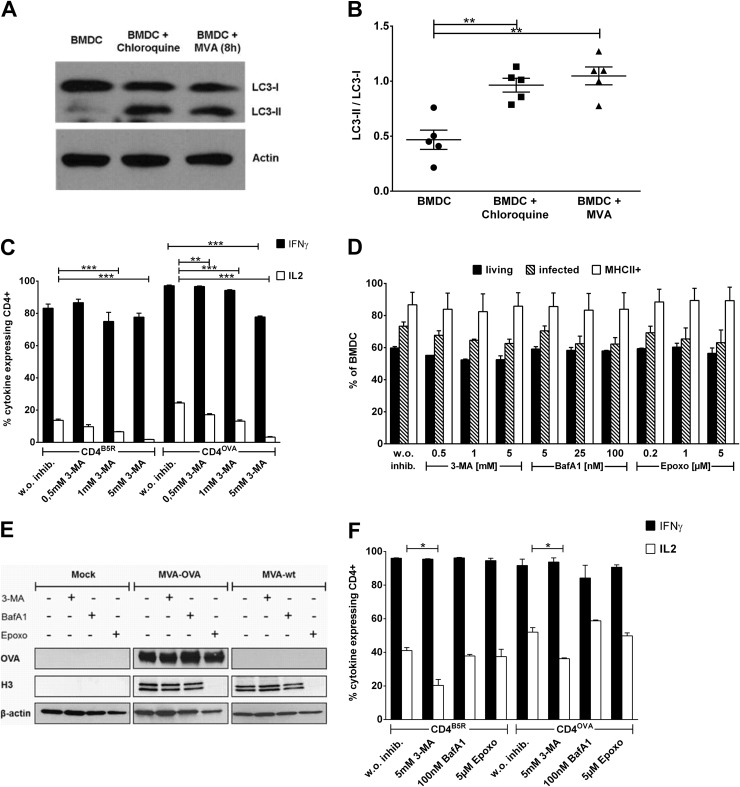
First, we analyzed whether autophagy is induced upon MVA infection in BMDCs. LC3 is a biochemical marker for this pathway, and the conversion of the cytosolic 18-kDa LC3-I to the membrane-bound 16-kDa LC3-II isoform indicates the induction of autophagy. Western blot analysis revealed that the amount of LC3-II was significantly increased in BMDCs upon MVA infection, reaching a level that is comparable to that in chloroquine-treated BMDCs that were used as positive controls for detection of LC3-I/LC3-II conversion (Fig. 3A and B). The possible role of autophagy for endogenous MHCII presentation in MVA-infected BMDCs was determined by using 3-methyladenine (3-MA) to block this pathway. Therefore, BMDCs were treated with the inhibitor, infected, and then used in the coculture system as APCs for stimulation of CD4B5R and CD4OVA. As shown in Fig. 3C, we observed a dose-dependent highly significant decrease in the stimulation of both CD4+ T-cell lines. For CD4B5R, the effect affected only the expression of IL-2, whereas for CD4OVA, the expression of IL-2 as well as IFN-γ was reduced (for IFN-γ only at the highest concentration [5 mM 3-MA]). The weaker inhibitory effect of 3-MA on CD4B5R was most likely due to the strong contribution of exogenous presentation for B5R peptide derived from virion-incorporated B5, which is not affected by 3-MA treatment. Of note, 3-MA did not impair viability, infection rates, MHCII surface expression, or the synthesis of virus-derived proteins like OVA and H3 in MVA-infected BMDCs (Fig. 3D and E). As depicted in Fig. 3F, 3-MA at the highest concentration did not affect the synthesis of IFN-γ but led to a reduction of IL-2 in CD4+ T cells. However, the decrease in IL-2 production by the direct effect of 3-MA on CD4+ T cells was considerably less than the reduction seen in the assay.
FIG 3.
Autophagy for endogenous MHCII presentation and effects of inhibitors on viability, infectibility, protein expression, and CD4+ T-cell activity. (A and B) MVA infection induces autophagy in BMDCs. (A) Western blot analysis revealed an increase of LC3-II in MVA-infected BMDCs (MOI, 10) compared to uninfected BMDCs (chloroquine treatment was used as a positive control). (B) The experiment whose results are presented in panel A was repeated 5 times (n = 5), and the increase in LC3-II/LC3-I ratio was quantified using Image J software. Error bars indicate SEM. (C) Inhibition of autophagy decreases endogenous MHCII presentation. BMDCs were treated with different concentrations of 3-MA followed by infection with MVA-OVA (MOI, 10). CD4B5R and CD4OVA were added to the culture for 14 h and the number of IFN-γ- and IL-2-expressing cells was quantified by ICS and FACS analysis. Data are means and SEM (n = 3, pooled from three experiments). (D) Inhibitors do not influence viability, infectibility, or MHCII expression in BMDCs. BMDCs were treated with the indicated inhibitors and then infected with MVA-OVA/EGFP (MOI, 10) for 6 h. Cells were subjected to live/dead staining and also stained for MHCII expression on their surfaces, and infection was assessed based on EGFP expression. FACS analysis was performed to quantify the percentage of cells that were living or infected or MHCII positive. (E) Inhibitors do not influence protein expression in BMDCs. BMDCs were treated with the indicated inhibitors (5 mM 3-MA, 100 nM BafA1, and 5 μM Epoxo) and then infected with MVA-OVA or MVA-wt (MOI, 10) for 14 h. Western blot analysis for expression of virus-derived ovalbumin and H3 was performed with β-actin as control. The blot is representative from three experiments. (F) 3-MA and epoxomicin exert a minimal effect on IL-2 expression in CD4+ T cells. BMDCs were treated with the indicated inhibitors (5 mM 3-MA, 100 nM BafA1, and 5 μM Epoxo) and pulsed with peptides. CD4B5R and CD4OVA were added to the culture for 14 h, and the numbers of IFN-γ- and IL-2-expressing CD4+ T cells were quantified by ICS and FACS analysis. Data are means and SEM (n = 3, pooled from three experiments). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (unpaired t test, two tailed).
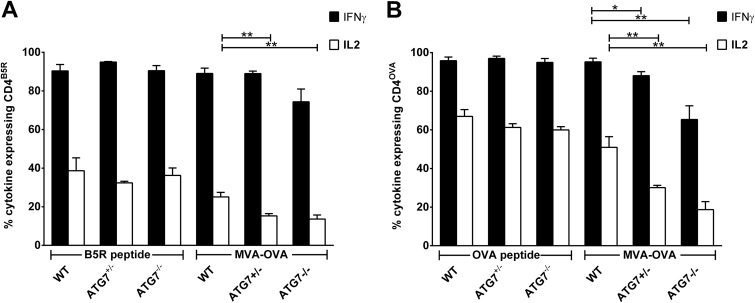
We could corroborate the results obtained with 3-MA by using hetero- or homozygous ATG7 knockout BMDCs (ATG7+/− and ATG7−/−) as APCs in the coculture system (Fig. 4A and B). Compared to wild-type littermates, BMDCs with a heterozygous ATG7 knockout exhibited a significant reduction in the stimulation of CD4+ T cells, and an even more pronounced reduction was observed in homozygous ATG7 knockout BMDCs. Of note, reverse transcription-qPCR (RT-qPCR) analysis revealed that the expression of ATG7-specific mRNA in ATG7−/− BMDCs was not completely absent but was reduced by 70 to 80% compared to the expression level in WT BMDCs, indicating that ATG7 was knocked out in only 70 to 80% of the cells, presumably due to inefficient Cre-mediated excision (data not shown). As with 3-MA, in ATG7 knockout mice CD4OVA showed significant reduction for both IL-2 and IFN-γ expression, while CD4B5R showed only reduction of IL-2 expression. Importantly, both CD4+ T-cell lines had a comparable response to peptide-pulsed WT, ATG7+/−, and ATG7−/− BMDCs.
FIG 4.
ATG7-deficient BMDCs possess an impaired ability to stimulate CD4+ T cells after infection with MVA. BMDCs from either ATG7flox/flox CD11c-Cre−/− (WT), ATG7+/flox CD11c-Cre+/− (ATG7+/−) or ATG7flox/flox CD11c-Cre+/− mice (ATG7−/−) were pulsed with peptide or were infected with MVA-OVA (MOI, 1). CD4B5R (A) and CD4OVA (B) were added to the culture for 14 h, and the numbers of IFN-γ- and IL-2-expressing CD4+ T cells were determined by ICS and FACS analysis. Data are means and SEM (n = 5 pooled from at least three experiments). *, P ≤ 0.05; **, P ≤ 0.01 (unpaired t test, two-tailed).
Taken together, our results indicate that autophagy is induced in MVA-infected BMDCs, which in turn contributes to the generation of antigenic peptides for presentation on MHCII and blockade of autophagy-impaired MHCII presentation.
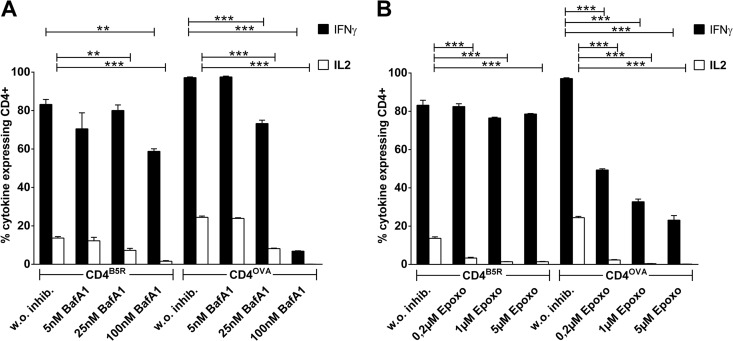
Given that autophagosomes fuse with lysosomes for degradation of the autophagic cargo, making it accessible for MHCII loading in late endosomal MHC class II loading compartments (MIIC), we hypothesized that blocking fusion of autophagic vacuoles with the lysosomal compartment would decrease presentation of endogenously expressed antigens on MHCII. To this end, we tested the effect of the lysosomotropic agent bafilomycin A. We found a strong and concentration-dependent inhibition of CD4+ T-cell stimulation for both specificities which was even more pronounced than 3-MA-induced inhibition of MHCII presentation (Fig. 5A). CD4B5R cells were less affected, indicating that the B5 antigen is predominantly presented by an exogenous route in infected APCs. In contrast, no significant impairment was observed in the controls, since bafilomycin A did not affect viability, infectibility, MHCII surface expression, synthesis of viral derived proteins in BMDCs, or the cytokine expression in the CD4+ T-cell lines (Fig. 3D, E, and F).
FIG 5.
Lysosomotropic agent and proteasome inhibitor decrease endogenous MHCII presentation. BMDCs were treated with different concentrations of bafilomycin A1 (BafA1) (A) or epoxomicin (B) and then infected with MVA-OVA (MOI, 10). CD4B5R and CD4OVA were added to the culture for 14 h, and the numbers of IFN-γ- and IL-2-expressing CD4+ T cells were quantified by ICS and FACS analysis. Data are means and SEM (n = 3, pooled from three experiments). **, P ≤ 0.01; ***, P ≤ 0.001 (unpaired t test, two-tailed).
The proteasome but neither TAP nor LAMP-2 is important for endogenous MHCII presentation of MVA-derived antigens in infected BMDCs.
As a further candidate pathway, proteasomal degradation was assessed for endogenous MHCII presentation using epoxomicin to block the proteasome. As depicted in Fig. 5B, epoxomicin decreased the ability of infected APCs to stimulate CD4B5R and CD4OVA in a concentration-dependent manner. This effect was much stronger for CD4OVA than CD4B5R, since the production of both IL-2 and IFN-γ was significantly reduced in these cells, indicating that the processing of OVA as a de novo-synthesized antigen was strictly dependent on proteasomal degradation. In contrast, the proteasomal processing of newly synthesized B5 contributed to a lesser extent to the overall presentation of this antigen. Epoxomicin did not adversely affect BMDCs within the cell culture assay (Fig. 3D and E) and had no significant influence on cytokine expression in CD4+ T cells even at the highest concentration of 5 μM (Fig. 3F).
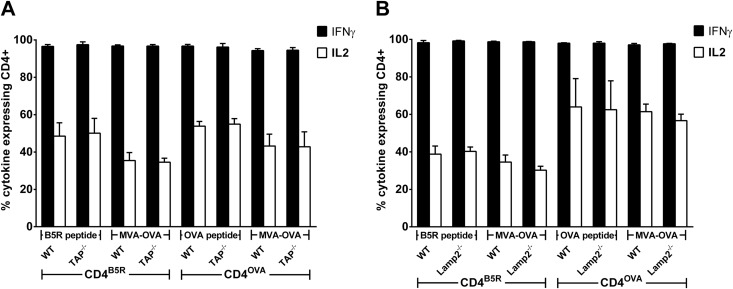
To test the role of further cytosolic proteolytic enzymes for endogenous MHCII presentation, we used PD150,606 to block calpains. However, no difference was observed for the activation of CD4+ T-cell lines between inhibitor-treated and untreated BMDCs. This result could be confirmed by using two other potent calpain inhibitors, MDL28170 (calpain inhibitor III) and Z-Leu-Leu-CHO (data not shown). Given that proteasome inhibition decreases endogenous CD4+ T-cell stimulation, we tried to identify the route which degraded cytosolic peptides might follow to be loaded on MHCII. We prepared BMDCs from knockout mice lacking expression of either the transporter associated with antigen presentation (TAP) or lysosome-associated membrane protein 2 (LAMP-2). TAP−/− (Fig. 6A) and Lamp-2−/− (Fig. 6B) BMDCs were able to stimulate CD4B5R and CD4OVA comparably to WT BMDCs after being pulsed with the corresponding peptides, indicating that MHCII expression on the surface is not altered in the absence of TAP or LAMP-2. Surprisingly, after infection with MVA-OVA, no inhibition of T-cell stimulation was observed for TAP−/− and Lamp-2−/− BMDCs for both CD4+ T-cell lines. Of note, Western blot analysis revealed no obvious reduction in LC3-II conversion upon starvation, chloroquine treatment, or MVA infection of BMDCs from Lamp-2−/− mice compared to WT control, arguing for no significant alterations of autophagy in Lamp-2−/− BMDCs (data not shown).
FIG 6.
TAP and LAMP-2 are not involved in endogenous MHCII presentation. BMDCs from TAP−/− mice (A) and Lamp-2−/− mice (B) were either pulsed with B5R or OVA peptide as a control or infected with MVA-OVA (MOI, 10). CD4B5R and CD4OVA were added to the culture for 14 h, and the numbers of IFN-γ- and IL-2-expressing CD4+ T cells were quantified by ICS and FACS analysis. Data are means and SEM (n = 4, pooled from at least 2 experiments). No significant differences (unpaired t test, two-tailed) were found for the stimulation of CD4+ T cells between WT and TAP−/− BMDCs and between WT and Lamp2−/− BMDCs in peptide-pulsed or MVA-OVA-infected groups for both CD4+ T-cell lines.
DISCUSSION
Due to their multiple functions the activation of CD4+ T cells is a key step in adaptive immunity and has broad-ranging consequences for the immune response. To date, two mechanistically distinct antigen presentation pathways have been identified for CD4+ T-cell activation. Apart from the classical pathway with presentation of phagocytosed exogenous antigens, there is emerging evidence for endogenous MHCII presentation by processing of cytosolic antigens in different infection models as well as for self and tumor antigens (9).
Several studies have investigated a possible role of endogenous MHCII presentation for antigens expressed by vaccinia viral vectors, but the results were quite inconsistent and may reflect differences in the vector backbone, choice of antigen, and detection systems used. One study investigating EBV nuclear antigens expressed by recombinant MVA in LCL cells found that CD4+ T-cell activation was not mediated via endogenous presentation but rather by intercellular antigen transfer (29). In contrast, other studies using recombinant vaccinia viruses expressing distinct forms of the M1 matrix protein from influenza A virus showed that endogenous MHCII presentation occurred and was independent of the proteasome for a long-lived form of M1 antigen (30, 31). Another study provides evidence that proteasome and TAP were important for endogenous MHCII presentation in MVA infection (32).
In this study, we characterized the molecular pathways of endogenous MHCII presentation in MVA infection using CD4+ T-cell activation by MVA-infected APCs which had been treated with inhibitors or were genetically modified to block intracellular antigen presentation pathways as a highly sensitive read-out system.
Given that ∼25% of MVA-infected cells in vivo are dendritic cells (25) which may serve as APCs for CD4+ T cells, BMDCs were chosen as APCs for the cell culture assay to closely resemble the in vivo situation. Since MVA is a widely applied boost vector in heterologous prime-boost vaccination strategies (33), we wanted to mimic the boost situation in vivo characterized by the presence of activated antigen-specific memory T cells. Thus, we generated CD4+ T-cell lines that do not need priming signals for activation as a read-out system. According to the CD4+ T-cell response in MVA-OVA-vaccinated mice, two immunodominant epitopes (among the 13 peptides tested) were used to establish CD4+ T-cell lines recognizing virus- and ovalbumin-derived epitopes, namely, B5R46–60 and OVA265–280, respectively. Both epitopes have been described as eliciting strong I-Ab-dependent CD4+ T-cell responses (34, 35). The CD4+ T-cell lines proved to be sensitive and specific indicators of the strength and quality of antigen processing and presentation by BMDCs, since we found a direct correlation between the CD4+ T-cell cytokine production and the antigen density on the APCs. This result is consistent with the observation that CD4+ T-cell lineage development and thus cytokine expression are regulated by different factors, like cytokine milieu, costimulatory signals, and TCR affinity, but also by antigen dose and ligand density (36). It has been shown that the antigen dose affects the level of cytokine production in primary CD4+ T-cell cultures (37). Interestingly, a higher MHCII-Ag density on the surface of BMDCs was required for induction of IL-2 expression than for induction of expression of IFN-γ.
Comprehensive validation of the assay system revealed that although uninfected cells were generally able to present soluble and to a much lesser extent cell-associated antigens via exogenous uptake and presentation, they did not contribute to the stimulation of CD4+ T cells in our assay system. This might be due to the fact that antigen or apoptotic BMDCs required for exogenous presentation by uninfected cells were not present in abundant amounts within the given assay time. More importantly, infected cells were completely unable to present exogenous cell-associated antigens. Presentation of soluble antigen, even when provided in abundant amounts, was significantly lower in infected cells.
Importantly, the results obtained with PUVA-treated virions indicate the existence and active involvement of distinct antigen presentation pathways for CD4+ T cell stimulation during MVA infection, differentiating between (i) virion-incorporated antigens such as B5 brought into the cell as part of the virion and (ii) ovalbumin-requiring de novo synthesis in infected cells. Psoralen intercalates into DNA and induces chemical cross-links upon exposure to UVA radiation (PUVA treatment) (38). Vaccinia virus treated with PUVA can still infect cells, but expression of larger early genes and late genes as well as replication is completely blocked (39). Given that B5R and OVA are not expressed upon PUVA treatment, the reduced but still existing response of CD4B5R after infection of BMDCs with PUVA-treated MVA indicates that the main antigenic source of B5 for the response is virions, and only a small proportion of the response is due to newly synthesized B5. This is conceivable, as B5R is a type I membrane glycoprotein incorporated into the membrane of the enveloped forms of vaccinia virus (intracellular enveloped virus [IEV], cell-associated enveloped virus [CEV], and EEV) (40). In contrast, when PUVA-treated MVA was used for infection, CD4OVA responses were completely abolished. This finding argues for the requirement of de novo synthesis of recombinant ovalbumin after infection to provide antigen for endogenous MHCII presentation. Therefore, CD4OVA activation seems to be completely based on endogenous MHCII presentation of de novo-synthesized OVA in the viral-vector-infected cell. In contrast, endogenous MHCII presentation of B5 which has been newly synthesized after infection is measurable but by far less efficient than B5 entering the cell via virions, which is likely to be processed via the classical route of MHCII presentation.
To elucidate the cellular pathways responsible for endogenous MHCII presentation in MVA-infected BMDCs, we used chemical inhibitors or BMDCs from genetically modified mice in which specific antigen presentation pathways were impaired. In professional antigen-presenting cells, autophagy is constitutively active, delivering antigens into MHCII loading compartments (41), and autophagy has already been shown to be an important pathway in endogenous MHCII presentation for intracellular self-antigens (17), model antigens (16), and viral antigens (18). Given that ectromelia virus (a related orthopoxvirus and the causative agent of smallpox in mice) has been shown to induce autophagy (42), we tested whether MVA also induces autophagy in BMDCs. LC3, the mammalian homolog of yeast Atg8, serves as a marker for autophagosome formation determined by the conversion of the cytosolic LC3-I into the membrane-bound LC3-II isoform (43). The strong increase in LC3-II in MVA-infected BMDCs revealed an induction of autophagy. 3-MA is a known inhibitor of phosphatidylinositol 3-phosphate kinase (PI3K), which is needed for induction of autophagy (44). The concentration-dependent decrease in the stimulation of both CD4+ T-cell lines by 3-MA indicates a contribution of autophagy for endogenous MHCII presentation of MVA-derived antigens in infected BMDCs. Degradation of autophagic cargo occurs by lysosomal proteases after fusion of autophagosomes with lysosomes (15). Bafilomycin A prevents lysosomal acidification, thereby inhibiting this fusion process as well as the activation of lysosomal enzymes. The decrease of CD4+ T-cell responses in infected BMDCs treated with this lysosomotropic agent strengthens the role of autophagy for endogenous MHCII presentation in MVA infection and could be confirmed by using infected ATG7-deficient BMDCs showing a significantly reduced ability to stimulate CD4+ T cells.
In search of further pathways, we found a role for proteasomes by using epoxomicin, a strong inhibitor highly specific for proteasomal degradation (45). Involvement of proteasomes in endogenous MHCII presentation in MVA infection has recently been demonstrated (32). Since B5R is expressed late and its expression is blocked by proteasome inhibitors (46), the reduced but readily detectable CD4B5R responses substantiate the idea that a high proportion of the antigenic source of B5R for MHCII presentation is brought into the cell as part of the virion. Beside the proteasome, calpains have been shown to be involved in endogenous presentation of a glutamate decarboxylase (GAD) epitope on MHCII (47). Although we used the calpain inhibitor PD150,606 as well as MDL28170 and Z-Leu-Leu-CHO, we could not detect any role for calpains in endogenous MHCII presentation for MVA-derived B5 and ovalbumin (data not shown). This could be explained by the different antigens used in both studies and the fact that calpains exhibit greater substrate selectivity than proteasomes (47, 48).
The stronger effect of bafilomycin A on T-cell activation than 3-MA indicated that lysosomal degradation for endogenous MHCII presentation occurs not only for autophagic cargo but also for antigens derived from other cellular compartments. Thus, we tested the hypothesis that proteasomally degraded antigens were directly guided into the endosomal/lysosomal compartment by investigating the role of different peptide transporters.
Proteasomally degraded proteins may be translocated into early endosomes via TAP to be loaded on recycling MHCII (32). However, we did not see involvement of TAP in endogenous presentation of the antigens investigated in this study, which could be explained by the different nature of antigens used in our study compared to the previously described antigens, considering the peptide specificity of TAP (49). This also excludes the possibility of TAP-mediated transfer of MHCII epitopes into the ER in our assay system (12).
LAMP-2 represents another reasonable candidate peptide transporter, since its role in endogenous MHCII presentation has been demonstrated (13). However, we could not find any evidence that LAMP-2 mediates endogenous MHCII presentation of the antigens investigated. The lack of the pentameric signal peptide sequence KFERQ, which is needed for recognition by LAMP-2 (14) in B5 and ovalbumin proteins, might explain this finding. LAMP-2 has been described as an important regulator of autophagosome maturation, and in LAMP-2-deficient mice, accumulation of autophagosomes is seen in many tissues, including liver, kidney, spleen, pancreas, and skeletal and heart muscles, due to an inefficient fusion with lysosomes (50). However, in macrophages and fibroblasts, LAMP-2 deficiency has no significant adverse effects on lysosomal structure and function (51). For BMDCs from Lamp-2-deficient mice, we also did not find a significant reduction of LC3-II conversion by Western blot analysis after infection, starvation, or chloroquine treatment in these cells, indicating no significant alterations of autophagy in Lamp-2−/− BMDCs. Given that TAP and LAMP-2 were not involved in antigen presentation, other peptide transporters, like TAPL (transporter associated with antigen processing like) (52), might translocate the peptides into the endosomal/lysosomal compartment and seem to be reasonable candidates for further studies to address this hypothesis.
Taken together, our data show that in MVA-infected dendritic cells, endogenous MHCII presentation of de novo-synthesized antigens occurs via two distinct routes that involve autophagic processing and/or proteasomal degradation, with further translocation into lysosomes. The involvement of calpain-mediated proteolysis as well as TAP- and LAMP-2-dependent transport was not relevant for the endogenous MHCII presentation of antigens used in this study, indicating that intrinsic features of antigens additionally determine the processing pathways that mediate endogenous MHCII loading.
The characterization of pathways contributing to presentation of CD4+ T-cell epitopes on MHCII in APCs could lead to a better understanding of immunogenic properties of pathogens. It may provide the basis for improvement of vaccine systems like MVA for enhanced CD4+ T-cell activation by directly targeting antigens for relevant MHCII presentation pathways, e.g., by fusion to LC3 (41) or to the invariant chain Ii (53) or by integrating the LAMP-2 signal sequence KFERQ to increase endogenous antigen processing and MHCII loading.
ACKNOWLEDGMENTS
Part of this work was funded by DFG grant SFB807 to R.A. and SFB456 and GRK1949 to I.D.
We thank Ronny Tao and Robert Baier for excellent technical assistance. We are much obliged to Thomas Misgeld (TUM Munich), Thomas Brocker (LMU Munich), Cytos Biotechnology AG (Schlieren, Switzerland), and Paul Saftig and Judith Blanz (University of Kiel) for providing us with ATG7flox/flox, CD11c-Cre, TAP−/−, and Lamp-2−/− mice, respectively.
REFERENCES
- 1.Trombetta ES, Mellman I. 2005. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 2.Andersen MH, Schrama D, thor Straten P, Becker JC. 2006. Cytotoxic T cells. J Investig Dermatol 126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Paul WE. 2008. CD4 T cells: fates, functions, and faults. Blood 112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Joffre OP, Segura E, Savina A, Amigorena S. 2012. Cross-presentation by dendritic cells. Nat Rev Immunol 12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 6.Rudensky A, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA Jr. 1991. Sequence analysis of peptides bound to MHC class II molecules. Nature 353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 7.Zwickey HL, Unternaehrer JJ, Mellman I. 2006. Presentation of self-antigens on MHC class II molecules during dendritic cell maturation. Int Immunol 18:199–209. doi: 10.1093/intimm/dxh363. [DOI] [PubMed] [Google Scholar]
- 8.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. 2008. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 9.Schmid D, Munz C. 2005. Immune surveillance of intracellular pathogens via autophagy. Cell Death Differ 12(Suppl 2):S1519–S1527. doi: 10.1038/sj.cdd.4401727. [DOI] [PubMed] [Google Scholar]
- 10.Eisenlohr LC. 2013. Alternative generation of MHC class II-restricted epitopes: not so exceptional? Mol Immunol 55:169–171. doi: 10.1016/j.molimm.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aichinger G, Karlsson L, Jackson MR, Vestberg M, Vaughan JH, Teyton L, Lechler RI, Peterson PA. 1997. Major histocompatibility complex class II-dependent unfolding, transport, and degradation of endogenous proteins. J Biol Chem 272:29127–29136. doi: 10.1074/jbc.272.46.29127. [DOI] [PubMed] [Google Scholar]
- 12.Malnati MS, Marti M, LaVaute T, Jaraquemada D, Biddison W, DeMars R, Long EO. 1992. Processing pathways for presentation of cytosolic antigen to MHC class II-restricted T cells. Nature 357:702–704. doi: 10.1038/357702a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. 2005. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Munz C. 2006. Autophagy and antigen presentation. Cell Microbiol 8:891–898. doi: 10.1111/j.1462-5822.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 15.He C, Klionsky DJ. 2009. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, Mautner J. 2003. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol 33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 17.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S. 2005. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A 102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. 2005. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 19.Levine B, Mizushima N, Virgin HW. 2011. Autophagy in immunity and inflammation. Nature 469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayr A, Hochstein-Mintzel V, Stickl H. 1975. Abstammung, Eigenschaften und Verwendung des attenuierten Vaccinia-Stammes MVA. Infection 3:6–14. doi: 10.1007/BF01641272. [DOI] [Google Scholar]
- 21.Meyer H, Sutter G, Mayr A. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol 72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 22.Drexler I, Heller K, Wahren B, Erfle V, Sutter G. 1998. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol 79:347–352. [DOI] [PubMed] [Google Scholar]
- 23.Drexler I, Staib C, Sutter G. 2004. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr Opin Biotechnol 15:506–512. doi: 10.1016/j.copbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verheust C, Goossens M, Pauwels K, Breyer D. 2012. Biosafety aspects of modified vaccinia virus Ankara (MVA)-based vectors used for gene therapy or vaccination. Vaccine 30:2623–2632. doi: 10.1016/j.vaccine.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Eitz Ferrer P, Potthoff S, Kirschnek S, Gasteiger G, Kastenmuller W, Ludwig H, Paschen SA, Villunger A, Sutter G, Drexler I, Hacker G. 2011. Induction of Noxa-mediated apoptosis by modified vaccinia virus Ankara depends on viral recognition by cytosolic helicases, leading to IRF-3/IFN-beta-dependent induction of pro-apoptotic Noxa. PLoS Pathog 7:e1002083. doi: 10.1371/journal.ppat.1002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastenmuller W, Gasteiger G, Gronau JH, Baier R, Ljapoci R, Busch DH, Drexler I. 2007. Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination. J Exp Med 204:2187–2198. doi: 10.1084/jem.20070489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staib C, Drexler I, Sutter G. 2004. Construction and isolation of recombinant MVA. Methods Mol Biol 269:77–100. doi: 10.1385/1-59259-789-0:077. [DOI] [PubMed] [Google Scholar]
- 28.Beeton C, Chandy KG. 2007. Preparing T cell growth factor from rat splenocytes. J Vis Exp 2007:402. doi: 10.3791/402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor GS, Long HM, Haigh TA, Larsen M, Brooks J, Rickinson AB. 2006. A role for intercellular antigen transfer in the recognition of EBV-transformed B cell lines by EBV nuclear antigen-specific CD4+ T cells. J Immunol 177:3746–3756. doi: 10.4049/jimmunol.177.6.3746. [DOI] [PubMed] [Google Scholar]
- 30.Jaraquemada D, Marti M, Long EO. 1990. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med 172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gueguen M, Long EO. 1996. Presentation of a cytosolic antigen by major histocompatibility complex class II molecules requires a long-lived form of the antigen. Proc Natl Acad Sci U S A 93:14692–14697. doi: 10.1073/pnas.93.25.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tewari MK, Sinnathamby G, Rajagopal D, Eisenlohr LC. 2005. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nat Immunol 6:287–294. doi: 10.1038/ni1171. [DOI] [PubMed] [Google Scholar]
- 33.Ramshaw IA, Ramsay AJ. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today 21:163–165. doi: 10.1016/S0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 34.Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, Peters B, Rafii-El-Idrissi Benhnia M, Hoffmann J, Su HP, Singh K, Garboczi DN, Head S, Grey H, Felgner PL, Crotty S. 2008. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity 28:847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizukami S, Kajiwara C, Ishikawa H, Katayama I, Yui K, Udono H. 2008. Both CD4+ and CD8+ T cell epitopes fused to heat shock cognate protein 70 (hsc70) can function to eradicate tumors. Cancer Sci 99:1008–1015. doi: 10.1111/j.1349-7006.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constant SL, Bottomly K. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol 15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 37.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. 1995. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med 182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson CV. 1992. Photochemical inactivation of viruses with psoralens: an overview. Blood Cells 18:7–25. [PubMed] [Google Scholar]
- 39.Tsung K, Yim JH, Marti W, Buller RM, Norton JA. 1996. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J Virol 70:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Earley AK, Chan WM, Ward BM. 2008. The vaccinia virus B5 protein requires A34 for efficient intracellular trafficking from the endoplasmic reticulum to the site of wrapping and incorporation into progeny virions. J Virol 82:2161–2169. doi: 10.1128/JVI.01971-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid D, Pypaert M, Munz C. 2007. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martyniszyn L, Szulc L, Boratynska A, Niemialtowski MG. 2011. Beclin 1 is involved in regulation of apoptosis and autophagy during replication of ectromelia virus in permissive L929 cells. Arch Immunol Ther Exp (Warsz) 59:463–471. doi: 10.1007/s00005-011-0149-7. [DOI] [PubMed] [Google Scholar]
- 43.Mizushima N, Yoshimori T, Levine B. 2010. Methods in mammalian autophagy research. Cell 140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo Y, Kanzawa T, Sawaya R, Kondo S. 2005. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 45.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. 1999. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A 96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mercer J, Snijder B, Sacher R, Burkard C, Bleck CK, Stahlberg H, Pelkmans L, Helenius A. 2012. RNAi screening reveals proteasome- and Cullin3-dependent stages in vaccinia virus infection. Cell Rep 2:1036–1047. doi: 10.1016/j.celrep.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Lich JD, Elliott JF, Blum JS. 2000. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med 191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorimachi H, Mamitsuka H, Ono Y. 2012. Understanding the substrate specificity of conventional calpains. Biol Chem 393:853–871. doi: 10.1515/hsz-2012-0143. [DOI] [PubMed] [Google Scholar]
- 49.Burgevin A, Saveanu L, Kim Y, Barilleau E, Kotturi M, Sette A, van Endert P, Peters B. 2008. A detailed analysis of the murine TAP transporter substrate specificity. PLoS One 3:e2402. doi: 10.1371/journal.pone.0002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. 2000. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 51.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. 2007. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J 26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bangert I, Tumulka F, Abele R. 2011. The lysosomal polypeptide transporter TAPL: more than a housekeeping factor? Biol Chem 392:61–66. doi: 10.1515/BC.2011.007. [DOI] [PubMed] [Google Scholar]
- 53.Diebold SS, Cotten M, Koch N, Zenke M. 2001. MHC class II presentation of endogenously expressed antigens by transfected dendritic cells. Gene Ther 8:487–493. doi: 10.1038/sj.gt.3301433. [DOI] [PubMed] [Google Scholar]