ABSTRACT
Highly pathogenic avian influenza virus infection is associated with severe mortality in both humans and poultry. The mechanisms of disease pathogenesis and immunity are poorly understood although recent evidence suggests that cytokine/chemokine dysregulation contributes to disease severity following H5N1 infection. Influenza A virus infection causes a rapid influx of inflammatory cells, resulting in increased reactive oxygen species production, cytokine expression, and acute lung injury. Proinflammatory stimuli are known to induce intracellular reactive oxygen species by activating NADPH oxidase activity. We therefore hypothesized that inhibition of this activity would restore host cytokine homeostasis following avian influenza virus infection. A panel of airway epithelial and immune cells from mammalian and avian species were infected with A/Puerto Rico/8/1934 H1N1 virus, low-pathogenicity avian influenza H5N3 virus (A/duck/Victoria/0305-2/2012), highly pathogenic avian influenza H5N1 virus (A/chicken/Vietnam/0008/2004), or low-pathogenicity avian influenza H7N9 virus (A/Anhui/1/2013). Quantitative real-time reverse transcriptase PCR showed that H5N1 and H7N9 viruses significantly stimulated cytokine (interleukin-6, beta interferon, CXCL10, and CCL5) production. Among the influenza-induced cytokines, CCL5 was identified as a potential marker for overactive immunity. Apocynin, a Nox2 inhibitor, inhibited influenza-induced cytokines and reactive oxygen species production, although viral replication was not significantly altered in vitro. Interestingly, apocynin treatment significantly increased influenza virus-induced mRNA and protein expression of SOCS1 and SOCS3, enhancing negative regulation of cytokine signaling. These findings suggest that apocynin or its derivatives (targeting host responses) could be used in combination with antiviral strategies (targeting viruses) as therapeutic agents to ameliorate disease severity in susceptible species.
IMPORTANCE Highly pathogenic avian influenza virus infection causes severe morbidity and mortality in both humans and poultry. Wide-spread antiviral resistance necessitates the need for the development of additional novel therapeutic measures to modulate overactive host immune responses after infection. Disease severity following avian influenza virus infection can be attributed in part to hyperinduction of inflammatory mediators such as cytokines, chemokines, and reactive oxygen species. Our study shows that highly pathogenic avian influenza H5N1 virus and low-pathogenicity avian influenza H7N9 virus (both associated with human fatalities) promote inactivation of FoxO3 and downregulation of the TAM receptor tyrosine kinase, Tyro3, leading to augmentation of the inflammatory cytokine response. Inhibition of influenza-induced reactive oxygen species with apocynin activated FoxO3 and stimulated SOCS1 and SOCS3 proteins, restoring cytokine homeostasis. We conclude that modulation of host immune responses with antioxidant and/or anti-inflammatory agents in combination with antiviral therapy may have important therapeutic benefits.
INTRODUCTION
Zoonotic, highly pathogenic avian influenza (HPAI) H5N1 virus remains an ongoing pandemic threat worldwide following its emergence in 1996 (∼60% mortality) (1). This virus has now become endemic in many regions of the globe ensuring ongoing opportunities for virus evolution through acquisition of point mutations or swapping of gene segments. More recently, a novel low-pathogenicity avian influenza (LPAI) H7N9 virus (A/Anhui/1/2013 H7N9) has emerged in China with a mortality rate of 31% (2). The H7N9 virus contains mutations that are associated with adaptation in mammals and respiratory droplet transmission in ferrets (Q226L in HA and E627K in PB2) (3, 4). If this virus also becomes endemic in poultry, as per H5N1, opportunities for human H7N9 transmission may increase given the lack of preexisting immunity in the population.
Cytokines are essential for the resolution of the infection. However, there is growing evidence suggesting that elevated host cytokine dysregulation (“cytokine storm”) contributes to many of the clinical signs associated with avian influenza virus infection. Elevated cytokine and chemokine levels (e.g., gamma interferon-induced protein 10 [IP-10], macrophage inflammatory protein 1β [MIP-1β], interleukin-6 [IL-6], interferon alpha [IFN-α], IFN-γ, monokine [MIG], monocyte chemoattractant protein 1 [MCP-1], and IL-8) have been noted in the serum of H7N9- and H5N1-infected patients (5, 6). Moreover, significantly higher concentrations of cytokines and chemokines have been found in fatal H5N1 and H7N9 virus infection cases (6, 7). This dysregulation has also been noted using in vitro human primary airway cell culture systems following HPAI H5N1 or H1N1 virus infection with elevated expression of IL-6, IP-10, IFN-β, tumor necrosis factor alpha (TNF-α), and RANTES (regulated on activation, normal T cell expressed and secreted) compared to LPAI virus controls (8).
A number of factors are thought to contribute to overall cytokine dysregulation, one of which is the expression of reactive oxygen species (ROS). Previous studies have demonstrated that infection with influenza A viruses induces a rapid influx of inflammatory cells into lungs resulting in the production ROS (9). ROS are essential, potent microbicidal agents that are known to kill ingested microorganisms within phagosomes. Excess production of ROS, however, has been associated with acute lung injury contributing significantly to morbidity and mortality following avian influenza virus infection (10).
Nox2 is the catalytic subunit of the phagocyte NADPH oxidase (NOX), a large multisubunit enzyme complex involved in phagocytic ROS production (11). Moreover, Nox2-containing NADPH oxidase is a major source of superoxide production in phagocytes, neutrophils, macrophages, and dendritic cells after acute infection (11). It is also important to note that Nox2 expression has been detected in human lung epithelial A549 cells (12). Previous studies using low-pathogenicity mouse-adapted H1N1 or H3N2 influenza virus strains have shown that administration of apocynin, a Nox2 inhibitor, improves disease outcomes after infection (9). The anti-inflammatory effects of apocynin administration observed in wild-type mice mimicked responses in Nox2−/y mice, suggesting the involvement of Nox2 in the regulation of cytokine production following influenza virus infection in mice (9). These results, along with others, have contributed to an increased focus on the development of intervention strategies with the ability to modulate deleterious host immune responses, such as those triggered by ROS, in an attempt to ameliorate disease severity (9). This is particularly important given the documentation of H5N1 and H7N9 influenza virus antiviral drug resistance (2, 13). The data presented here provide important mechanistic evidence to suggest that apocynin or its derivatives could be used therapeutically to reduce immunopathology following H5N1 or H7N9 avian virus infection, resulting in amelioration of disease severity.
The suppressors of cytokine signaling (SOCS) family members are key regulators of cytokine homeostasis. Their expression is tightly controlled to avoid excessive inflammatory damage while maintaining effective control of pathogens. SOCS1 and SOCS3 are two potent signaling suppressors that can be induced by IL-6 and IFNs (types I and II), and both have the ability to regulate IL-6 and IFN signaling in vitro (14). It is therefore not surprising that one of the mechanisms used by influenza A viruses to counteract host antiviral immunity is the suppression of type I IFN signaling via induction of SOCS1 and SOCS3.
Although significant progress has been made in our understanding of H5N1 and H7N9 viruses at the molecular level, the mechanisms that determine disease pathogenesis and immunity in the host are poorly understood. We undertook a comprehensive comparative analysis of immune responses in airway epithelial cells and macrophages derived from different host backgrounds following HPAI H5N1, LPAI H7N9, LPAI H5N3, and PR8 H1N1 virus infection. Our results suggested that treatment with the ROS inhibitor apocynin abrogated inflammatory responses via a SOCS1- and SOCS3-mediated mechanism and involved the transcription factor FoxO3 and the TAM receptor tyrosine kinase, Tyro3. Our findings not only highlight the importance and complexity of host-pathogen interactions, but also identify pathways that can be targeted for the development of immunomodulatory strategies to control overactive host immune responses. As such, we propose and support the synergistic use of host-targeting strategies with currently available antiviral therapies to ameliorate severe disease following avian influenza virus infection.
MATERIALS AND METHODS
Cell culture.
All cell lines were provided by the tissue culture facility of CSIRO Australian Animal Health Laboratory (AAHL). A549 adenocarcinomic human alveolar basal epithelial cells, Madin-Darby canine kidney (MDCK) cells, chicken HD-11 macrophage and DF-1 embryo fibroblast were maintained in Ham F-12 K medium (Gibco), RPMI 1640 medium (Invitrogen), and Dulbecco modified Eagle medium (Gibco), respectively, supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco), and maintained at 37°C, 5% CO2.
Viruses.
Viral stocks of A/Puerto Rico/8/1934 H1N1 (PR8) were obtained from the University of Melbourne. LPAI A/duck/Victoria/0305-2/2012 H5N3 virus (H5N3) was obtained from the State of Victoria Department of Primary Industries, the HPAI A/chicken/Vietnam/0008/2004 H5N1 (H5N1) and LPAI A/Anhui/1/2013 H7N9 (H7N9) viruses were obtained from CSIRO AAHL and the World Health Organization (WHO) Collaborating Centre for Reference and Research on Influenza (Victoria, Australia), respectively, and were prepared using standard methods in 10-day-old embryonated eggs. A single stock of virus was prepared for use in all assays. All H5N3, H7N9, and H5N1 experiments were performed in biosafety level 3 laboratories (BSL3) at CSIRO AAHL.
In vitro infection studies.
Cells were infected with influenza A viruses at multiplicities of infection (MOI) of 0.01 or 2 as indicated in the text and as previously described (8). Fifty percent tissue culture infectious dose (TCID50) assays were carried out on MDCK cells to determine virus replication in various cell lines. The endpoint of viral dilution leading to cytopathic effect in 50% of inoculated wells was estimated by using the Spearman and Kärber method (15). For low-pathogenicity virus (PR8, H5N3, and H7N9) infection studies, a final concentration of 0.5 μg/ml TPCK (l-1-tosylamide-2-phenylethyl chloromethyl ketone)-treated trypsin (Worthington) was included in the medium postinoculation.
Detection of ROS and immunofluorescent staining.
Monolayers of cells were infected with influenza virus in the presence of 1% dimethyl sulfoxide (DMSO) control or 1 mM apocynin. At 24 h postinfection (hpi), ROS levels in the cytoplasm of live cells was measured by adding 5 μM CellROX Deep Red reagent according to the manufacturer's instructions. In parallel experiments, cells were fixed with 4% Paraformaldehyde, permeabilized, and incubated with anti-influenza nucleoprotein (NP) monoclonal antibody (kindly provided by Paul Monaghan, CSIRO AAHL) and Alexa Fluor 488-coupled secondary goat anti-mouse IgG(H+L) antibody (Dako). Nuclei were rapidly stained with DAPI (4′,6′-diamidino-2-phenylindole). Fluorescence staining was digitally scanned using a Thermo Fisher Scientific CellInsight personal cell imager, and intensities were quantified by CellInsight software (Thermo Fisher Scientific).
Quantitative real-time reverse transcriptase PCR (qRT-PCR).
Confluent monolayers of A549 cells were infected at an MOI of 2, while DF-1 and HD-11 cells that are more sensitive to HPAI influenza virus infection were consistently infected with all influenza virus strains with an MOI of 0.01. Total RNA was extracted from cells using RNeasy minikit (Qiagen) and treated with RNase-free DNase (Promega) according to the manufacturer's instructions. cDNA was prepared using SuperScript III first-strand synthesis SuperMix (Invitrogen). The mRNA concentrations of genes of interest were assessed using TaqMan gene expression assays (Applied Biosystems) with commercial TaqMan primers and probes, with the exception of chicken β-actin (forward primer, 5′-TGCGTGACATCAAGGAGAAG-3′; reverse primer, 5′-GACCATCAGGGAGTTCATAGC-3′; probe, 5′-FAM-TGTGCTACGTCGCACTGGATTTCGA-NFQ-3′) (16) and influenza matrix (M) gene (forward primer, 5′-CTTCTAACCGAGGTCGAAACGTA-3′; reverse primer, 5′-GGTGACAGGATTGGTCTTGTCTTTA-3′; probe, 5′-FAM-TCAGGCCCCCTCAAAGCCGAG-NFQ-3′) (17). Probes that expand exons were chosen wherever possible. All assays were performed in duplicate using an Applied Biosystems StepOnePlus real-time PCR system. The PCR conditions were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Gene expression was normalized to β-actin mRNA using the 2–ΔΔCT method where expression levels were determined relative to uninfected cell controls. Copy numbers of M gene were determined by generation of a standard curve.
Western blot analysis.
Infected or uninfected A549 cells were lysed in RIPA buffer supplemented with protease inhibitors (cOmplete, Mini, EDTA-free protease inhibitor cocktail tablets; Roche). Equal amount of proteins were loaded and resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Bio-Rad). Membranes were probed with antibodies against SOCS1 (mouse monoclonal antibody; MBL), SOCS3 (mouse monoclonal antibody; BioLegend), phospho-FoxO3 phosphorylated at serine 253 (rabbit polyclonal antibody; Cell Signaling) (18), followed by incubation with horseradish peroxidase (HRP)-conjugated sheep anti-mouse secondary antibody (Millipore) or goat anti-rabbit IgG secondary antibody (Life Technologies) wherever appropriate. β-Actin (rabbit monoclonal antibody, HRP conjugated; Cell Signaling) or GAPDH (glyceraldehyde-3-phosphate dehydrogenase; rabbit monoclonal antibody; Cell Signaling) were used as loading controls. Proteins were visualized using Pierce ECL Plus Western blotting substrate (Thermo Scientific). The protein band intensity was quantified using Fiji software (version 1.49J10) (19), normalized against β-actin or GAPDH, and expressed as fold changes compared to the control.
Statistical analysis.
Differences in cytokine and chemokine mRNA levels between experimental groups were evaluated by two-way analysis of variance (ANOVA), followed by a Bonferroni multiple-comparison test. Differences in immunofluorescence or protein densitometry between two experimental groups were evaluated by using the Student t test. Differences were considered significant at a P value of <0.05. The data are shown as means ± the standard deviations (SD). The statistical analyses were performed by using GraphPad Prism for Windows (v5.02).
RESULTS
In vitro influenza A virus replication kinetics.
The in vitro replication kinetics of A/Puerto Rico/8/1934 H1N1 (PR8), A/duck/Victoria/0305-2/2012 (H5N3) and A/chicken/Vietnam/0008/2004 (H5N1) viruses in all five cell lines was determined and compared using TCID50 assays. The H7N9 virus emerged in 2013 and, although asymptomatic in birds, shows significant mortality in humans (5). As such, H7N9 replication kinetics were included in our analysis, and replication was compared in the A549 and HD-11 cell lines. Virus replication in all cell lines followed a similar pattern: H5N1 > PR8 ≥ H5N3 (Table 1). H5N1 viruses had comparable replication kinetics in DF-1 and HD-11 cells, both of which were higher than that observed for A549 cells (Table 1).
TABLE 1.
Comparative analysis of viral replication in supernatants of influenza virus-infected cells using the TCID50 assay
| Virus and time postinfection (h) | Mean log(TCID50/ml) ± SDa |
||
|---|---|---|---|
| A549 | DF-1 | HD-11 | |
| PR8 H1N1 | |||
| 3 | 3.50 ± 0.82 | 2.88 ± 0.52# | 3.00 ± 0.53 |
| 6 | 2.75 ± 0.50### | 3.13 ± 0.52## | 3.25 ± 0.71# |
| 18 | 4.25 ± 0.50### | 4.13 ± 1.06### | 4.88 ± 1.41### |
| 24 | 4.75 ± 0.50### | 4.25 ± 0.89### | 6.25 ± 0.71**###††† |
| LPAI H5N3 | |||
| 3 | 1.66 ± 0.35### | 1.75 ± 0.50### | 1.50 ± 0.00### |
| 6 | 1.63 ± 0.35### | 3.25 ± 0.50*** | 2.25 ± 0.50###† |
| 18 | 2.00 ± 0.58### | 3.25 ± 0.50**### | 2.75 ± 0.50### |
| 24 | 3.50 ± 0.76### | 3.75 ± 0.50### | 8.00 ± 0.58**#††† |
| HPAI H5N1 | |||
| 3 | 4.25 ± 0.71 | 4.00 ± 0.58 | 4.00 ± 0.58 |
| 6 | 4.75 ± 0.50 | 4.50 ± 0.00 | 4.50 ± 0.00 |
| 18 | 6.50 ± 0.93 | 9.75 ± 0.50*** | 9.50 ± 0.00*** |
| 24 | 7.13 ± 0.92 | 9.25 ± 0.50*** | 9.50 ± 0.00*** |
| LPAI H7N9 | |||
| 3 | 2.25 ± 0.35### | NDb | 1.00 ± 0.87### |
| 6 | 2.50 ± 0.00### | ND | 3.50 ± 0.87 |
| 18 | 4.00 ± 0.71 | ND | 5.50 ± 1.75### |
| 24 | 7.13 ± 0.18 | ND | 6.42 ± 1.88### |
*, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to the corresponding virus infection in A549 cells at the same time point). #, P < 0.05; ##, P < 0.01; ###, P < 0.001 (compared to the H5N1 counterparts within the same cell line). †, P < 0.05, †††, P < 0.001 (DF-1 versus HD-11). Data are expressed as the means from three individual experiments and were analyzed with two-way ANOVA using a Bonferroni post-test.
ND, not determined.
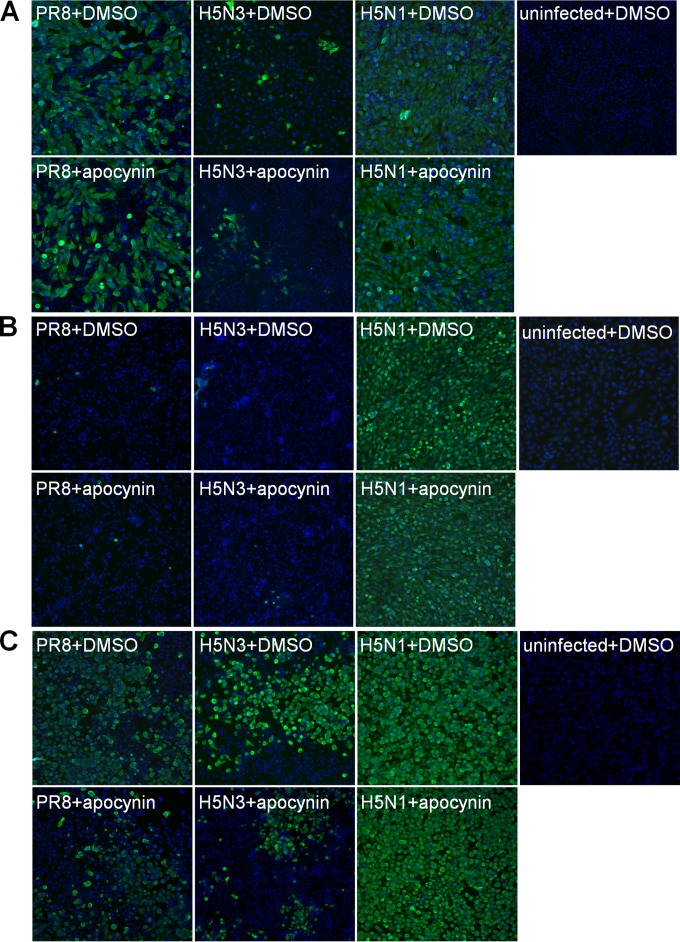
The sensitivity of avian cell lines to H5N1 virus was validated through the use of anti-influenza NP immunofluorescence staining. At an MOI of 0.01, NP protein expression in infected chicken cell lines (Fig. 1B and C) was comparable to that observed in A549 (Fig. 1A). It is important to note that A549 cells were infected at an MOI of 2. These results clearly suggest that H5N1 viruses are capable of infecting avian and mammalian cell lines from a range of hosts and that avian cells are more susceptible to avian influenza virus infection.
FIG 1.
Immunofluorescence staining of influenza-infected cells. The influenza viral NP protein (green) was detected by immunofluorescence using a mouse anti-NP monoclonal antibody in A549 (A), DF-1 (B), and HD-11 (C) cells infected with PR8, H5N3, or H5N1 in the presence of 1% DMSO vehicle control or 1 mM apocynin at 24 hpi. Uninfected cells treated with 1% DMSO for 24 h were used as negative controls. DAPI staining (blue) shows the total nuclei.
The low-pathogenicity H5N3 avian virus replicated most efficiently in the chicken macrophage HD-11 cell line (Table 1). This was consistent with stronger NP protein immunofluorescence staining (Fig. 1C). Our data suggest that avian macrophages are primary targets for influenza viruses.
H7N9 virus had comparable replication kinetics in A549 and HD-11 cells (Table 1), suggesting that differences in human and avian pathogenicity is not related to replication capacity in these two hosts under these conditions. Compared to H5N1 virus, H7N9 replication kinetics were significantly lower in HD-11 cells, and at early time points (3 and 6 hpi) in A549 cells (Table 1).
Expression of cytokines and chemokines following infection with influenza A viruses.
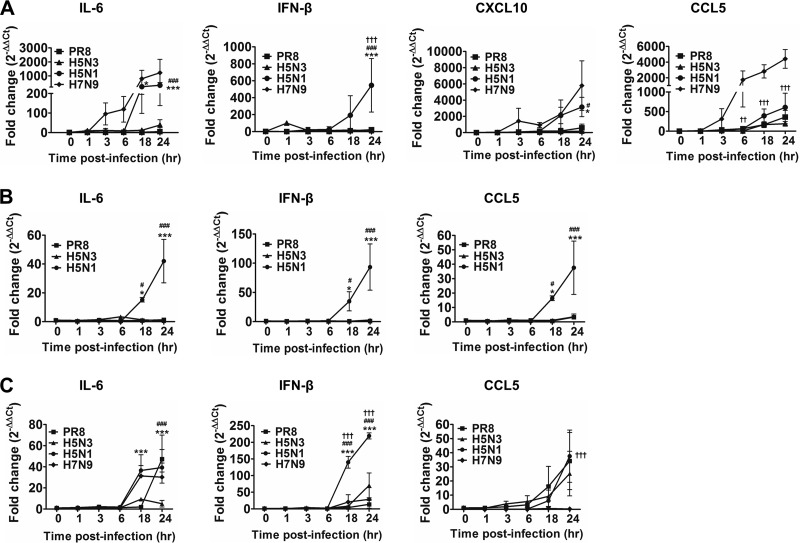
IL-6, IFN-β, CXCL10, and CCL5 levels have previously been shown to be elevated in patients and animals infected with H5N1 or 1918 “Spanish Flu” (6, 20). We therefore set out to determine expression of these above-mentioned cytokines and chemokines following PR8, H5N3, and H5N1 virus infection in all cell lines using qRT-PCR. It is important to note that the chemokine CXCL10 is not expressed in chickens (21). As such, CXCL10 was not assessed in DF-1 and HD-11 cells. Finally, H7N9-induced cytokine and chemokine production was only examined in A549 airway epithelial cell lines and HD-11 macrophage cell lines, two cell types that are major targets of influenza A viruses.
In general, among the virus strains tested, H5N1 consistently induced significantly higher levels of IL-6, IFN-β, and CXCL10 compared to PR8- and H5N3-infected cells (Fig. 2), which is consistent with elevated cytokine profiles described in the literature for H5N1 infection in patients (6) and animals (20). Comparison of the two avian cell lines showed that only minimal cytokine production was induced in DF-1 cells by PR8 and H5N3 viruses (Fig. 2B).
FIG 2.
Cytokine and chemokine gene expression profiles in influenza virus-infected human and chicken cell lines. (A to C) A549 cells (A), DF-1 cells (B), and HD-11 (C) cells were infected with influenza viruses as indicated. Differences were expressed as the fold change compared to uninfected cells calculated using the 2–ΔΔCT method. The data shown are the means ± the SD (*, P < 0.05; ***, P < 0.001 [PR8 versus H5N1]; #, P < 0.05; ###, P < 0.001 [H5N1 versus H5N3]; †††, P < 0.001 [H5N1 versus H7N9]).
CXCL10, also known as IP-10, is produced by various cell types in response to IFN-γ and is elevated during viral infection (22). In A549 cells, CXCL10 induction by H5N1 virus was considerably higher than that observed for PR8 or H5N3 virus at ≥18 hpi (Fig. 2A). Interestingly, H5N1 and H7N9 induced similar levels of IL-6 and CXCL10 mRNA expression in A549 cells (Fig. 2A) and IL-6 in HD-1 cells (Fig. 2C), which is consistent with clinical observations of patients infected with H5N1 and H7N9 where similar levels of IL-6 and CXCL10 were detected in sera (5). IFN-β mRNA expression was not significantly elevated following H7N9 infection in both A549 and HD-11 cells (Fig. 2A and C).
CCL5, also known as RANTES, is induced in response to inflammatory stimuli, such as respiratory syncytial virus (23) and influenza A virus infection (24), acting as a potent chemoattractant for leukocytes at inflammatory sites. Interestingly, although not statistically different, the trend associated with CCL5 expression favored H5N1 > PR8 > H5N3 viruses in A549 airway epithelial cells (Fig. 2A) and chicken macrophage HD-11 cells (Fig. 2C). Only H5N1 significantly upregulated CCL5 in the DF-1 chicken fibroblast cells (Fig. 2B). Interestingly, following H7N9 infection, CCL5 mRNA expression was not altered in HD-11 cells but was highly upregulated in A549 cells (5,310-fold increase) compared to H5N1 infection (870-fold increase) (Fig. 2A). The contrast in CCL5 mRNA expression in mammalian versus avian cell lines is intriguing as H7N9 infection can be fatal in humans yet asymptomatic in birds. These results suggest that CCL5 levels mirror the state of the host inflammatory response. Indeed, high levels of CCL5 in airway epithelial cells have been previously correlated with respiratory virus infection (23, 24). We therefore propose that CCL5 could be used as a potential biomarker for monitoring the state of the host immune response during avian influenza virus infection in mammalian and avian species.
Inhibition of influenza A virus-induced cytokine and chemokine production by the Nox2 inhibitor, apocynin.
Previous studies involving Nox2-knockout mice infected with laboratory strains of influenza virus (X31, H3N2; PR8, H1N1) showed reduced inflammatory infiltrates, decreased production of ROS, and amelioration of disease compared to wild-type controls (9). This response was also mimicked in vivo using the Nox2 inhibitor, apocynin. With this in mind, we analyzed influenza virus-induced cytokine and chemokine production after in vitro influenza virus infection in the presence or absence of apocynin. In A549 cells, H5N1-induced IL-6 (330-fold), IFN-β (546-fold), and CXCL10 (2,844-fold) mRNA expression was significantly reduced following the addition of apocynin (Fig. 3A). PR8- and H5N3-induced cytokine production in A549 cells was not markedly affected (Fig. 3A). H5N1-induced IL-6 mRNA expression in DF-1 cells, as well as IL-6, IFN-β, and CCL5 mRNA expression in HD-11 cells was significantly reduced by the addition of apocynin (Fig. 3B). Interestingly, elevated IFN-β expression was only halved by apocynin in HD-11 cells (Fig. 3C), suggesting that apocynin treatment could preserve antiviral activity while reducing overproduction of inflammatory cytokines in chicken cells (Fig. 3C).
FIG 3.
Anti-inflammatory effects of apocynin in human and chicken cell lines infected with influenza viruses. (A to C) A549 cells (A), DF-1 cells (B), and HD-11 (C) cells were infected with influenza viruses as indicated in the presence of 1% DMSO control or 1 mM apocynin for 24 h. Negative controls included uninfected cells cultured in medium supplemented with 1% DMSO or 1 mM apocynin. The level of influenza M gene was expressed as the log of its cDNA copy number relative to 106 cells. The data shown are the means ± the SD (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [PR8 versus H5N1]; #, P < 0.05; ##, P < 0.01; ###, P < 0.001 [H5N1 versus H5N3]; †, P < 0.05; ††, P < 0.01; †††, P < 0.001 [H5N1 versus H7N9]).
Apocynin treatment of mice in vivo has been shown to reduce inflammation and ROS production and was associated with a small decrease in overall virus titer in the lung (9). We therefore assessed the effects of apocynin on viral replication using qRT-PCR and immunofluorescence staining for each virus and cell type. Apocynin treatment did not affect matrix (M) gene copy number in vitro with numbers comparable between DMSO control and apocynin-treated groups after infection in all cell types at 24 hpi (Fig. 3). These observations were further validated using anti-influenza NP immunofluorescence staining. Similar levels of influenza NP protein were detected between DMSO control and apocynin-treated cells (A549 [Fig. 1A], DF-1 [Fig. 1B], and HD-11 cells [Fig. 1C]) infected with PR8, H5N3, or H5N1 virus at 24 hpi.
Apocynin treatment reduces ROS production in influenza virus-infected cells.
Activated inflammatory cells and infected epithelial cells are thought to produce large amounts of ROS after infection with influenza virus leading to the induction of NF-κB and the production of a variety of cytokines (25, 26). Inhibition of Nox2-mediated ROS production may therefore facilitate reductions in cytokine expression following infection. Our observations show that influenza-stimulated ROS production correlated with elevated levels of cytokine. Moreover, H5N1 infection resulted in greater ROS production compared to that induced by PR8 or H5N3 (Fig. 4). Apocynin treatment significantly inhibited influenza virus-induced ROS production when used with all three viruses and all cell types (Fig. 4).
FIG 4.
Apocynin reduced ROS production in influenza-infected human and chicken cell lines. (A to C) A549 cells (A), DF-1 cells (B), and HD-11 cells (C) were infected with influenza viruses as indicated in the presence of 1% DMSO control or 1 mM apocynin for 24 h. Uninfected cells cultured in medium containing 1% DMSO were used as negative controls. ROS production (red) was detected by immunofluorescence with CellROX Deep Red reagent, and the total nuclei were shown with DAPI staining (blue). Fluorescence was analyzed and quantified using a CellInsight system. ROS fluorescence was normalized against DAPI staining. The data represent the mean ROS fluorescence ± the SD from three experiments (**, P < 0.01; ***, P < 0.001 [compared to H5N1-infected cells with the same DMSO or apocynin treatment]; ##, P < 0.01; ###, P < 0.001 [compared to H5N1-infected cells treated with DMSO control]).
Apocynin increases mRNA and protein expression of SOCS1 and SOCS3 in influenza A virus-infected A549 cells.
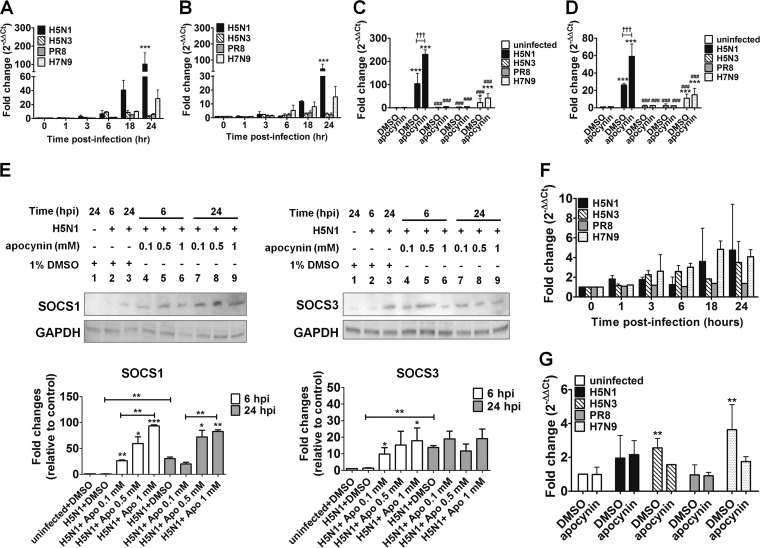
SOCS1 and SOCS3 are the key negative regulators of cytokine signaling. Previous research suggests that influenza viruses manipulate SOCS expression and function to modulate antiviral cytokine production to promote virus replication and survival (27, 28). In A549 cells, SOCS1 (Fig. 5A) and SOCS3 (Fig. 5B) mRNA was detected over a 24-h time course with all influenza viruses tested, although the kinetics of expression were clearly different for each virus. SOCS1 and SOCS3 mRNA levels were elevated slightly but not significantly by PR8 and H5N3 influenza virus infection (Fig. 5A and B). In contrast, H5N1 infection significantly upregulated SOCS1 (101-fold increase) and SOCS3 (45-fold increase) mRNA expression, peaking at 24 hpi compared to uninfected controls (Fig. 5A and B). The H7N9 virus stimulated similar patterns of SOCS1 and SOCS3 gene expression as H5N1, but ∼4-fold less (Fig. 5A and B).
FIG 5.
Expression of SOCS1, SOCS3, and SOCS2 in A549 cells infected with PR8, H5N3, H5N1, or H7N9. SOCS1 (A) and SOCS3 (B) gene expression levels in A549 cells after influenza virus infection were measured by qRT-PCR over a 24-h time course. The effects of apocynin on SOCS1 (C) and SOCS3 (D) gene expression in uninfected and infected A549 cells were also determined at 24 hpi. The data shown are the means ± the SD. *, P < 0.05; ***P < 0.001 (compared to uninfected cells in medium with 1% DMSO); ###, P < 0.001 (compared to H5N1); †††, P < 0.001 (significantly different between infected cells treated with DMSO control and apocynin). (E) Protein expression of SOCS1 and SOCS3 in A549 cells infected with H5N1 in the absence or presence of 0.1, 0.5, or 1 mM apocynin at 6 or 24 hpi. Representative Western blots and quantification of protein band intensity from three individual experiments are shown. The protein band intensity data represent the means ± the SD (*, P < 0.05; **, P < 0.01; ***, P < 0.001). SOCS2 gene expression levels were also analyzed during a 24-h time course (F) or at 24 hpi in the presence of 1% DMSO or 1 mM apocynin (G). The “0” time point represents uninfected cells cultured in medium only.
We next investigated whether the inhibition on cytokine production by apocynin involved upregulation of SOCS1 and SOCS3 gene expression in influenza A virus-infected A549 cells. Both PR8 and H5N3 failed to stimulate significant SOCS1 and SOCS3 mRNA expression and treatment with apocynin did not alter expression levels (Fig. 5C and D). H7N9 infection significantly increased SOCS1 (23-fold increase) and SOCS3 (11-fold increase) mRNA expression but was not affected by apocynin (Fig. 5C and D). In contrast, apocynin significantly enhanced H5N1-induced upregulation of both SOCS1 (230-fold increase; Fig. 5C) and SOCS3 mRNA expression (60-fold increase; Fig. 5D).
As H5N1 infection induced high levels of SOCS1 and SOCS3 mRNA that were further augmented by apocynin treatment, we next analyzed their protein levels by Western blotting in A549 cells treated with apocynin (0.1, 0.5, and 1 mM) at 6 and 24 hpi to validate our gene expression data. The SOCS1 and SOCS3 protein expression profile strongly correlated with the kinetics of mRNA expression following H5N1 infection (Fig. 5A and B). Minimal amounts of SOCS1 and SOCS3 proteins were detected at 6 hpi, but were clearly visible at 24 hpi (Fig. 5E). The addition of apocynin enhanced SOCS1 protein expression in a time- and dose-dependent manner (Fig. 5E). Similar results were also observed for SOCS3, although SOCS3 was not as highly promoted as SOCS1 (Fig. 5E). The impact of apocynin on SOCS1 and SOCS3 protein expression occurred rapidly (6 hpi), suggesting that posttranslational protein modification, but not de novo protein synthesis, was affected. These results suggest that the addition of apocynin to cultures contributes to the inhibition of influenza virus-induced hypercytokinemia through an as yet fully defined mechanism involving regulation of SOCS1 and SOCS3 gene expression. Moreover, they indicate that restoration of cytokine homeostasis may be possible following HPAI H5N1 infection.
To ensure that upregulation of SOCS1 and SOCS3 mRNA expression in H5N1-infected, apocynin-treated A549 cells was highly specific and not generally applicable to all SOCS gene family members, SOCS2 mRNA expression was evaluated in influenza virus-infected A549 cells. SOCS2 gene expression was not induced by influenza virus infection (Fig. 5F). Moreover, apocynin had no significant impact on SOCS2 gene expression following infection (Fig. 5G). This strongly suggests that apocynin manipulates a pathway involving upregulation of SOCS1 and SOCS3 to control cytokine/chemokine production.
H5N1 and H7N9 influenza viruses induce cytokine production by modulating the transcription factor FoxO3 and the TAM receptor tyrosine kinase, Tyro3.
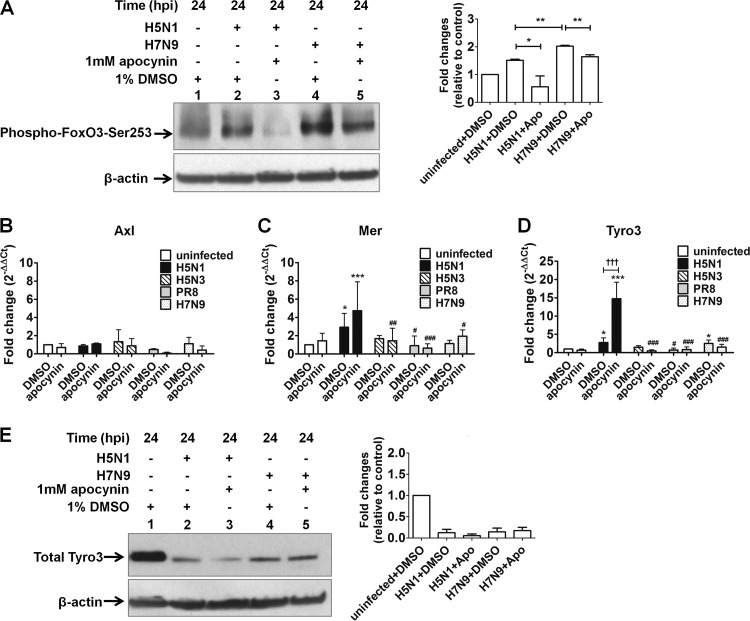
Several factors involved in regulating the expression of SOCS proteins have been described in the literature. One such regulator is Forkhead box O transcription factor 3 (FoxO3), a member of the FoxO family. FoxO3 plays a critical role in preventing overactive antiviral inflammatory responses (29). It has also been shown to bind the SOCS3 promoter, resulting in augmentation of its expression in a mouse pro-B cell line (30). Phosphorylation of FoxO3 by Akt at serine 253 (phospho-FoxO3-Ser253) has previously been shown to result in cytoplasmic retention of FoxO3, preventing target gene activation (18). We therefore investigated the involvement of FoxO3 in the SOCS regulatory pathway (identified by our apocynin/ROS studies) by measuring the level of endogenous phospho-FoxO3-Ser253 using Western blot analysis. The results showed that phospho-FoxO3-Ser253 levels increased after H5N1 infection in A549 cells at 24 hpi compared to mock-treated, uninfected cells. This increased phosphorylation was abolished by apocynin treatment (Fig. 6A). Interestingly, H7N9 infection resulted in significantly greater accumulation of phospho-FoxO3-Ser253 compared to H5N1 infection, which was also reduced by the addition of apocynin (Fig. 6A). These results highlight the fact that H5N1 and H7N9 influenza virus infection may be driving the phosphorylation of FoxO3 at Ser253, reducing SOCS3 expression, thus contributing to the overproduction of cytokines and chemokines. The elevated SOCS3 protein expression shown in Fig. 5E is likely to reflect the overall result of host-pathogen interactions, suggesting that unknown host regulatory mechanisms may be involved in the upregulation of SOCS1 and SOCS3 proteins. More importantly, apocynin may increase SOCS3 expression through modulation of ROS production, which in turn may decrease the phosphorylation of FoxO3 at Ser253 via Akt (a serine/threonine-specific protein kinase) after H5N1 or H7N9 virus infection.
FIG 6.
Effects of apocynin on the expression of phospho-FoxO3-Ser253 and TAM receptors in influenza virus-infected A549 cells. (A) The presence of phospho-FoxO3-Ser253 protein was determined in A549 cells infected with H5N1 and H7N9 in the presence of 1% DMSO or 1 mM apocynin at 24 hpi. Gene expression levels of the TAM receptors Axl (B), Mer (C), and Tyro3 (D) were analyzed by qRT-PCR. *, P < 0.05, ***, P < 0.001 (compared to uninfected controls); #, P < 0.05; ##, P < 0.01; ###, P < 0.001 (compared to H5N1 virus infection group); †††, P < 0.001 (significantly different between infected cells treated with DMSO control and apocynin). (E) Total endogenous Tyro3 protein expression was analyzed in A549 cells infected with H5N1 and H7N9 in the presence of 1% DMSO or 1 mM apocynin at 24 hpi. Representative Western blots and quantification of protein band intensity from three individual experiments are shown. The protein band intensity data represent mean ± the SD (*, P < 0.05; **, P < 0.01).
The TAM receptor protein tyrosine kinases, Tyro3, Axl, and Mer, have also been associated with regulation of SOCS1 and SOCS3 expression (31). These tyrosine kinases are activated upon infection and initiate the transcription of SOCS1 and SOCS3 genes through activation of the Toll-like receptor signaling to limit cytokine overproduction and inflammation (32). Moreover, activation of TAM receptors can be modulated by cytokines that are produced in response to infection (32) and by ROS (33). We therefore set out to investigate the possible involvement of TAM receptors in the SOCS regulatory pathway in A549 cells at 24 hpi. Addition of apocynin alone had no effect on the gene expression of TAM receptors, as shown in the uninfected A549 cells (Fig. 6B to D). Both Tyro3 and Mer mRNA expression was significantly induced following H5N1 infection compared to uninfected controls, but only Tyro3 expression was further enhanced with the addition of apocynin (Fig. 6C and D). H7N9 infection also increased Tyro3 expression; however, apocynin had no additive effects (Fig. 6D). Further analysis of total Tyro3 protein expression showed a marked reduction following H5N1 and H7N9 infection of A549 cells compared to uninfected controls (Fig. 6E). The effect of apocynin on total Tyro3 protein expression in H5N1-infected A549 cells was minimal and completely absent in H7N9-infected A549 cells (Fig. 6E), suggesting ROS does not play a major role in regulating Tyro3 protein expression. These results highlight an unexpected inverse correlation between Tyro3 mRNA and protein expression in H5N1- and H7N9-infected A549 cells. It is important to note here that Tyro3 is a substrate of the E3 ubiquitin ligase Cbl-b (34) and that the NS1 protein of H5N1 has been shown to affect protein ubiquitination in order to evade immune surveillance (35). The inverse correlation between Tyro3 mRNA and protein in H5N1- and H7N9-infected A549 cells may suggest that infection results in the enhancement of Tyro3 ubiquitination and degradation, resulting in the disruption of innate immunity. A recent study analyzing TAM receptor expression in lung tissues from PR8-infected mice demonstrated increased gene expression levels of all three TAM receptors at the acute stage of infection (day 2) (36). Moreover, TAM receptor expression was differentially modulated by PR8 virus in mouse myeloid-derived macrophages and DCs in vitro (36). Together, these results suggest that the expression of TAM receptors may be modulated differentially depending on the influenza virus strain, cell type, and/or in vivo system. In addition to promoting phosphorylation of FoxO3 at Ser253 by Akt as demonstrated above, overproduction of cytokines and chemokines following H5N1 and H7N9 influenza virus infection may be associated with inhibition of Tyro3 protein expression and its downstream targets, SOCS1 and SOCS3. The elevation of SOCS1 and SOCS3 expression following influenza virus infection, as shown in the present studies (Fig. 5E) and in other literature (27, 28, 37), is likely to reflect the combined effect of a dynamic interaction between the host and the pathogen, resulting in the modulation of multiple signaling pathways (Fig. 7). Additional studies will be necessary to determine the exact role of TAM receptors/Tyro3 protein in the host-pathogen interaction.
FIG 7.
Schematic diagram showing the proposed mechanism for influenza virus-induced cytokine dysregulation and the anti-inflammatory effects of apocynin. SOCS1 and SOCS3 expression are positively regulated by FoxO3 and the TAM receptor, Tyro3. The infection of influenza virus, especially HPAI H5N1 and LPAI H7N9 influenza viruses, causes increased ROS production and hypercytokinemia. Influenza virus-mediated cytokine dysregulation can be induced by enhancing the accumulation of the inactivated phospho-FoxO3-Ser253 (Fig. 6A). This in turn reduces SOCS3 expression and contributes to the overproduction of cytokines and chemokines following influenza virus infection. H5N1 and H7N9 infection also inhibit Tyro3 expression in infected cells (Fig. 6E), interrupting the transcription of SOCS1 and SOCS3, resulting in cytokine dysregulation. In addition, yet to be identified host factors may also influence gene expression in an attempt to restore cytokine homeostasis, resulting in an upregulation of SOCS1 and SOCS3 (Fig. 5E, 24 hpi). Treatment of influenza virus-infected cells with apocynin significantly reduces influenza virus-stimulated cytokine/chemokine overproduction (Fig. 3) via the upregulation of SOCS1 and SOCS3 (Fig. 5E). One of the underlying mechanisms suggests that apocynin influences the host immune response by reducing influenza virus-induced ROS production, which in turn reduces phospho-FoxO3-Ser253 accumulation (Fig. 6A) and upregulation of SOCS3 (Fig. 5E). Apocynin also elevates SOCS1 expression through a yet-to-be-determined mechanism.
DISCUSSION
The emergence and high mortality rates associated with HPAI H5N1 and LPAI H7N9 avian influenza viruses make these viruses a pandemic threat. Moreover, disease severity and high fatality rates reported in humans have been associated with virus-induced cytokine dysregulation and hyperactive inflammatory responses in the host (5–7). A positive correlation between cytokine levels and virus pathogenicity has also been observed in ducks (38) and chickens (39) infected with HPAI H5N1. Our data clearly demonstrate that HPAI H5N1 consistently stimulates more cytokine production (especially IL-6, IFN-β, and CXCL10) than low pathogenic influenza viruses in various cell lines. The contribution of “cytokine storm” or “hypercytokinemia” to pathogenesis of HPAI H5N1 influenza virus itself remains controversial. Variable levels of cytokine expression (either markedly elevated or undetectable) have been demonstrated in lung tissue extracted from deceased patients following HPAI H5N1 virus infection (40). Other literature suggests that suppression of the cytokine response alone may not be sufficient to provide adequate protection, as has been noted in mice infected with human H5N1 isolates (41). On the other hand, however, there is clear evidence of hypercytokinemia in H5N1- and H7N9-infected humans (5–7) and chickens (39). Moreover, functional microarray analysis of lung samples obtained from infected ferrets has shown overexpression of genes involved in IFN signaling, especially CXCL10 (42). Treatment of H5N1-infected ferrets with a potent antagonist of CXCL10 receptor resulted in reduced viral loads in the lungs, improved clinical symptoms, and prolonged survival (42). There have also been elevated levels of IL-6 documented in cerebrospinal fluid and serum/plasma samples associated with influenza virus-induced neurological disorders in children (43, 44). As such, the overall contribution of host inflammatory cytokine and chemokine responses to the severity of H5N1 influenza virus pathogenesis in various host species remains to be fully elucidated. Ideal therapies for avian influenza virus infection may therefore require a combination of antiviral treatment and modulation of the host immune response to improve disease outcomes. In the present study, we demonstrated for the first time that apocynin treatment significantly inhibited influenza virus-induced ROS production and cytokine upregulation in various cell lines, including human airway epithelial A549 cells, chicken fibroblasts, and macrophage cell lines, possibly via a SOCS1- and SOCS3-mediated mechanism of action at least in A549 cells. The downstream effects of ROS activation are complex since ROS concentration has the ability to differentially affect the same signaling pathways (45). Our studies suggest that apocynin treatment may modulate multiple pathways (e.g., FoxO3 signaling) involved in regulation of SOCS1 and SOCS3 expression in an attempt to restore ROS homeostasis through the downregulation of cytokine/chemokine responses following influenza virus infection.
The impact of host responses on disease outcomes is an area of considerable interest. Taubenberger and coworkers used a ROS scavenger, EUK-207, to reduce lung pathology and improve survival following infection of mice with the reconstructed 1918 “Spanish Flu” virus (46), further validating our apocynin-based observations. Both EUK-207 and apocynin treatment resulted in decreased oxidation and infiltration of inflammatory cell in the lungs of infected mice (9, 46). In a similar fashion, neither EUK-207 nor apocynin had impact on viral replication in vitro. In our in vitro system, apocynin specifically inhibits influenza virus-induced deleterious ROS overproduction in infected cells, leading to upregulation of SOCS1 and SOCS3 and inhibition of cytokine production. We concede that the largely simplified clonal cell line infection model described herein may not reflect the complexity of interactions in vivo after apocynin treatment of a whole organism. As such, the impact on immune cell populations via cell-specific signaling pathways or regulatory factors may not be fully elucidated and requires further investigation. Future studies will analyze host innate and adaptive immune responses after apocynin treatment in numerous influenza virus-infected animal models.
The present study identified the involvement of two signaling molecules, FoxO3 and Tyro3, in the regulation of SOCS3 and/or SOCS1 expression (30, 32) (Fig. 7). FoxO transcription factors are involved in the regulation of diverse physiological and pathological processes, including immunity, apoptosis, oncogenesis, and damage repair in response to oxidative stress (47). As such, the FoxO family are downstream targets of multiple signaling pathways that tightly control subcellular localization, activation, and phosphorylation of FoxOs (47). Accumulating evidence suggests that FoxO plays an important role in protecting cells from oxidative damage and maintaining homeostasis of cellular ROS levels. Two signaling pathways, the PI3K/PKB (Akt) (influences cytoplasmic retention and inactivation of FoxO) and C-Jun N-terminal protein kinase (JNK; influences nuclear translocation and activation of FoxO) pathways, have been implicated in this process (18). The activation of both pathways depends on the state and overall level of stress (e.g., nutrition starvation and oxidative stress level) (48). ROS regulation of FoxO activity is extremely complex. ROS production can influence posttranslational modifications on FoxO proteins, leading to either activation or inactivation of FoxO activity and/or a shift in transcriptional targets (45). Phosphorylation of FoxO3 by PKB at Thr32, Ser253, or Ser315 has been shown to result in cytoplasmic retention and inactivation of FoxO3 (18). PKB activity is promoted by ROS and correlates with increased levels of inactivated, phosphorylated FoxO3 at Thr32 and Ser253 (48). In contrast, stress-responsive JNK-mediated FoxO phosphorylation stimulated upon ROS exposure promotes nuclear localization and activation of FoxO transcriptional activity resulting in upregulation of antioxidant enzymes (manganese superoxide dismutase [MnSOD] and catalase) and reduction of cellular ROS levels (45). The present study demonstrated that H5N1 infection can stimulate significant ROS production in infected cells, which can be reduced through exposure to apocynin. This coincided with an increase in phospho-FoxO3-Ser253 (inactivated FoxO3) that was also reduced with apocynin treatment of A549 cells at 24 hpi. Our results therefore suggest that ROS are involved in the regulation of FoxO3 phosphorylation. Moreover, we suspect that inhibition of influenza virus-induced ROS production by apocynin may in fact adjust ROS level to a point enabling inactivation of the PI3K/PKB pathway and enhancement of FoxO3 activity. This in turn drives SOCS3 expression to reduce cytokine production following H5N1 infection (Fig. 7).
TAM receptors are negative regulators of innate immunity after infection, acting in part, via the upregulation of SOCS1 and SOCS3 to maintain homeostasis of the host inflammatory responses (32). Previous studies have demonstrated elevated TAM receptor expression levels after PR8 infection in a mouse model (36). Our in vitro gene expression observations (Fig. 6C and D) produced similar results and we have now extended these initial findings to include Tyro3 protein expression. Interestingly, we noted that Tyro3 protein expression was inhibited by H5N1 and H7N9 viruses at 24 hpi (in contrast to Tyro3 gene expression) and might be responsible for cytokine overproduction by decreasing SOCS1 and SOCS3 protein expression (Fig. 7). These findings in combination with the accumulation of influenza-induced phospho-FoxO3-Ser253 strengthen the argument that SOCS1 and SOCS3 protein expression levels (Fig. 5E) are influenced by a complex and dynamic interaction between the host and pathogen. The interplay between a virus and host is complex and therefore difficult to simplify and, as such, the field has been unable to carefully tease apart the mechanisms driving aberrant cytokine and chemokine responses in severe disease. A clear “power struggle” exists where, on the one hand, influenza A viruses attempt to downregulate SOCS1/SOCS3 through enhancement of phospho-FoxO3-Ser253 expression and inhibition of Tyro3 expression resulting in cytokine dysregulation, while, on the other hand, currently unidentified host cell factor(s) partially counteract this action through upregulation of SOCS1/SOCS3 in an attempt to restore cytokine homeostasis. When the host is not completely overwhelmed by the infection as is the case in our in vitro system, the overall result is increased SOCS1 and SOCS3 protein expression. Apocynin assists the host by inhibiting ROS production, reducing phospho-FoxO3-Ser253, and increasing SOCS1 and SOCS3 expression. It is therefore not surprising that variable SOCS1 and SOCS3 mRNA and protein expression levels have been reported in human cells (27, 28, 37) following influenza virus infection given the fact that various cell types, influenza virus strains, and/or experimental conditions may influence outcomes. Future studies will need to be conducted in biologically relevant innate immune cells to fully characterize the role of FoxO and TAM receptors following influenza virus infection.
Our present study and emerging evidence from others (9, 46) clearly demonstrates that antioxidant agents or ROS scavengers are attractive and promising therapeutics agents for the treatment of influenza virus infection that could be used in combination with current antiviral agents. We have demonstrated that apocynin exhibits anti-inflammatory properties in various cell types (avian versus mammalian; epithelial versus macrophage) and is especially efficacious in cells infected with highly pathogenic viruses, such as H5N1. We show the involvement of two potential pathways: PKB/Akt-FoxO3 signaling and the TAM receptor, Tyro3. Apocynin may modulate multiple pathways (e.g., FoxO3 phosphorylation) via manipulation of ROS production, resulting in increased expression of the negative regulators of cytokine signaling, SOCS1 and SOCS3. Since SOCS1, SOCS3, FoxO3, and TAM receptors all play important roles in regulating innate immunity, the anti-inflammatory effects of apocynin may be applicable to other inflammatory diseases, such as arthritis (49) and asthma (50). In addition, these four proteins are highly conserved across species, including human and chicken (the protein identities were assessed using NCBI BLAST: SOCS1, 61%; SOCS3, 81%; FoxO3, 89%; and Tyro3, 69%). It is likely that the involvement of PKB/Akt-FoxO3 and TAM receptor signaling pathways in influenza virus-induced cytokine dysregulation also exists in chicken and requires further investigation. In conclusion, we support the use of agents (e.g., apocynin or ROS scavengers) that minimize immunopathology induced by excessive ROS production or cytokine dysregulation in order to improve survival rates following avian influenza A virus infection.
ACKNOWLEDGMENTS
This study was supported by the CSIRO Biosecurity Flagship Collaboration Fund (to J.S.) and by an Alfred Deakin Postdoctoral Research Fellowship (to S.Y.).
We thank Paul Monaghan and Diane Green for technical assistance and for providing anti-influenza NP monoclonal antibody. We thank Celine Deffrasnes for providing technical assistance with image analysis using CellInsight Imager. We thank the State of Victoria Department of Primary Industries, the WHO Collaborating Centre for Reference and Research, CSIRO/AAHL, and the University of Melbourne for providing viruses.
REFERENCES
- 1.Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, Krauss S, Shortridge KF, Webster RG. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 2.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 3.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Wang D, Gao R, Zhao B, Song J, Qi X, Zhang Y, Shi Y, Yang L, Zhu W, Bai T, Qin K, Lan Y, Zou S, Guo J, Dong J, Dong L, Wei H, Li X, Lu J, Liu L, Zhao X, Huang W, Wen L, Bo H, Xin L, Chen Y, Xu C, Pei Y, Yang Y, Zhang X, Wang S, Feng Z, Han J, Yang W, Gao GF, Wu G, Li D, Wang Y, Shu Y. 2013. Biological features of novel avian influenza A (H7N9) virus. Nature 499:500–503. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
- 6.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D, Li Y, Yao X, Zhang Y, Wu H, Zheng S, Diao H, Xia S, Chan KH, Tsoi HW, Teng JL, Song W, Wang P, Lau SY, Zheng M, Chan JF, To KK, Chen H, Li L, Yuen KY. 2013. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam WY, Yeung AC, Chu IM, Chan PK. 2010. Profiles of cytokine and chemokine gene expression in human pulmonary epithelial cells induced by human and avian influenza viruses. Virol J 7:344. doi: 10.1186/1743-422X-7-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, Selemidis S. 2011. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog 7:e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. 2008. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedard K, Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 12.Kolarova H, Bino L, Pejchalova K, Kubala L. 2010. The expression of NADPH oxidases and production of reactive oxygen species by human lung adenocarcinoma epithelial cell line A549. Folia Biol (Praha) 56:211–217. [PubMed] [Google Scholar]
- 13.Govorkova EA, Baranovich T, Seiler P, Armstrong J, Burnham A, Guan Y, Peiris M, Webby RJ, Webster RG. 2013. Antiviral resistance among highly pathogenic influenza A (H5N1) viruses isolated worldwide in 2002-2012 shows need for continued monitoring. Antivir Res 98:297–304. doi: 10.1016/j.antiviral.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. 2003. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 15.Hierholzer JC, Killington RA. 1996. Virus isolation and quantitation, p 25–46 InBrian HOK, Mahy WJ (ed), Virology methods manual. Academic Press, New York, NY. [Google Scholar]
- 16.Ebers KL, Zhang CY, Zhang MZ, Bailey RH, Zhang S. 2009. Transcriptional profiling avian beta-defensins in chicken oviduct epithelial cells before and after infection with Salmonella enterica serovar Enteritidis. BMC Microbiol 9:153. doi: 10.1186/1471-2180-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchamp MB, Casalegno JS, Gillet Y, Frobert E, Bernard E, Escuret V, Billaud G, Valette M, Javouhey E, Lina B, Floret D, Morfin F. 2010. Pandemic A(H1N1)2009 influenza virus detection by real-time RT-PCR: is viral quantification useful? Clin Microbiol Infect 16:317–321. doi: 10.1111/j.1469-0691.2010.03169.x. [DOI] [PubMed] [Google Scholar]
- 18.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 19.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. 2007. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser P, Poh TY, Rothwell L, Avery S, Balu S, Pathania US, Hughes S, Goodchild M, Morrell S, Watson M, Bumstead N, Kaufman J, Young JR. 2005. A genomic analysis of chicken cytokines and chemokines. J Interferon Cytokine Res 25:467–484. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, Stiles JK. 2011. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev 22:121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Culley FJ, Pennycook AM, Tregoning JS, Dodd JS, Walzl G, Wells TN, Hussell T, Openshaw PJ. 2006. Role of CCL5 (RANTES) in viral lung disease. J Virol 80:8151–8157. doi: 10.1128/JVI.00496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsukura S, Kokubu F, Kubo H, Tomita T, Tokunaga H, Kadokura M, Yamamoto T, Kuroiwa Y, Ohno T, Suzaki H, Adachi M. 1998. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol 18:255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 25.Vlahos R, Stambas J, Selemidis S. 2012. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol Sci 33:3–8. doi: 10.1016/j.tips.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. 2000. Role of oxidants in NF-κB activation and TNF-α gene transcription induced by hypoxia and endotoxin. J Immunol 165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 27.Jia D, Rahbar R, Chan RW, Lee SM, Chan MC, Wang BX, Baker DP, Sun B, Peiris JS, Nicholls JM, Fish EN. 2010. Influenza virus nonstructural protein 1 (NS1) disrupts interferon signaling. PLoS One 5:e13927. doi: 10.1371/journal.pone.0013927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pauli EK, Schmolke M, Wolff T, Viemann D, Roth J, Bode JG, Ludwig S. 2008. Influenza A virus inhibits type I IFN signaling via NF-κB-dependent induction of SOCS-3 expression. PLoS Pathog 4:e1000196. doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litvak V, Ratushny AV, Lampano AE, Schmitz F, Huang AC, Raman A, Rust AG, Bergthaler A, Aitchison JD, Aderem A. 2012. A FOXO3-IRF7 gene regulatory circuit limits inflammatory sequelae of antiviral responses. Nature 490:421–425. doi: 10.1038/nature11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. 2007. Regulation of suppressor of cytokine signaling 3 (SOC3) by growth hormone in pro-B cells. Mol Endocrinol 21:2503–2515. doi: 10.1210/me.2006-0498. [DOI] [PubMed] [Google Scholar]
- 31.Lemke G, Rothlin CV. 2008. Immunobiology of the TAM receptors. Nat Rev Immunol 8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. 2007. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 33.Cavet ME, Smolock EM, Menon P, Konishi A, Korshunov VA, Berk BC. 2010. Gas6-Axl pathway: the role of redox-dependent association of Axl with nonmuscle myosin IIB. Hypertension 56:105–111. doi: 10.1161/hypertensionaha.109.144642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, Jamieson AM, Langdon WY, Ikeda F, Fededa JP, Cronin SJ, Nitsch R, Schultz-Fademrecht C, Eickhoff J, Menninger S, Unger A, Torka R, Gruber T, Hinterleitner R, Baier G, Wolf D, Ullrich A, Klebl BM, Penninger JM. 2014. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 507:508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajsbaum R, Albrecht RA, Wang MK, Maharaj NP, Versteeg GA, Nistal-Villan E, Garcia-Sastre A, Gack MU. 2012. Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein. PLoS Pathog 8:e1003059. doi: 10.1371/journal.ppat.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata T, Habiel DM, Coelho AL, Kunkel SL, Lukacs NW, Hogaboam CM. 2014. Axl receptor blockade ameliorates pulmonary pathology resulting from primary viral infection and viral exacerbation of asthma. J Immunol 192:3569–3581. doi: 10.4049/jimmunol.1302766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelli RK, Dunham SP, Kuchipudi SV, White GA, Baquero-Perez B, Chang P, Ghaemmaghami A, Brookes SM, Brown IH, Chang KC. 2012. Mammalian innate resistance to highly pathogenic avian influenza H5N1 virus infection is mediated through reduced proinflammation and infectious virus release. J Virol 86:9201–9210. doi: 10.1128/JVI.00244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei L, Jiao P, Song Y, Cao L, Yuan R, Gong L, Cui J, Zhang S, Qi W, Yang S, Liao M. 2013. Host immune responses of ducks infected with H5N1 highly pathogenic avian influenza viruses of different pathogenicities. Vet Microbiol 166:386–393. doi: 10.1016/j.vetmic.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K, Okada H, Itoh T, Tada T, Mase M, Nakamura K, Kubo M, Tsukamoto K. 2009. Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses. J Virol 83:7475–7486. doi: 10.1128/JVI.01434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng R, Lu M, Korteweg C, Gao Z, McNutt MA, Ye J, Zhang T, Gu J. 2008. Distinctly different expression of cytokines and chemokines in the lungs of two H5N1 avian influenza patients. J Pathol 216:328–336. doi: 10.1002/path.2417. [DOI] [PubMed] [Google Scholar]
- 41.Salomon R, Hoffmann E, Webster RG. 2007. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A 104:12479–12481. doi: 10.1073/pnas.0705289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron CM, Cameron MJ, Bermejo-Martin JF, Ran L, Xu L, Turner PV, Ran R, Danesh A, Fang Y, Chan PK, Mytle N, Sullivan TJ, Collins TL, Johnson MG, Medina JC, Rowe T, Kelvin DJ. 2008. Gene expression analysis of host innate immune responses during Lethal H5N1 infection in ferrets. J Virol 82:11308–11317. doi: 10.1128/JVI.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aiba H, Mochizuki M, Kimura M, Hojo H. 2001. Predictive value of serum interleukin-6 level in influenza virus-associated encephalopathy. Neurology 57:295–299. doi: 10.1212/WNL.57.2.295. [DOI] [PubMed] [Google Scholar]
- 44.Ito Y, Ichiyama T, Kimura H, Shibata M, Ishiwada N, Kuroki H, Furukawa S, Morishima T. 1999. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol 58:420–425. doi:. [DOI] [PubMed] [Google Scholar]
- 45.de Keizer PL, Burgering BM, Dansen TB. 2011. Forkhead box o as a sensor, mediator, and regulator of redox signaling. Antioxid Redox Signal 14:1093–1106. doi: 10.1089/ars.2010.3403. [DOI] [PubMed] [Google Scholar]
- 46.Kash JC, Xiao Y, Davis AS, Walters KA, Chertow DS, Easterbrook JD, Dunfee RL, Sandouk A, Jagger BW, Schwartzman LM, Kuestner RE, Wehr NB, Huffman K, Rosenthal RA, Ozinsky A, Levine RL, Doctrow SR, Taubenberger JK. 2013. Treatment with the reactive oxygen species scavenger EUK-207 reduces lung damage and increases survival during 1918 influenza virus infection in mice. Free Radic Biol Med 67:235–247. doi: 10.1016/j.freeradbiomed.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzivion G, Dobson M, Ramakrishnan G. 2011. FoxO transcription factors: regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta 1813:1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Nemoto S, Finkel T. 2002. Redox regulation of Forkhead proteins through a p66shc-dependent signaling pathway. Science 295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 49.Lafeber FP, Beukelman CJ, van den Worm E, van Roy JL, Vianen ME, van Roon JA, van Dijk H, Bijlsma JW. 1999. Apocynin, a plant-derived, cartilage-saving drug, might be useful in the treatment of rheumatoid arthritis. Rheumatology 38:1088–1093. doi: 10.1093/rheumatology/38.11.1088. [DOI] [PubMed] [Google Scholar]
- 50.Peters EA, Hiltermann JT, Stolk J. 2001. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 31:1442–1447. doi: 10.1016/S0891-5849(01)00725-0. [DOI] [PubMed] [Google Scholar]