Abstract
For the first time, we report the whole-genome sequence analysis of Chryseobacterium oranimense G311, a multidrug-resistant bacterium, from a cystic fibrosis patient in France, including resistance to colistin. Whole-genome sequencing of C. oranimense G311 was performed using Ion Torrent PGM, and RAST, the EMBL-EBI server, and the Antibiotic Resistance Gene-ANNOTation (ARG-ANNOT) database were used for annotation of all genes, including antibiotic resistance (AR) genes. General features of the C. oranimense G311 draft genome were compared to the other available genomes of Chryseobacterium gleum and Chryseobacterium sp. strain CF314. C. oranimense G311 was found to be resistant to all β-lactams, including imipenem, and to colistin. The genome size of C. oranimense G311 is 4,457,049 bp in length, with 37.70% GC content. We found 27 AR genes in the genome, including β-lactamase genes which showed little similarity to the known β-lactamase genes and could likely be novel. We found the type I polyketide synthase operon followed by a zeaxanthin glycosyltransferase gene in the genome, which could impart the yellow pigmentation of the isolate. We located the O-antigen biosynthesis cluster, and we also discovered a novel capsular polysaccharide biosynthesis cluster. We also found known mutations in the orthologs of the pmrA (E8D), pmrB (L208F and P360Q), and lpxA (G68D) genes. We speculate that the presence of the capsular cluster and mutations in these genes could explain the resistance of this bacterium to colistin. We demonstrate that whole-genome sequencing was successfully applied to decipher the resistome of a multidrug resistance bacterium associated with cystic fibrosis patients.
INTRODUCTION
Cystic fibrosis (CF) is the most common autosomal recessive genetic disease and results from mutations within the gene coding for the cystic fibrosis transmembrane regulator (CFTR) protein. This life-threatening disease affects all racial and ethnic groups, though it is more common among Caucasians (1, 2). CF is characterized by hyperproduction of viscous mucus by the affected glands, resulting mainly in impaired respiratory and pancreatic functions. The most common complication of CF involves the chronic respiratory infections caused by bacterial pathogens (3), which are the main reason for the high morbidity and mortality of the disease (4). Traditionally, only a few bacteria were involved in CF lung infections, including Staphylococcus aureus, Pseudomonas aeruginosa, Haemophilus influenzae, and Streptococcus pneumoniae. However, many new or emerging opportunistic bacteria have been described in CF patients over the past decade, for instance, Burkholderia cepacia complex, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, Pandoraea spp., Ralstonia spp., Inquilinus limosus, and nontuberculosis mycobacteria, as well as fungi (5). Chronic microbial infection, along with P. aeruginosa infections, leads to excessive airway inflammation and the eventual loss of pulmonary function. Colistin is an extremely important antibiotic used in patients with CF upon the first acquisition and for maintenance of chronic Pseudomonas infections. Consequently, polymyxin-resistant P. aeruginosa clinical isolates are increasingly being reported in CF patients (6, 7). However, although aggressive antimicrobial therapy has often helped to eradicate or minimize the deterioration of lung infections, it has eventually led to the emergence of new and/or atypical multidrug resistance bacteria, including colistin-resistant bacteria in CF. Several colistin resistance bacteria have been reported recently in CF patients, such as I. limosus (8), Brevundimonas diminuta (9), Ochrobactrum anthropi (9), S. maltophilia, and A. xylosoxidans (8–12).
Members of the genus Chryseobacterium, mainly Chryseobacterium indologenes, have been documented as opportunistic pathogens known to be associated with nosocomial infections in infants and immunocompromised patients of all age groups and are resistant to colistin (13, 14). There are about 283 reported cases of infections associated with C. indologenes (15, 16). In a report by Chen et al., 215 clinical isolates of multidrug-resistant C. indologenes were identified after the increasing clinical use of colistin and tigecycline (16), a risk for patients who have undergone extensive administration of antibiotics for a long period (17). Although the source of infection of this microbe is not clear, it has been reported to be acquired nosocomially via medical devices and contaminated water supplies in hospitals (18). C. indologenes was also reported from a cohort of CF patients in Italy (19). Thirty-five clinical isolates of Chryseobacterium spp. (C. indologenes, Elizabethkingia meningoseptica [formerly Chryseobacterium meningosepticum], and Chryseobacterium gleum) were reported from CF patients who were also coinfected by one of the dominating pathogens of CF (P. aeruginosa or Burkholderia cepacia complex) (20). Furthermore, Chryseobacterium spp. only susceptible to cotrimoxazole and quinolones were reported in Italian CF patients who had received colistin therapy because of coinfection with P. aeruginosa or B. cepacia (21). The genetic basis of these multidrug-resistant bacteria remains unknown. Nonetheless, bacterial whole-genome sequencing is an economically feasible tool for deciphering the resistome (22) and has provided unprecedented insight into the evolution of antibiotic resistance (AR) (23).
Here, we report the whole-genome sequence used to decipher the resistome and genomic properties of Chryseobacterium oranimense G311, a colistin-resistant Gram-negative bacterium isolated for the first time from the sputum of a 26-month-old child with CF. It should be noted that the patient was coinfected with S. maltophilia and P. aeruginosa and had received colistin treatment prior to the isolation of this colistin-resistant bacterium. We speculate that colistin therapy led to the selection of this colistin-resistant bacterium; however, we could not isolate any other strain to perform the comparison. The true significance of isolating C. oranimense G311 in terms of clinical evolution is difficult to establish; however, it could be clinically significant, especially in immunocompromised patients. We also performed a comparison of the C. oranimense G311 genome with the genomes of closely related C. gleum ATCC 35910 and Chryseobacterium sp. strain CF314.
MATERIALS AND METHODS
Growth conditions and identification.
C. oranimense was isolated on Columbia agar with 5% sheep blood COS (bioMérieux) medium and was identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Microflex; Bruker Daltonic, Bremen, Germany) by using the flex control software (Bruker Daltonics), as previously described (24), and 16S rRNA gene amplification and sequencing as well. Growth was also assessed under other conditions and by using brain heart infusion medium with different salt concentrations, ranging from 0 to 10% NaCl, Trypticase soy agar (TSA) (bioMérieux), extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae medium (ChromID ESBL) (bioMérieux), and Burkholderia cepacia-specific CEPACIA medium (AES Laboratory, Combourg, France). Gram staining and electron microscopy were performed.
Antibiotic susceptibility test.
Antibiotic susceptibility testing was performed on Mueller-Hinton agar medium (MH) (bioMérieux) according to the Committee for Antimicrobial Testing of the French Society for Microbiology using a Vitek2 auto system (bioMérieux, Marcy l'Etoile, France), and MICs were determined by the Etest method (bioMérieux).
Screening for metallo-β-lactamase activity was performed using the modified imipenem-EDTA (IMI-EDTA) double-disc synergy test and modified Hodge test as described previously (25, 26). Carbapenemase activity was assessed by MALDI-TOF assay. The colistin and imipenem susceptibilities were determined using the Etest strip (bioMérieux) and a 0.5 McFarland inoculum grown on TSA, as previously described (27). The antibiotics used for this study were amoxicillin, ticarcillin, amoxicillin-clavulanic acid, ticarcillin-clavulanic acid, cefoxitin, cefotaxime, ceftriaxone, ceftazidime, aztreonam, gentamicin, tobramycin, amikacin, ciprofloxacin, ofloxacin, and trimethoprim-sulfamethoxazole.
DNA isolation and genome sequencing.
C. oranimense G311 was grown in Columbia agar with 5% sheep blood (bioMérieux) medium at 37°C for 24 h. The overnight bacterial culture was treated with 500 μl of TE buffer (25 mM Tris-HCl [pH 8.0], 10 mM EDTA [pH 8.0], and 10 mM NaCl) and 1 mg/ml of proteinase K at 37°C, and the genomic DNA was extracted using phenol-chloroform and alcohol precipitation. DNA was then visualized on an ethidium bromide-stained 0.7% agarose gel. The DNA concentration was quantified using the Quant-iT PicoGreen kit (Invitrogen). Bacterial genome sequencing was performed using the Ion Torrent PGM (Life Technologies, Saint Aubin, France) on 1 μg of DNA. A DNA library was constructed using enzymatic fragmentation and adaptor ligation with the Ion Xpress Plus fragment library kit (Life Technologies). Fragment size selection was performed using agarose gel electrophoresis. The distribution of DNA fragment sizes was analyzed with a Bioanalyzer using the High Sensitivity kit (Agilent, Santa Clara, CA). After dilution of the library at 11.62 pM, template preparation, emulsion PCR, and ion sphere particle (ISP) enrichment were performed using the Ion One Touch 200 template kit v.2. The quality of the resulting ISPs was assessed using the Qubit 2.0 fluorometer (Life Technologies), and the ISPs were loaded and sequenced on a 316 chip (Life Technologies). No prior quality filtering was used for the de novo assembly, which was performed using Newbler version 2.3 software (Roche) with 90% identity and 50% coverage as overlap.
Genome annotation.
For C. oranimense G311 genome annotation, contigs were submitted to the Rapid Annotation using Subsystems Technology (RAST) online bioserver (http://rast.nmpdr.org/) (28), and more of the genome was annotated using the EMBL-EBI (The European Bioinformatics Institute) server using default parameters and the standard procedure. ORFans were confirmed by BLASTP (E value 10E−3; identity of ≥30%; coverage of ≥50%) against the nonredundant protein (nr) database of the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov). The tRNA and rRNA genes were also verified on the tRNAscan-SE search server (http://lowelab.ucsc.edu/tRNAscan-SE) and RNammer (http://www.cbs.dtu.dk/services/RNAmmer/). All the antimicrobial resistance genes in C. oranimense G311 were predicted using our local database Antibiotic Resistance Gene-ANNOTation (29).
Nucleotide sequence accession numbers.
The genome sequence was deposited in EMBL under accession numbers CDHM01000001 to CDHM01000015 (EBI accession numbers CEJ67725 to CEJ72111).
RESULTS
Phenotypic properties.
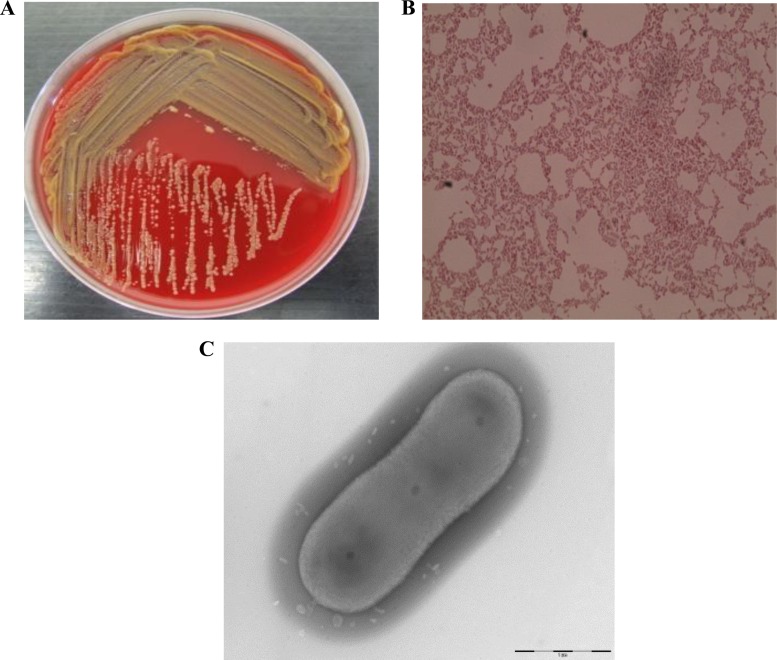
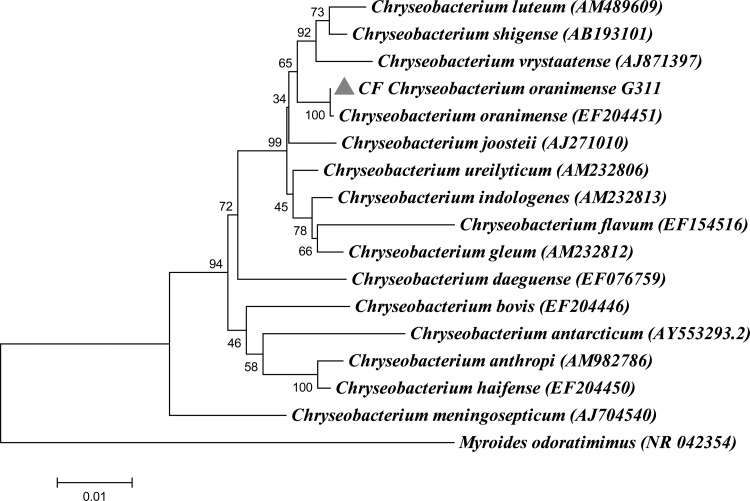
C. oranimense G311 (Collection de Souches de l'Unité des Rickettsies [CSUR] reference no. P277) (104 CFU/ml), along with P. aeruginosa (104 CFU/ml) and S. maltophilia (105 CFU/ml), was isolated from the sputum sample of a 26-month-old girl in August 2012. The isolate was pigmented yellow when isolated on Columbia agar with 5% sheep blood COS (bioMérieux) medium at 37°C after 24 h of incubation and correctly identified by MALDI-TOF as C. oranimense with a good score (>2.0). The colony size of the CF isolate varied from 0.5 to 1 mm in diameter and was capsulated (Fig. 1). This aerobic, Gram-negative, nonmotile bacillus grew well at 29°C, and growth was also observed at 18°C, 10°C, and 4°C after 2 days, 4 days, and 8 days of incubation, respectively. The isolate was able to grow microaerophilically and in the presence of 5% CO2 but not under an anaerobic condition. Growth was also observed on Trypticase soy agar, extended spectrum-β-lactamase-producing Enterobacteriaceae, and cepacia media at 37°C and 29°C. The isolate also grew at a salt concentration of up to 2% after 24 h of incubation. Although Chryseobacterium species have been described to be present in CF patients, C. oranimense has not been reported so far, and it was not isolated from any other CF clinical sample in our lab as well. 16S rRNA PCR amplification and sequencing confirmed that the sequence was 99.7% similar to that of C. oranimense H8T (30). The genome sequence confirmed the identification. The phylogenetic tree based on the 16S rRNA sequence was constructed to show the phylogenetic position of CF C. oranimense G311 (Fig. 2).
FIG 1.
(A) Chryseobacterium oranimense G311 yellow isolate on Columbia agar with 5% sheep blood (bioMérieux) at 37°C; (B) Gram staining image of Chryseobacterium oranimense G311 viewed at ×100 magnification; (C) transmission electron microscopic image of Chryseobacterium oranimense G311 using a Morgani 268D TEM (Philips) at an operating voltage of 60 kV.
FIG 2.
Phylogenetic tree based on 16S rRNA sequence highlighting the phylogenetic position of CF Chryseobacterium oranimense G311. Myroides odoratimimus was used as an outgroup. Sequences were aligned using ClustalX, and phylogenetic inferences were obtained using the neighbor-joining method within Mega 5 software. Bootstrap values are expressed by percentage of 1,000 replicates with a Kimura 2 parameter test and shown at the branching points. The branches of the tree are indicated by the genus and species name of the type strains followed by the NCBI gene accession numbers.
General features of the C. oranimense G311 genome.
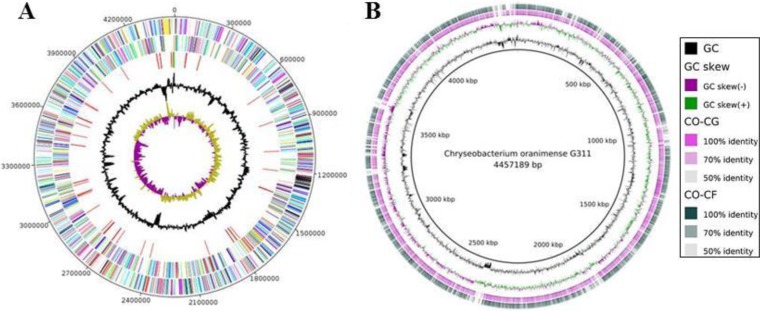
A total of 2,764,904 reads were obtained, leading to 511,490,430 bp of sequence data. The size of the C. oranimense G311 genome is 4,457,049 bp, comprising one circular chromosome, with a 37.7% GC content, assembled into 15 contigs. No plasmid was detected. A total of 4,475 genes were predicted, including 4,387 protein-coding genes (EBI accession number CEJ67725 to CEJ72111) and 88 RNAs (3 rRNA operons and 85 tRNAs). Of the 4,387 protein-coding genes, 3,004 (68.47%) were assigned a putative function, whereas 216 (4.92%) genes were identified as ORFans and 1,100 were annotated as hypothetical proteins (26.60%). As many as 541 genes had a signal peptide, and 896 transmembrane proteins were detected. A comparison among the general features of the genomes of three Chryseobacterium spp. is shown in Table 1. The average nucleotide identities of the C. oranimense G311 genome with C. gleum ATCC 35910 and Chryseobacterium sp. CF314 genomes were 80.68% and 79.72%, respectively. The distribution of genes into COG functional categories and the comparison of C. oranimense with C. gleum ATCC 35910 and Chryseobacterium sp. strain CF using the BLAST Ring Image Generator (31) (BRIG) are presented in Fig. 3, and the distribution of COG categories is presented in Table S1 in the supplemental material.
TABLE 1.
General features of the Chryseobacterium oranimense G311 genome in comparison to the Chryseobacterium gleum ATCC 35910 and Chryseobacterium sp. strain CF314 genomes
| Species | Database accession no.a | Genome size (bp) | % GC content | No. of CDSb | No. of RNA | Avg nt identity | No. of C. oranimense G311 proteins with: |
|
|---|---|---|---|---|---|---|---|---|
| Any similarity | Up to 80% similarity | |||||||
| Chryseobacterium oranimense G311 | CDHM01000001–CDHM01000015 | 4,457,049 | 37.70 | 4,387 | 88 | |||
| Chryseobacterium gleum ATCC 35910 | ACKQ02000001–ACKQ02000007 | 5,569,640 | 36.80 | 5,304 | 79 | 80.68 | 1,355 | 2,435 |
| Chryseobacterium sp. strain CF314 | AKJY01000001–AKJY01000119 | 4,484,672 | 36.62 | 4,182 | 54 | 79.72 | 1,449 | 1,952 |
The genome sequences were deposited in the Whole Genome Sequence (WGS) database: 15 contigs for Chryseobacterium oranimense G311, 7 contigs for Chryseobacterium gleum ATCC 35910, and 119 contigs for Chryseobacterium sp. strain CF314.
CDS, coding sequences.
FIG 3.
(A) Graphical circular map of the Chryseobacterium oranimense G311 genome. Circle range is from 1 (outer) to 5 (inner). Circle 1, positive strand; circle 2, negative strand; circle 3, tRNA (red) and rRNA (green); circle 4, GC; circle 5, GC skew. All genes are color coded according to Cluster of Orthologous Group (COG) functions, shown in the table with number of genes for each COG using BRIG software. (B) Comparison of the Chryseobacterium oranimense G311 (CO) genome with Chryseobacterium gleum ATCC 35910 (CG) and Chryseobacterium sp. strain CF314 (CF) genomes using RAST.
Resistome of C. oranimense G311.
The C. oranimense G311 isolate was found to be highly multidrug resistant. The isolate was resistant to colistin (MIC of 24 μg/ml) and imipenem (MIC of 12 μg/ml) and also to amoxicillin, ticarcillin, amoxicillin-clavulanic acid, ticarcillin-clavulanic acid, second-generation cephalosporin (cefoxitin), and third-generation cephalosporins (cefotaxime, ceftriaxone, ceftazidime, and aztreonam). C. oranimense G311 was also found to be resistant to the aminoglycoside tobramycin but was susceptible to gentamicin and amikacin and was also susceptible to fluoroquinolones ciprofloxacin, ofloxacin, and trimethoprim-sulfamethoxazole (Table 2). The resistome of this multidrug-resistant C. oranimense G311 revealed the presence of 27 antibacterial-resistant genes using the Antibiotic Resistance Gene-ANNOTation (ARG-ANNOT) database (29). This isolate possesses three different types of β-lactamases, i.e., Amber class A ESBL genes (blaCME-like), Amber class B metallo-β-lactamase (MBL) genes (blaGOB- and blaIND-like), and Amber class C ESBL genes (blaACC-, blaampH-, and blaCMY-like). There was little homology between blaACC-like genes and other beta-lactamases genes (Table 3); thus, there is a possibility that these genes could be novel. They are likely to contribute to the resistance of this bacterium to beta-lactam compounds. We also performed the analysis of the 10-kb sequence upstream and downstream of each AR gene. We found only one putative, 138-amino-acid (aa)-long Holiday junction resolvase (39.1%), located 740 bp upstream of the blaACC gene, and a 304-aa-long integrase (38.9%), located 2.839 kb downstream of the blaampH-like gene. Apart from these, there were many hypothetical proteins flanking the AR genes with no BLAST hit; hence, we believe that there is a possibility of those sequences to carry unknown insertion elements or transposases by which the genes were acquired. Tetracycline resistance genes, such as the otr-like, tetC, and tetX genes, were also found, as well as aminoglycoside adenyltransferase genes (aac6 and aadK). Other AR genes included genes conferring resistance to macrolide-lincosamide-streptogramin B (MLS-like), phenicols (cfr- and cmlv-like), sulfonamide (sulIII-like), and rifampin (arr5-like) (Table 3). Conversely, C. oranimense was found to be susceptible to rifampin (MIC of 0.38 μg/ml) and to fluoroquinolones. We did not find any mutations in the known genes (gyrA, rpoB, parC), which play a role in imparting resistance to these antibiotics. Lastly, a genetic analysis of putative candidate target genes associated with polymyxin resistance (pmrA, pmrB, phoP, and phoQ genes) revealed that the pmrA gene harbors a single substitution at position eight (E8D),that the pmrB gene harbors two substitutions (L208F and P360Q), and that lpxA harbors a single substitution (G68D), as shown in Table 4. We speculate that these mutations could likely play a role in colistin resistance exhibited by C. oranimense G311.
TABLE 2.
Antibiotic susceptibility pattern in the Chryseobacterium oranimense G311 genome
| Antibiotica | Pattern |
|---|---|
| AMX | 9/R |
| TIC | 10/R |
| AMC | 10/R |
| TCC | 15/R |
| FOX | 11/R |
| CTX | 9/R |
| CRO | 14/R |
| CAZ | 25/S |
| ATM | 7/R |
| IMP | 15/R (12 μg/ml) |
| CN | 40/S |
| TOB | 9/R |
| AK | 30/S |
| CIP | 38/S |
| OFX | 30/S |
| SXT | 37/S |
| CT | 10/R (24μg/ml) |
AMX, amoxicillin; TIC, ticarcillin; AMC, amoxicillin-clavulanic acid; TCC, ticarcillin-clavulanic acid; FOX, cefoxitin; CTX, cefotaxime; CRO, ceftriaxone; CAZ, ceftazidime; ATM, aztreonam; IMP, imipenem; CN, gentamicin; TOB, tobramycin; AK, amikacin; CIP, ciprofloxacin; OFX, ofloxacin; SXT, sulfamethoxazole-trimethoprim; CT, colistin.
TABLE 3.
Antibiotic resistance genes in the Chryseobacterium oranimense G311 genome
| Antibiotic class | ORF/EBI gene identifier | Putative gene | GC (%) | Size (aa) | Function | Best BLAST hit organism in GenBank | % aa identity | E value |
|---|---|---|---|---|---|---|---|---|
| Beta-lactams | 55/CEJ67779 | penA-like | 41.5 | 663 | Penicillin-binding protein | Chryseobacterium gleum ATCC 35910 | 95.8 | 0.0 |
| 1366/CEJ69070 | blaIND-like | 40.2 | 241 | Metallo-beta-lactamase IND-4 | Chryseobacterium indologenes | 93.7 | 3.00E–165 | |
| 2824/CEJ70497 | blaACC-like | 40.9 | 514 | Beta-lactamase | Chryseobacterium luteum | 95.2 | 0.0 | |
| 4186/CEJ71833 | blaampH-like | 38.6 | 420 | Beta-lactamase | Chryseobacterium vrystaatense | 94.7 | 0.0 | |
| 390/CEJ68108 | blamecA-like | 41.3 | 668 | Penicillin-binding protein 2a (PBP-2a) | Chryseobacterium indologenes NBRC 14944 | 86.0 | 0.0 | |
| 1132/CEJ68841 | blaCME-like | 37.5 | 292 | Beta-lactamase | Chryseobacterium vrystaatense | 87.0 | 0.0 | |
| 1161/CEJ68870 | blaGOB-like | 39.3 | 330 | Beta-lactamase | Chryseobacterium luteum | 88.1 | 0.0 | |
| 2092/CEJ69789 | blaACC-like | 36.5 | 462 | Beta-lactamase | Chitinophaga pinensis | 41.6 | 2.00E–106 | |
| 3736/CEJ71390 | blaCMY-like | 34.5 | 439 | Penicillin-binding protein beta-lactamase class C | Chryseobacterium hispalense | 91.5 | 0.0 | |
| Aminoglycoside | 1104/CEJ68813 | aac6 | 39.5 | 91 | Aminoglycoside N6 acetyltransferase | Chryseobacterium luteum | 83.5 | 5.00E–50 |
| 2703/CEJ70393 | aadK | 41.7 | 287 | Aminoglycoside 6-adenylyltransferase | Chryseobacterium luteum | 79.4 | 3.00E–168 | |
| Tetracycline | 3882/CEJ71533 | otr-like | 43.6 | 524 | emrB-qacA family drug resistance transporter | Chryseobacterium gleum | 97.6 | 0.0 |
| 1319/CEJ69027 | otr-like | 39.2 | 601 | GTP-binding protein of typA-bypA | Chryseobacterium daeguense | 97.6 | 0.0 | |
| 2344/CEJ70041 | tetC | 42.1 | 412 | Tetracycline efflux protein | Chryseobacterium luteum | 93.9 | 0 | |
| 2428/CEJ70124 | tetX-like | 42.6 | 383 | FAD-binding monooxygenase/tetracycline resistance protein | Pedobacter heparinus DSM 2366 | 70.3 | 0.0 | |
| MLS | 752/CEJ68465 | MLS-like | 38.7 | 540 | ABC transporter ATP-binding protein | Chryseobacterium indologenes NBRC14944 | 98.7 | 0.0 |
| 956/CEJ68667 | MLS-like | 40.7 | 233 | ABC transporter-related protein | Chryseobacterium luteum | 98.2 | 5.00E–162 | |
| 3478/CEJ71135 | MLS-like | 39.7 | 642 | ABC superfamily ATP-binding cassette transporter | Chryseobacterium vrystaatense | 94.8 | 0.0 | |
| 4328/CEJ71973 | ole-like | 42.3 | 239 | ABC-type multidrug transport system | Chryseobacterium luteum | 96.2 | 2.00E–165 | |
| 2084/CEJ69781 | ole-like | 41.6 | 303 | ATP-binding cassette transporter | Chryseobacterium luteum | 94.0 | 0.0 | |
| 3848/CEJ71499 | vga-like | 39.4 | 295 | ABC superfamily ATP-binding cassette transporter | Chryseobacterium indologenes NBRC 14944 | 78.3 | 2.00E–164 | |
| Phenicols | 925/CEJ68636 | cfr-like | 42.9 | 344 | Cfr family radical SAM enzyme | Chryseobacterium gleum | 95.9 | 0.0 |
| 1679/69381 | cmlv-like | 44.0 | 404 | Major facilitator family protein/chloramphenicol resistance protein | Chryseobacterium vrystaatense | 93.9 | 0.0 | |
| Glycopeptide | 2837/CEJ70510 | vanL-like | 37.9 | 330 | d-alanine–d-alanine ligase | Chryseobacterium luteum | 97.5 | 0.0 |
| Fluoroquinolones | 908/CEJ68619 | qepA-like | 43.1 | 462 | Drug resistance transporter | Chryseobacterium vrystaatense | 91.1 | 0.0 |
| Sulfonamide | 2063/CEJ69760 | sulIII-like | 37.9 | 293 | Dihydropteroate synthase | Chryseobacterium vrystaatense | 89.1 | 3.00E–167 |
| Rifampin | 3000/CEJ70672 | arr5-like | 42.0 | 141 | Rifampin ADP-ribosyl transferase | Chryseobacterium daeguense | 89.3 | 5.00E–81 |
| Multidrug efflux pumps | 248/CEJ67966 | 41.5 | 396 | MFS superfamily, putative drug resistance transporter | Chryseobacterium indologenes NBRC 14944 | 81.4 | 0.0 | |
| 1382/CEJ69086 | acrB | 42.9 | 1061 | Acriflavin resistance protein | Chryseobacterium daeguense | 96.9 | 0.0 | |
| 1653/CEJ69355 | norM | 44.4 | 467 | MatE efflux family protein | Chryseobacterium vrystaatense | 94.0 | 0.0 | |
| 1766/CEJ69467 | acrB | 42.0 | 790 | Multidrug transporter AcrB | Chryseobacterium gleum | 96.9 | 0.0 | |
| 1767/CEJ69468 | 44.4 | 234 | Putative efflux system protein | Chryseobacterium gleum | 97.2 | 1.00E–145 | ||
| 1829/CEJ69527 | acrB | 44.0 | 1052 | Acriflavin resistance protein | Chryseobacterium vrystaatense | 99.9 | 0.0 | |
| 1830/CEJ69528 | 42.7 | 368 | RND transporter | Epilithonimonas lactis | 100 | 0.0 | ||
| 1911/CEJ69609 | acrB | 38.4 | 1454 | Acriflavin resistance protein B | Epilithonimonas lactis | 99.9 | 0.0 | |
| 2652/CEJ70346 | 41.4 | 483 | RND transporter | Chryseobacterium luteum | 94.1 | 0.0 | ||
| 2653/CEJ70347 | acrB | 42.3 | 1064 | Multidrug transporter | Chryseobacterium hispalense | 98.8 | 0.0 | |
| 3124/CEJ70786 | matE | 41.6 | 464 | Multidrug transporter MatE | Chryseobacterium luteum | 96.9 | 0.0 | |
| 3569/CEJ71225 | 43.3 | 386 | RND transporter | Chryseobacterium luteum | 93.5 | 0.0 | ||
| 3570/CEJ71226 | acrB | 43.2 | 1059 | Multidrug transporter | Chryseobacterium luteum | 97.4 | 0.0 | |
| 3571/CEJ71227 | 41.9 | 470 | RND transporter | Chryseobacterium vrystaatense | 95.7 | 0.0 | ||
| 4072/CEJ71722 | matE | 39.7 | 451 | Multidrug transporter MatE | Chryseobacterium luteum | 96.8 | 0.0 | |
| 1440/CEJ69143 | emrB | 40.8 | 520 | ABC superfamily ATP-binding cassette transporter | Chryseobacterium gleum | 90.5 | 0.0 | |
| 1945/CEJ69643 | 43.0 | 349 | Putative major facilitator superfamily transporter | Chryseobacterium indologenes NBRC 14944 | 84.5 | 0.0 | ||
| 2257/CEJ69954 | 43.2 | 352 | MFS transporter | Chryseobacterium vrystaatense | 95.4 | 0.0 | ||
| 2307/CEJ70004 | 38.6 | 387 | MFS transporter | “Candidatus Solibacter usitatus” | 26.3 | 4.00E–26 | ||
| 3981/CEJ71631 | araJ | 42.3 | 310 | Putative major facilitator superfamily transporter | Chryseobacterium vrystaatense | 83.2 | 2.00E–176 | |
| 3138/CEJ70800 | mdlB | 38.4 | 553 | Putative ABC transporter ATP-binding/permease protein | Chryseobacterium luteum | 96.9 | 0.0 | |
| 882/CEJ68593 | 39.7 | 730 | Putative ABC transporter ATP-binding protein | Chryseobacterium luteum | 97.2 | 0.0 | ||
| 1090/CEJ68799 | 40.6 | 396 | ABC transporter ATP permease protein | Chryseobacterium vrystaatense | 93.6 | 0.0 |
TABLE 4.
Known mutations in genes conferring resistance to colistin and corresponding variants in Chryseobacterium oranimense G311, C. gleum ATCC 35910, and Chryseobacterium sp. strain CF314 variants
| Gene | Known mutation (reference) | Organism | Mutation |
||
|---|---|---|---|---|---|
| C. oranimense G311 (EBI gene identifier no.) | C. gleum ATCC 35910 | Chryseobacterium sp. strain CF314 | |||
| pmrA | E8D (65) | Acinetobacter baumannii | E8D (CEJ70734) | E8D | E8D |
| G53S (62) | Enterobacter aerogenes | No mutation (CEJ70734) | No mutation | No mutation | |
| pmrB | L208F (64) | Acinetobacter baumannii | L208F (CEJ70733) | No mutation | No mutation |
| P360Q (64) | Acinetobacter baumannii | P360Q (CEJ70733) | No mutation | No mutation | |
| lpxA | G68D (65) | Acinetobacter baumannii | G68D (CEJ70978) | G68D | G68D |
| Q72K (65) | Acinetobacter baumannii | No mutation (CEJ70978) | No mutation | No mutation | |
| H159D (65) | Acinetobacter baumannii | No mutation (CEJ70978) | No mutation | No mutation | |
| Insertional inactivation (66) | Acinetobacter baumannii | No mutation (CEJ70978) | No mutation | No mutation | |
| lpxC | I38T (67) | E. coli | No mutation (CEJ70979) | No mutation | No mutation |
| P30L (66) | Acinetobacter baumannii | No mutation (CEJ70979) | No mutation | No mutation | |
| Insertional inactivation (66) | Acinetobacter baumannii | No mutation (CEJ70979) | No mutation | No mutation | |
| lpxD | M290A (68) | E. coli | No mutation (CEJ70980) | No mutation | No mutation |
| phoP | NDa | ||||
| phoQ | D179(L/A) (4) | E. coli | No mutation (CEJ67802) | No mutation | No mutation |
ND, not detected.
Specific features of the C. oranimense G311 genome.
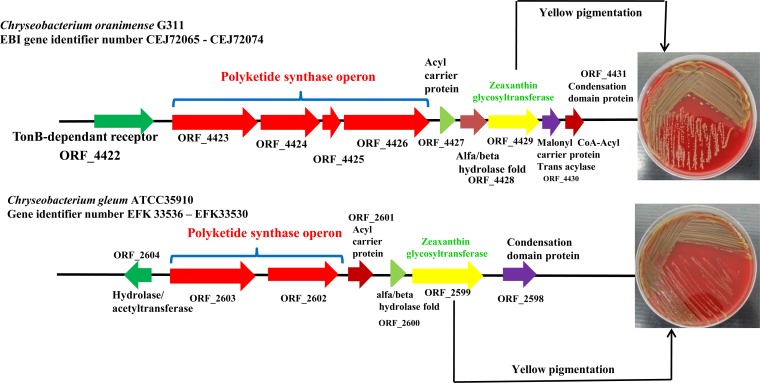
We found an operon (20,162 bp) comprised of modular polyketide synthase genes and a zeaxanthin glycosyltransferase gene. We noted a similar arrangement in the size of 18,518 bp in C. gleum ATCC 35910 (Fig. 4), though we did not find this arrangement in the Chryseobacterium sp. strain CF314 genome. The presence of zeaxanthin, a carotenoid pigment, in the genome likely explains the yellowish color of our CF isolate. We located the O-antigen biosynthesis cluster in the genome of the C. oranimense isolate, as shown in Fig. S1 in the supplemental material (open reading frame [ORF] 569 [CEJ68283] to ORF 601 [CEJ68319]). Similar clusters are present in the genomes of C. gleum ATCC 35910 and Chryseobacterium sp. strain CF314 (see Fig. S1 in the supplemental material). In addition, a new capsular polysaccharide (CPS) biosynthesis was identified in the genome of C. oranimense (see Fig. S2 in the supplemental material) (ORF 1284 [CEJ68992] to ORF 1317 [CEJ69025]). We could not find a similar cluster in the other genomes analyzed. The list of all the genes of O-antigen-like and K-antigen-like clusters is shown in Table S2 in the supplemental material.
FIG 4.
Polyketide synthase operon arrangement in Chryseobacterium oranimense G311 and Chryseobacterium gleum ATCC 35910.
DISCUSSION
Chryseobacterium species are found in a variety of habitats and essentially ubiquitous, though some are opportunist pathogens (32). C. oranimense has been reported to be isolated from raw milk (30), yet this is the first report of C. oranimense in humans, i.e., from a CF patient. Chryseobacterium species are multidrug resistant, with most intrinsically resistant to penicillin, first- and second-generation cephalosporin, aztreonam (14, 33), and colistin (16), and have been reported to be acquired nosocomially via medical devices and contaminated water supplies in hospitals (18). Using a polyphasic approach, some studies have reported the presence of unusual bacteria, such as Acinetobacter spp., Bordetella spp., Comamonas spp., Rhizobium spp., Herbaspirillum spp., Moraxella spp., I. limosus, and Chryseobacterium spp., in the sputum samples from CF patients (34). Although the emergence of new multidrug-resistant, Gram-negative bacteria in CF lung infections has been relatively low, the incidence is increasing considerably, presenting a serious challenge for the development of effective and appropriate antibiotic therapies when they are misidentified. It is known that Chryseobacterium spp. cause infections in immunocompromised patients (13, 14), and their existence in CF airways has been reported over the last 10 years (34). One study reports E. meningoseptica and C. indologenes as the most frequently isolated species, followed by C. gleum and coinfections with at least one Gram-negative bacterium, such as P. aeruginosa, A. xylosoxidans, S. maltophilia, or B. cepacia complex in CF patients (34). Many of the isolates in the above-named study were found to be resistant to imipenem but were not checked for resistance to colistin, whereas our multidrug isolate was found to be resistant to both imipenem and colistin. As the life expectancy of CF patients has increased, antimicrobial pressure has also experienced an increase, and, consequently, more multidrug-resistant microorganisms are being isolated from the CF lung microbiota. Importantly, as these bacteria have developed multiple mechanisms of antibiotic resistance, they must be identified correctly for designing therapeutic treatments.
The genomic comparison of C. oranimense G311 with the available genomes of Chryseobacterium gleum ATCC 35910 and Chryseobacterium sp. strain CF314 (35) revealed similar genome sizes and GC contents, and none of them harbored any plasmid (Table 1). Apart from deciphering the resistome of this atypical bacterium, which will be discussed in details below, we identified three specific features in the C. oranimense G311 genome. First, the presence of PKS might play a role in the synthesis of zeaxanthin, a secondary metabolite imparting the yellowish pigmentation of the isolate. Flavobacterium multivorum has been widely studied for the production of the xanthophyll carotenoid zeaxanthin, as this species could be used as a commercial source of zeaxanthin (36). High intake of foods providing zeaxanthin is related with lower incidence of age-related macular degeneration (ARMD), mostly for ocular and retinal health. They are used as supplemental antioxidants in treating ARMD (37). The presence of this bacterium, which produces beta-carotene, in a clinical isolate in the context of CF was unexpected, and the role this bacterium may play in the lung microbiota remains to be studied in the future. Second, the lipopolysaccharide (LPS) cluster in the genome of this bacterium could be acquired laterally and to the best of our knowledge was unknown in this genus. This cluster consisted of glycosyltransferases (see Table S2 in the supplemental material) that likely contribute to modification of LPS (38), which is a well-known phenomenon associated with resistance to polymyxins (38–41). Third, C. oranimense G311 also harbors a new capsular polysaccharide biosynthesis (K-antigen) gene cluster that was unique to this genome and also acquired laterally. Within this cluster, the wza and wzc genes have been described as outer membrane lipoprotein and integral inner membrane protein/protein tyrosine kinase, respectively, in some human pathogens, such as K. pneumoniae K2 and Escherichia coli K-12 (42–45). Another gene product, ugd, was identified, which is involved in the production of UDP-4-amino-4-deoxy-l-arabinose, a compound that renders E. coli resistant to cationic antimicrobial peptides (46). The ugd produces UDP-glucuronic acid (UDPGA), which plays a role in the production of a sugar derivative, UDP-4-amino-4-deoxy-l-arabinose (l-Ara4N), which is vital for bacterial resistance to polymyxin (38, 47). Capsular clusters in the genus Flavobacterium have been reported in Flavobacterium columnare ATCC 43622 (48), Flavobacterium psychrophilum strain 259-93 (49), and Zunongwangia profunda SM-A87 (50).
The resistome of C. oranimense G311 comprises a reservoir of diverse β-lactamases, including a class A β-lactamase gene, blaCME, and the cme-1 gene has been reported to be structurally divergent from other class A enzymes (51) in E. meningoseptica. The cme-1 gene encodes a clavulanic acid-susceptible extended-spectrum β-lactamase that hydrolyzes most of the cephalosporins, such as cefotaxime and ceftazidime, and monobactams, such as aztreonam, though it does not hydrolyze cephamycins and carbapenems. The C. oranimense G311 cme-like gene clustered with the cme-1 gene reported from E. meningoseptica (data not shown). Another class A β-lactamase gene, penA, encodes penicillin-binding protein PBP-2a, which is a mecA gene product that can result in ceftazidime and amoxicillin-clavulanic acid resistance if it is overproduced or mutated (52, 53). We discovered two genes for class B metallo-β-lactamases: a blaGOB-like gene and a putative metallo-β-lactamase blaIND gene. Class B lactamases (generically termed metallo-β-lactamases) employ one or two Zn(II) ions for cleaving the β-lactam ring. The Gob-18 is fully active against a broad range of β-lactam substrates and has been reported from E. meningoseptica (54); many more variants of gob genes have recently been reported from this species, which is known to be intrinsically resistant to most β-lactams, including carbapenems (55). The blaIND-4 gene found in the C. oranimense G311 genome is 93.7% similar to blaIND-4 from C. indologenes 009 (56), an enzyme that is able to hydrolyze carbapenems. We also discovered many class C extended-spectrum β-lactamases (ESBLs), such as blaACC-, blaampH-, blaACC-4-, and blaCMY-like; however, certain genes, such as blaACC-like, showed similarities with the reported genes from plant sources (57).
The most common mechanism of resistance to colistin is modification of the LPS structure (58). Intrinsic resistance to polymyxins in Burkholderia cenocepacia and Proteus mirabilis has been linked to alterations in their lipid A structure with the addition at the 4′-phosphate moiety of the LPS of 4-amino-l-arabinopyranose and 4-amino-l-arabinose (l-Ara4N), respectively (59, 60). Such modifications have also been reported for K. pneumoniae and E. coli (58). In K. pneumoniae, the resistance to polymyxin is due to increased production of capsular polysaccharides (61). Recently, it has been demonstrated that in Acinetobacter baumannii or in Enterobacter aerogenes, acquired resistance to colistin may also be due to mutations in the pmrA-pmrB two-component systems (62, 63). Finally loss of LPS production by mutations in the three genes lpxA, lpxC, and lpxD has been associated with the resistance to Acinetobacter baumannii (64). Here, we found similar variants of pmrA (E8D) (63), pmrB (L208F, P360Q) (64), and lpxA (G68D) (Table 4) (65) that confer resistance to colistin in Acinetobacter baumannii (63–65). Thus, we believe the intrinsic resistance of C. oranimense G311 to colistin is due to both alterations in LPS and production of capsular polysaccharides.
Conclusion.
In conclusion, we believe that the increased clinical use of nebulized colistin in patients with CF might have led to the selection of this specific colistin-resistant bacterium. Our findings provide insight into the mechanism of colistin resistance in the genus Chryseobacterium, as it is well known that many clinically significant species from this genus are intrinsically resistant to many antimicrobial agents. This bacterium could be considered an opportunistic human pathogen in immunocompromised patients. We demonstrate that whole-genome sequencing was successfully applied to completely decipher the resistome of this multidrug-resistant bacterium associated with CF patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Linda Hadjadj for technical assistance.
There are no conflicts of interest to declare.
This work was partly funded by CNRS.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02417-14.
REFERENCES
- 1.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N. 1989. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MB, Gilligan PH. 2002. Laboratory aspects of management of chronic pulmonary infections in patients with cystic fibrosis. J Clin Microbiol 41:4009–4015. doi: 10.1128/JCM.41.9.4009-4015.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LiPuma JJ. 2010. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denton M, Kerr K, Mooney L, Keer V, Rajgopal A, Brownlee K, Arundel P, Conway S. 2002. Transmission of colistin-resistant Pseudomonas aeruginosa between patients attending a pediatric cystic fibrosis center. Pediatr Pulmonol 34:257–261. doi: 10.1002/ppul.10166. [DOI] [PubMed] [Google Scholar]
- 7.Johansen HK, Moskowitz SM, Ciofu O, Pressler T, Hoiby N. 2008. Spread of colistin resistant non-mucoid Pseudomonas aeruginosa among chronically infected Danish cystic fibrosis patients. J Cyst Fibros 7:391–397. doi: 10.1016/j.jcf.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bittar F, Leydier A, Bosdure E, Toro A, Reynaud-Gaubert M, Boniface S, Stremler N, Dubus JC, Sarles J, Raoult D, Rolain JM. 2008. Inquilinus limosus and cystic fibrosis. Emerg Infect Dis 14:993–995. doi: 10.3201/eid1406.071355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menuet M, Bittar F, Stremler N, Dubus JC, Sarles J, Raoult D, Rolain JM. 2008. First isolation of two colistin-resistant emerging pathogens, Brevundimonas diminuta and Ochrobactrum anthropi, in a woman with cystic fibrosis: a case report. J Med Case Rep 2:373. doi: 10.1186/1752-1947-2-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, Ramsey BW, Clausen CR. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis 27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 11.Hogardt M, Schmoldt S, Gotzfried M, Adler K, Heesemann J. 2004. Pitfalls of polymyxin antimicrobial susceptibility testing of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother 54:1057–1061. doi: 10.1093/jac/dkh470. [DOI] [PubMed] [Google Scholar]
- 12.Spencer RC. 1995. The emergence of epidemic, multiple-antibiotic-resistant Stenotrophomonas (Xanthomonas) maltophilia and Burkholderia (Pseudomonas) cepacia. J Hosp Infect 30(Suppl):453–464. [DOI] [PubMed] [Google Scholar]
- 13.Smith J, Han R, Mailman T, MacDonald N. 2012. Chryseobacterium indologenes: distinguishing pathogen from contaminant in a neonate. J Ped Infect Dis 7:31–35. doi: 10.3233/JPI-2012-0337. [DOI] [Google Scholar]
- 14.Reynaud I, Chanteperdrix V, Broux C, Pavese P, Croize J, Maurin M, Stahl JP, Jacquot C. 2007. A severe form of Chryseobacterium indologenes pneumonia in an immunocompetent patient. Med Mal Infect 37:762–764. doi: 10.1016/j.medmal.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Chou DW, Wu SL, Lee CT, Tai FT, Yu WL. 2011. Clinical characteristics, antimicrobial susceptibilities, and outcomes of patients with Chryseobacterium indologenes bacteremia in an intensive care unit. Jpn J Infect Dis 64:520–524. [PubMed] [Google Scholar]
- 16.Chen FL, Wang GC, Teng SO, Ou TY, Yu FL, Lee WS. 2013. Clinical and epidemiological features of Chryseobacterium indologenes infections: analysis of 215 cases. J Microbiol Immunol Infect 46(6):425–432. doi: 10.1016/j.jmii.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Vandamme P, Bernardet JF, Segers P, Kersters K, Holmes B. 1994. New perspectives in the classification of the flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov, and Empedobacter norn. rev. Int J Syst Bacteriol 44:827–831. [Google Scholar]
- 18.Maravic A, Skocibusic M, Samanic I, Puizina J. 2013. Profile and multidrug resistance determinants of Chryseobacterium indologenes from seawater and marine fauna. World J Microbiol Biotechnol 29:515–522. doi: 10.1007/s11274-012-1205-0. [DOI] [PubMed] [Google Scholar]
- 19.Lambiase A, Raia V, Del Pezzo M, Sepe A, Carnovale V, Rossano F. 2006. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis 6:4. doi: 10.1186/1471-2334-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambiase A, Del Pezzo M, Raia V, Sepe A, Ferri P, Rossano F. 2007. Chryseobacterium respiratory tract infections in patients with cystic fibrosis. J Infect 55:518–523. doi: 10.1016/j.jinf.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Lambiase A, Raia V, Stefani S, Sepe A, Ferri P, Buonpensiero P, Rossano F, Del Pezzo M. 2007. Burkholderia cepacia complex infection in a cohort of Italian patients with cystic fibrosis. J Microbiol 45:275–279. [PubMed] [Google Scholar]
- 22.Zankari E, Hasman H, Kaas RS, Seyfarth AM, Agerso Y, Lund O, Larsen MV, Aarestrup FM. 2013. Genotyping using whole-genome sequencing is a realistic alternative to surveillance based on phenotypic antimicrobial susceptibility testing. J Antimicrob Chemother 68:771–777. doi: 10.1093/jac/dks496. [DOI] [PubMed] [Google Scholar]
- 23.Snitkin ES, Zelazny A, Gupta J, Palmore TN, Murray PR, Segre JA. 2013. Genomic insights into the fate of colistin resistance and Acinetobacter baumannii during patient treatment. Genome Res 23(7):1155–1162. doi: 10.1101/gr.154328.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, Raoult D. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 25.Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect 7:88–91. doi: 10.1046/j.1469-0691.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 26.Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. 2002. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol 40:3798–3801. doi: 10.1128/JCM.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard L, Vaudaux P, Rohner P, Huggler E, Armanet M, Pittet D, Lew DP, Schrenzel J. 2004. Comparative analysis and validation of different assays for glycopeptide susceptibility among methicillin-resistant Staphylococcus aureus strains. J Microbiol Methods 57:231–239. doi: 10.1016/j.mimet.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta SK, Padmanabhan B, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, Rolain J-M. 2014. ARG-ANNOT (Antibiotic Resistance Gene-ANNOTation), a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hantsis-Zacharov E, Shaked T, Senderovich Y, Halpern M. 2008. Chryseobacterium oranimense sp. nov., a psychrotolerant, proteolytic and lipolytic bacterium isolated from raw cow's milk. Int J Syst Evol Microbiol 58:2635–2639. doi: 10.1099/ijs.0.65819-0. [DOI] [PubMed] [Google Scholar]
- 31.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodford N, Palepou MF, Babini GS, Holmes B, Livermore DM. 2000. Carbapenemases of Chryseobacterium (Flavobacterium) meningosepticum: distribution of blaB and characterization of a novel metallo-beta-lactamase gene, blaB3, in the type strain, NCTC 10016. Antimicrob Agents Chemother 44:1448–1452. doi: 10.1128/AAC.44.6.1448-1452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasmin S, Garcia G, Sylvester T, Sunenshine R. 2013. Chryseobacterium indologenes in a woman with metastatic breast cancer in the United States of America: a case report. J Med Case Rep 7:190. doi: 10.1186/1752-1947-7-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coenye T, Goris J, Spilker T, Vandamme P, LiPuma JJ. 2002. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J Clin Microbiol 40:2062–2069. doi: 10.1128/JCM.40.6.2062-2069.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown SD, Utturkar SM, Klingeman DM, Johnson CM, Martin SL, Land ML, Lu TY, Schadt CW, Doktycz MJ, Pelletier DA. 2012. Twenty-one genome sequences from Pseudomonas species and 19 genome sequences from diverse bacteria isolated from the rhizosphere and endosphere of Populus deltoides. J Bacteriol 194:5991–5993. doi: 10.1128/JB.01243-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhosale P, Bernstein PS. 2004. Beta-carotene production by Flavobacterium multivorum in the presence of inorganic salts and urea. J Ind Microbiol Biotechnol 31:565–571. doi: 10.1007/s10295-004-0187-9. [DOI] [PubMed] [Google Scholar]
- 37.Krishnadev N, Meleth AD, Chew EY. 2010. Nutritional supplements for age-related macular degeneration. Curr Opin Ophthalmol 21:184–189. doi: 10.1097/ICU.0b013e32833866ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raetz CR, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem 76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bornet C, Davin-Regli A, Bosi C, Pages JM, Bollet C. 2000. Imipenem resistance of Enterobacter aerogenes mediated by outer membrane permeability. J Clin Microbiol 38:1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiolas A, Bollet C, La Scola B, Raoult D, Pages JM. 2005. Successive emergence of Enterobacter aerogenes strains resistant to imipenem and colistin in a patient. Antimicrob Agents Chemother 49:1354–1358. doi: 10.1128/AAC.49.4.1354-1358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingram BO, Sohlenkamp C, Geiger O, Raetz CR. 2010. Altered lipid A structures and polymyxin hypersensitivity of Rhizobium etli mutants lacking the LpxE and LpxF phosphatases. Biochim Biophys Acta 1801:593–604. doi: 10.1016/j.bbalip.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulsen IT, Beness AM, Saier MH Jr. 1997. Computer-based analyses of the protein constituents of transport systems catalysing export of complex carbohydrates in bacteria. Microbiology 143(Part 8):2685–2699. [DOI] [PubMed] [Google Scholar]
- 43.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. 1995. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol 177:1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perna NT, Plunkett G III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta ET, Potamousis KD, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 45.Reeves PR, Hobbs M, Valvano MA, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz CR, Rick PD. 1996. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol 4:495–503. doi: 10.1016/S0966-842X(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 46.Lacour S, Bechet E, Cozzone AJ, Mijakovic I, Grangeasse C. 2008. Tyrosine phosphorylation of the UDP-glucose dehydrogenase of Escherichia coli is at the crossroads of colanic acid synthesis and polymyxin resistance. PLoS One 3:e3053. doi: 10.1371/journal.pone.0003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trent MS, Ribeiro AA, Lin S, Cotter RJ, Raetz CR. 2001. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-l-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J Biol Chem 276:43122–43131. doi: 10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- 48.MacLean LL, Perry MB, Crump EM, Kay WW. 2003. Structural characterization of the lipopolysaccharide O-polysaccharide antigen produced by Flavobacterium columnare ATCC 43622. Eur J Biochem 270:3440–3446. doi: 10.1046/j.1432-1033.2003.03736.x. [DOI] [PubMed] [Google Scholar]
- 49.MacLean LL, Vinogradov E, Crump EM, Perry MB, Kay WW. 2001. The structure of the lipopolysaccharide O-antigen produced by Flavobacterium psychrophilum (259-93). Eur J Biochem 268:2710–2716. doi: 10.1046/j.1432-1327.2001.02163.x. [DOI] [PubMed] [Google Scholar]
- 50.Qin QL, Zhang XY, Wang XM, Liu GM, Chen XL, Xie BB, Dang HY, Zhou BC, Yu J, Zhang YZ. 2010. The complete genome of Zunongwangia profunda SM-A87 reveals its adaptation to the deep-sea environment and ecological role in sedimentary organic nitrogen degradation. BMC Genomics 11:247. doi: 10.1186/1471-2164-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossolini GM, Franceschini N, Lauretti L, Caravelli B, Riccio ML, Galleni M, Frere JM, Amicosante G. 1999. Cloning of a Chryseobacterium (Flavobacterium) meningosepticum chromosomal gene (blaA(CME)) encoding an extended-spectrum class A beta-lactamase related to the Bacteroides cephalosporinases and the VEB-1 and PER beta-lactamases. Antimicrob Agents Chemother 43:2193–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schweizer HP. 2012. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 7:1389–1399. doi: 10.2217/fmb.12.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim C, Milheirico C, Gardete S, Holmes MA, Holden MT, de Lencastre H, Tomasz A. 2012. Properties of a novel PBP2A protein homolog from Staphylococcus aureus strain LGA251 and its contribution to the beta-lactam-resistant phenotype. J Biol Chem 287:36854–36863. doi: 10.1074/jbc.M112.395962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moran-Barrio J, Gonzalez JM, Lisa MN, Costello AL, Peraro MD, Carloni P, Bennett B, Tierney DL, Limansky AS, Viale AM, Vila AJ. 2007. The metallo-beta-lactamase GOB is a mono-Zn(II) enzyme with a novel active site. J Biol Chem 282:18286–18293. doi: 10.1074/jbc.M700467200. [DOI] [PubMed] [Google Scholar]
- 55.Yum JH, Lee EY, Hur SH, Jeong SH, Lee H, Yong D, Chong Y, Lee EW, Nordmann P, Lee K. 2010. Genetic diversity of chromosomal metallo-beta-lactamase genes in clinical isolates of Elizabethkingia meningoseptica from Korea. J Microbiol 48:358–364. doi: 10.1007/s12275-010-9308-5. [DOI] [PubMed] [Google Scholar]
- 56.Bellais S, Poirel L, Leotard S, Naas T, Nordmann P. 2000. Genetic diversity of carbapenem-hydrolyzing metallo-beta-lactamases from Chryseobacterium (Flavobacterium) indologenes. Antimicrob Agents Chemother 44:3028–3034. doi: 10.1128/AAC.44.11.3028-3034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glavina Del Rio T, Abt B, Spring S, Lapidus A, Nolan M, Tice H, Copeland A, Cheng JF, Chen F, Bruce D, Goodwin L, Pitluck S, Ivanova N, Mavromatis K, Mikhailova N, Pati A, Chen A, Palaniappan K, Land M, Hauser L, Chang YJ, Jeffries CD, Chain P, Saunders E, Detter JC, Brettin T, Rohde M, Goker M, Bristow J, Eisen JA, Markowitz V, Hugenholtz P, Kyrpides NC, Klenk HP, Lucas S. 2010. Complete genome sequence of Chitinophaga pinensis type strain (UQM 2034). Stand Genomic Sci 2:87–95. doi: 10.4056/sigs.661199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. 2012. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther 10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 59.Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin Microbiol Rev 21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamad MA, Di Lorenzo F, Molinaro A, Valvano MA. 2012. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia. Mol Microbiol 85:962–974. doi: 10.1111/j.1365-2958.2012.08154.x. [DOI] [PubMed] [Google Scholar]
- 61.Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA. 2004. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun 72:7107–7114. doi: 10.1128/IAI.72.12.7107-7114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diene SM, Merhej V, Henry M, El FA, Roux V, Robert C, Azza S, Gavory F, Barbe V, La SB, Raoult D, Rolain JM. 2013. The rhizome of the multidrug-resistant Enterobacter aerogenes genome reveals how new “killer bugs” are created because of a sympatric lifestyle. Mol Biol Evol 30:369–383. doi: 10.1093/molbev/mss236. [DOI] [PubMed] [Google Scholar]
- 63.Rolain JM, Diene SM, Kempf M, Gimenez G, Robert C, Raoult D. 2013. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob Agents Chemother 57:592–596. doi: 10.1128/AAC.01314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. 2011. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St. Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. 2011. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother 55:3022–3024. doi: 10.1128/AAC.01732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clements JM, Coignard F, Johnson I, Chandler S, Palan S, Waller A, Wijkmans J, Hunter MG. 2002. Antibacterial activities and characterization of novel inhibitors of LpxC. Antimicrob Agents Chemother 46:1793–1799. doi: 10.1128/AAC.46.6.1793-1799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartling CM, Raetz CR. 2009. Crystal structure and acyl chain selectivity of Escherichia coli LpxD, the N-acyltransferase of lipid A biosynthesis. Biochemistry 48:8672–8683. doi: 10.1021/bi901025v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.