Abstract
Forty percent of the world's population is threatened by malaria, which is caused by Plasmodium parasites and results in an estimated 200 million clinical cases and 650,000 deaths each year. Drug resistance has been reported for all commonly used antimalarials and has prompted screens to identify new drug candidates. However, many of these new candidates have not been evaluated against the parasite stage responsible for transmission, gametocytes. If Plasmodium falciparum gametocytes are not eliminated, patients continue to spread malaria for weeks after asexual parasite clearance. Asymptomatic individuals can also harbor gametocyte burdens sufficient for transmission, and a safe, effective gametocytocidal agent could also be used in community-wide malaria control programs. Here, we identify 15 small molecules with nanomolar activity against late-stage gametocytes. Fourteen are diaminonaphthoquinones (DANQs), and one is a 2-imino-benzo[d]imidazole (IBI). One of the DANQs identified, SJ000030570, is a lead antimalarial candidate. In contrast, 94% of the 650 compounds tested are inactive against late-stage gametocytes. Consistent with the ineffectiveness of most approved antimalarials against gametocytes, of the 19 novel compounds with activity against known anti-asexual-stage targets, only 3 had any strong effect on gametocyte viability. These data demonstrate the distinct biology of the transmission stages and emphasize the importance of screening for gametocytocidal activity. The potent gametocytocidal activity of DANQ and IBI coupled with their efficacy against asexual parasites provides leads for the development of antimalarials with the potential to prevent both the symptoms and the spread of malaria.
INTRODUCTION
Effective chemotherapy remains a critical component of current malaria control strategies and is essential to treat severe malaria (1). The introduction of artemisinin combination therapies (ACTs) has successfully lowered malaria mortality but does not effectively control the spread of the disease, because ACTs do not eliminate the sexual stages of the parasite that are required for malaria transmission (2, 3). As a consequence, patients remain infectious for over a week after asexual parasite clearance and the cessation of symptoms. Moreover, the identification of parasite lines with delayed parasite clearance following ACT treatment has spurred the effort to identify new antimalarials (4). Several recent screens of novel small-molecule libraries against asexual parasites have expanded the repertoire of potential candidates for treating acute malaria, but the analysis of their effects on the sexual stages is just beginning and has been focused on the 400 molecules included in the malaria box (5–13). Only 12 of the 260 antimalaria compounds analyzed in this study are also present in the malaria box.
Both gametocytes and asexual parasites develop within the erythrocyte, but they have distinct developmental patterns that contribute to their differential sensitivity to common antimalarials (14, 15). While Plasmodium falciparum asexual stages undergo 4 to 5 rounds of DNA replication to produce 16 to 32 new parasites over the course of 48 h, gametocytes differentiate through 5 morphologically distinct stages (stages I to V) into a single male or female gametocyte over 10 to 12 days (16). To completely block transmission, all these stages need to be eliminated during the course of treatment. The lack of DNA replication during gametocyte development provides resistance to drugs that target nucleic acid production, such as sulfadoxine-pyrimethamine, atovaquone, and dihydroorotate dehydrogenase (DHODH) inhibitors (17). Additionally, stage III to V gametocytes are no longer affected by compounds that block hemoglobin digestion, such as the 4-aminoquinolines and cysteine protease inhibitors (17, 18). Gametocytes are also resistant to sorbitol lysis, suggesting a reduction in permeability pathways, such as the plasmodial surface anion channel (PSAC) (19–21). The lack of PSAC could affect drug accessibility, as shown for blasticidin and leupeptin (22). Likewise, gametocytes are not cleared by antibacterial agents that target the apicoplast, such as clindamycin and tetracycline analogs (23). Additional apicoplast-specific enzyme systems have not yet been evaluated in gametocytes (24). In contrast, proteasome and protein synthesis inhibitors are quite effective against all parasite stages (18, 25–27), including late-stage gametocytes, which indicates the presence of shared pathways that could be targets of drugs with activities against both asexual- and sexual-stage parasites.
Here, we used the gametocyte viability assay that we developed (28) and validated (29) to screen a library of 260 lead-like compounds with activity against asexual parasites. The results indicate that the majority of the anti-asexual-stage compounds tested were inactive (>80% viability after treatment), including novel inhibitors of hemozoin formation and pyrimidine synthesis. This finding is consistent with the limited gametocytocidal activity of commonly used antimalarials and also demonstrates the specificity of the assay for late-stage gametocytes. However, 9% of the compounds (23/260) did decrease gametocyte viability by more than 50%, suggesting the presence of targets that are important for both asexual and sexual development. These 23 gametocytocidal compounds are members of five different chemotypes: diaminonaphthoquinones (DANQ), dihydropyridines (DHP), bisphenylbenzimidazoles (BPBI), carbazoleaminopropanols (CAP), and iminobenzimidazoles (IBI). Two of these scaffolds, DANQ and DHP, have been identified as leads against asexual parasites (30). Follow-up studies that screened 390 additional compounds to define structure-gametocytocidal activity profiles identified 15 compounds with 50% effective concentrations (EC50s) in the nanomolar range.
MATERIALS AND METHODS
Chemical preparation.
All compounds used in these studies were purchased from vendors and used without further purification. Prior to use, the identity of each compound was confirmed by ultra-high-performance liquid chromatography (UPLC)-mass spectrometry (MS), and their purities were confirmed to be greater than 95% by UPLC–evaporative light-scattering detection (ELSD)-UV–MS. Stock solutions were prepared at a nominal concentration of 10 mM in dimethyl sulfoxide (DMSO), and the concentrations were confirmed by chemiluminescent nitrogen detection (CLND) prior to use.
P. falciparum gametocytocidal assay. (i) alamarBlue viability assay.
The gametocyte induction and gametocytocidal assays were performed using P. falciparum strain 3D7 as described previously (28). Briefly, parasite cultures were maintained in complete RPMI (RPMI 1640, 25 mM HEPES, 25 mM NaHCO3 [pH 7.3], 100 μg ml−1 hypoxanthine, and 5 μg ml−1 gentamicin [KD Biomedical, Columbia, MD]) supplemented with 10% human serum (Interstate Blood Bank, Memphis, TN). Gametocyte cultures were set up at 0.1% parasitemia and 6% hematocrit on day 0. On day 3, the hematocrit was reduced to 3% by increasing the amount of medium added during the daily feed. Following N-acetylglucosamine (NAG; 50 mM) treatment on days 8 to 10 to eliminate asexual parasites, stage III/IV/V gametocytes were purified on a 65% Percoll gradient and returned to culture. The next day, the parasites were resuspended at 10% gametocytemia, 0.5% hematocrit and aliquoted into a 96-well plate containing the test compounds or positive (30 nM epoxomicin) and negative (DMSO) controls. After incubation at 37°C for 3 days, 1/10 volume of the fluorescent viability indicator dye alamarBlue was added, and 24 h later, the fluorescence was determined at 590/35 nm following excitation at 530/25 nm. For compounds that interfere with alamarBlue reduction, wash steps were added before the addition of alamarBlue to dilute the compound 2,500-fold. To do this, 100 μl of incomplete medium was added to each well at the end of the incubation period instead of alamarBlue, resulting in a 2-fold dilution. After centrifugation at 1,860 × g for 2 min, 150 μl of supernatant was removed from each well and 200 μl of incomplete medium was added, resulting in a 5-fold dilution. After centrifugation, 200 μl of supernatant was removed and replaced with 200 μl of incomplete medium, resulting in another 5-fold dilution, and this procedure was repeated twice more. After the last centrifugation, 200 μl of supernatant was removed and 50 μl/well complete medium (10% human serum) was added, resulting in a 2-fold dilution, for a final dilution of 2,500 (2 × 5 × 5 × 5 × 5 × 2) before the addition of 1/10 volume of alamarBlue.
(ii) Gametocytocidal confirmation assays.
Zero, 12, 24, 48, and 72 h after the addition of test compounds, samples (5% gametocytemia and 2 to 3% hematocrit) were washed 3 times with complete RPMI and analyzed using alamarBlue, Giemsa-stained smears, or MitoProbe DiIC1(5), a membrane potential-sensitive cyanine dye (Life Technologies). Samples probed with alamarBlue were incubated for 24 h before the fluorescent signal was determined as previously described. For MitoProbe DiIC1(5) staining, 20 μl of the washed, compound-treated sample was diluted to 200 μl with buffer containing 1.67 mg ml–1 glucose, 8 mg ml–1 NaCl, 8 mM Tris-Cl (pH 8.2) and incubated with 50 nM MitoProbe DiIC1(5) for 30 min prior to flow cytometry (AccuriC6; BD). Uninfected red blood cells (RBCs) incubated with MitoProbe DiIC1(5) and unstained P. falciparum-infected RBCs were used as controls to determine the threshold for MitoProbe DiIC1(5)-positive single, intact cells (640 nm laser excitation and FL4 emission filter [675/25 nm]). All experiments were done in triplicate.
(iii) Exflagellation assay.
Twenty-four to 48 h after Percoll purification, gametocytes were diluted to 10% parasitemia using fresh human RBCs. Parasites were resuspended to 0.5% hematocrit with complete RMPI 1640 medium containing 10% human serum and test compounds at different concentrations or carrier alone. The cultures were gassed with 90% N2, 5% O2, 5% CO2 and allowed to incubate at 37°C for 72 h. To measure exflagellation, a 500-μl aliquot was pelleted by centrifugation (900 × g) and resuspended in 10 μl room temperature human serum with 100 μM xanthurenic acid. Following a 15-min incubation, 5 μl was applied to a hemocytometer and the number of exflagellation centers counted in 50 fields using a 40× objective.
(iv) P. falciparum asexual growth assay.
Asynchronous parasites were maintained in culture based on the method of Trager and Jensen (31). Parasites were grown in the presence of fresh group O-positive erythrocytes (Key Biologics, LLC, Memphis, TN) in petri dishes at a hematocrit of 4 to 6% in complete RPMI 1640 supplemented with 0.5% AlbuMAX II (Life Technologies). Cultures were incubated at 37°C in a gas mixture of 90% N2, 5% O2, 5% CO2. For EC50 determinations, 20 μl of RPMI 1640 with 5 μg ml−1 gentamicin were dispensed per well in a 384-well assay plate (product number 8807BC; Corning). An amount of 40 nl of compound, previously serially diluted in a separate 384-well white polypropylene plate (product number 8748BC; Corning), was dispensed to the assay plate by hydrodynamic pin transfer (FP1S50H PinHead; V&P Scientific), and then an amount of 20 μl of a synchronized culture suspension (1% rings and 4% hematocrit) was added per well, thus resulting in a final hematocrit and parasitemia of 2% and 1%, respectively. Assay plates were incubated for 72 h, and the parasitemia was determined by a method previously described (32). Briefly, an amount of 10 μl of the following solution in phosphate-buffered saline (PBS) was added per well: 10× SYBR green I, 0.5% Triton X-100 [vol/vol], 0.5 mg ml−1 saponin. Assay plates were shaken for 1 min, incubated in the dark for 90 min, and then read with the EnVision spectrophotometer at excitation/emission wavelengths of 485 nm/535 nm. EC50s were calculated with the Robust Investigation of Screening Experiments (RISE) application with four-parameter logistic equation.
Drug susceptibility assay on human cell lines.
BJ and HepG2 cell lines were purchased from ATCC (American Type Culture Collection, Manassas, VA) and were cultured according to ATCC's recommendations. Cell culture media were purchased from ATCC. Cells were routinely tested for mycoplasma contamination using the MycoAlert Mycoplasma detection kit (Lonza). Exponentially growing cells (BJ cells at 1,000 cells/25 μl/well and HepG2 cells at 400 cells/25 μl/well) were plated in Corning 384-well white custom assay plates and incubated overnight at 37°C in a humidified, 5% CO2 incubator. DMSO inhibitor stock solutions were added the following day to a maximum final concentration of 25 μM, 0.25% DMSO and then diluted 1/3 for a total of 10 testing concentrations. Cytotoxicity was determined following a 72-h incubation using Promega CellTiter Glo reagent according to the manufacturer's recommendations. Luminescence was measured on an EnVision plate reader (PerkinElmer).
Data analysis.
Dose-response curves were calculated from normalized percent activity values and log10-transformed concentrations using the proprietary RISE application written in Pipeline Pilot (Accelrys, version 8.5) and the R program (http://www.R-project.org/) (33). Briefly, nonlinear regression was performed using the R drc package with the four-parameter log-logistic function (LL2.4) (34). The median value from replicates for each compound was fit three separate times by varying the parameters that were fixed during regression as follows: (i) all parameters free, (ii) high response fixed to 100, and (iii) low response fixed to 0. The best fit from these three nested models was selected using the anova.drc function. Confidence intervals of 95% were produced based on this fit. Dose-response curves were assigned a quality score according to the following heuristic. Compounds that failed to fit to any curve or with curves having an efficacy of <25% or >150% or hill slope of <0.5 or >25 were designated class D1. Compounds passing these first criteria with curves having an efficacy of <50%, calculated EC50 of more than the highest concentration tested, lower and upper EC50 confidence limits of >10-fold EC50, or slope at the highest concentration tested of >75% (nonsaturating) were designated class C1. Compounds passing the previous criteria with curves having lower and upper EC50 confidence limits of >5-fold EC50 or slope at the highest concentration tested of >25% (not completely saturating) were designated class B1. All remaining curves were designated A1, which is indicative of ideal, well-behaved sigmoidal response. In general, only A-class curves were assigned potencies for the manuscript. Curves that were inverted (activity decreased as concentration increased) were prefixed with the letter N, e.g., NA1. In tabulating data, a single EC50 was reported only for A1 and B1 class curves. C1 and D1 curves were assigned an arbitrary value greater than the highest concentration tested.
RESULTS
Primary screening with anti-asexual-stage compounds.
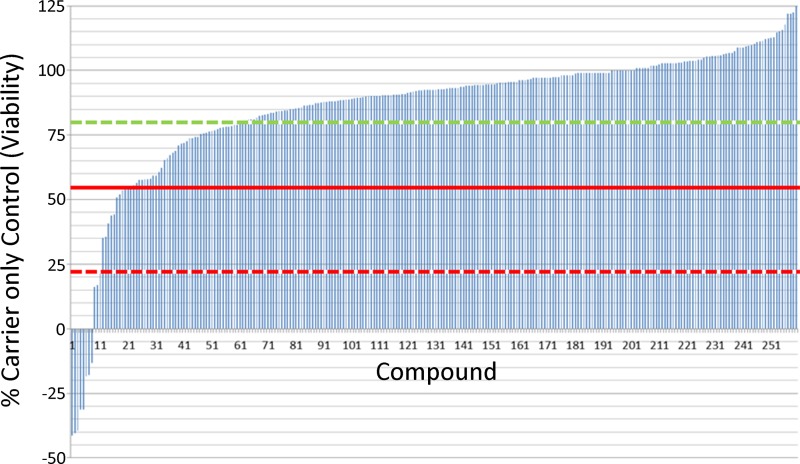
For primary screening, 260 antimalarial compounds were selected from 309,474 compounds in the St. Jude chemical library that was tested against asexual P. falciparum parasites (5). These 260 compounds inhibited asexual growth by >80% at a concentration of 2 μM in the original screen. To evaluate their efficacy against late-stage (stage III to V) gametocytes, they were tested at a single concentration (10 μM), and 24 compounds were found to decrease viability to <55% (Fig. 1; see also Table S1 in the supplemental material). Importantly, gametocytes were insensitive (>80% viability) to the majority of the 260 anti-asexual-stage compounds (200/260), demonstrating the distinct biology of late-stage gametocytes, as well as the specificity of the assay for gametocytes (Fig. 1; see Table S1). The library included 19 compounds that have targets previously shown not to affect gametocyte viability (DHODH, dihydrofolate reductase [DHFR], cytochrome bc1 complex, and hemozoin formation) (5, 35), and only one of these, a bisphenylbenzimidazole (SJ000111341), an inhibitor of hemozoin formation, decreased gametocyte viability to <22% (see Fig. S1 in the supplemental material). Twelve of the 260 compounds were included in the malaria box, and none of these decreased viability to <70% (see Table S2). In all, derivatives from just 3 molecular scaffolds, DANQ, IBI, and BPBI, decreased gametocyte viability to <22% at 10 μM. The 8 most effective compounds were DANQ derivatives and will be discussed separately below. Based on the first screen, 390 additional compounds were selected as structural analogs to five chemotypes (see Table S2). Fifteen derivatives decreased viability to <55% at 6.25 μM (see Table S2). In total, the structure-activity relationships of 479 unique compounds were analyzed, 22 (4.6%) of which decreased gametocyte viability to <50% (Table 1; see also Fig. S2).
FIG 1.
Gametocyte viability. Late-stage gametocyte viability after incubation with the 260 compounds (10 μM) with activity against asexual-stage parasites from the St. Jude antimalarial compound library was assayed using the fluorescent indicator alamarBlue as described in Materials and Methods. Gametocyte viability is presented as the percentage of the carrier (DMSO)-only control signal after subtracting the background fluorescence signal remaining after treatment with 30 nM epoxomicin. The dashed green line indicates 80% gametocyte viability, the red line indicates 55% gametocyte viability, and the dashed red line indicates 22% gametocyte viability.
TABLE 1.
Gametocytocidal activities of selected chemotypesa

The chemotype (group) and backbone structure (backbone) are listed in addition to the number of derivatives of each chemotype that were tested and reduced gametocyte viability to <50% or 50 to 70%.
Gametocytocidal activity and human cell cytotoxicity of DANQ derivatives.
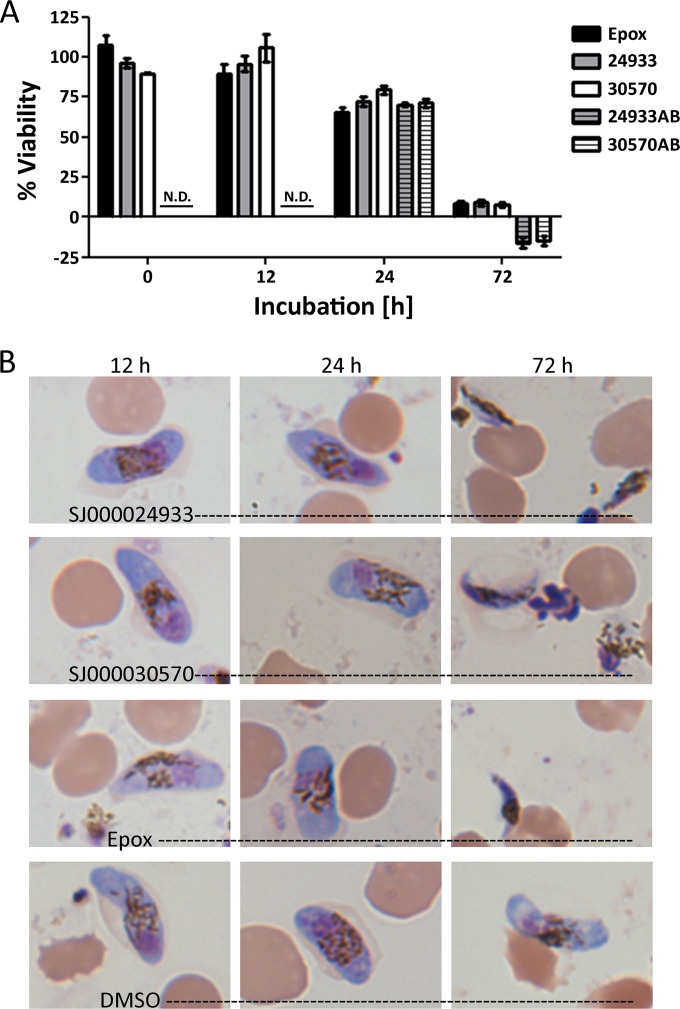
In the initial 260-compound screen, the 8 most potent compounds were all DANQ derivatives (see Table S1 in the supplemental material). However, the fluorescent signals were lower than those in positive-control wells that contained 30 nM epoxomicin, a potent gametocytocidal agent, raising concern that the compounds were affecting the alamarBlue indicator. Consequently, a series of wash steps were included in the protocol and two approaches were taken to confirm the gametocytocidal activity of the compounds (Fig. 2). Gametocyte viability was tested immediately after the addition of drug using the modified alamarBlue protocol that included wash steps and a new flow cytometry protocol using a membrane potential-sensitive dye [MitoProbe DiIC1(5)] (Fig. 2). In contrast to the 24-h incubation period needed for alamarBlue, MitoProbe DiIC1(5) staining only requires 30 min, allowing more rapid screening of gametocyte viability. The results indicate that gametocyte viability remains high 12 h after the addition of drug and then gradually decreases until viable gametocytes are no longer detected at 72 h. At 24 h, both assays detected ∼20 to 30% reductions in viability for SJ000030570 [71% ± 3% viability for alamarBlue and 80% ± 5% viability for DiIC1(5)] and SJ000024933 [70% ± 2% viability for alamarBlue and 71% ± 6% viability for DiIC1(5)], indicating that, following the wash steps at early time points, alamarBlue could detect viable gametocytes even when high concentrations of DANQ SJ000030570 (18 μM) and SJ000024933 (23 μM) were used. In contrast, at 72 h, none of the gametocytes were viable in either assay or in Giemsa-stained smears. The time courses of gametocyte elimination were similar for SJ000030570, SJ000024933, and epoxomicin, and this modified alamarBlue protocol was then used to determine the structure-activity relationships of the DANQ scaffold using 21 DANQ derivatives (Table 2; Fig. 3). The asexual-parasite EC50s were also determined using the SYBR green assay (Table 2). Derivative SJ000030570 showed the best potency against both gametocytes and asexual-stage parasites (gametocytocidal activity, EC50 = 0.061 μM) (Table 2), while three additional derivatives had EC50s of 0.1 μM. The dose-response curves are shown in Fig. S3 in the supplemental material.
FIG 2.
Gametocytocidal activities of DANQ derivatives. Cultures were assayed for viability using flow cytometry (solid bars), alamarBlue fluorescence (striped bars) (A), and Giemsa-stained blood smears (B) at the indicated times after the addition of epoxomicin (black bar), SJ000024933 (gray bars), and SJ000030570 (white bars). The data are presented as percentages of the DMSO vehicle control value. N.D., not determined.
TABLE 2.
Biological activities of DANQ derivatives
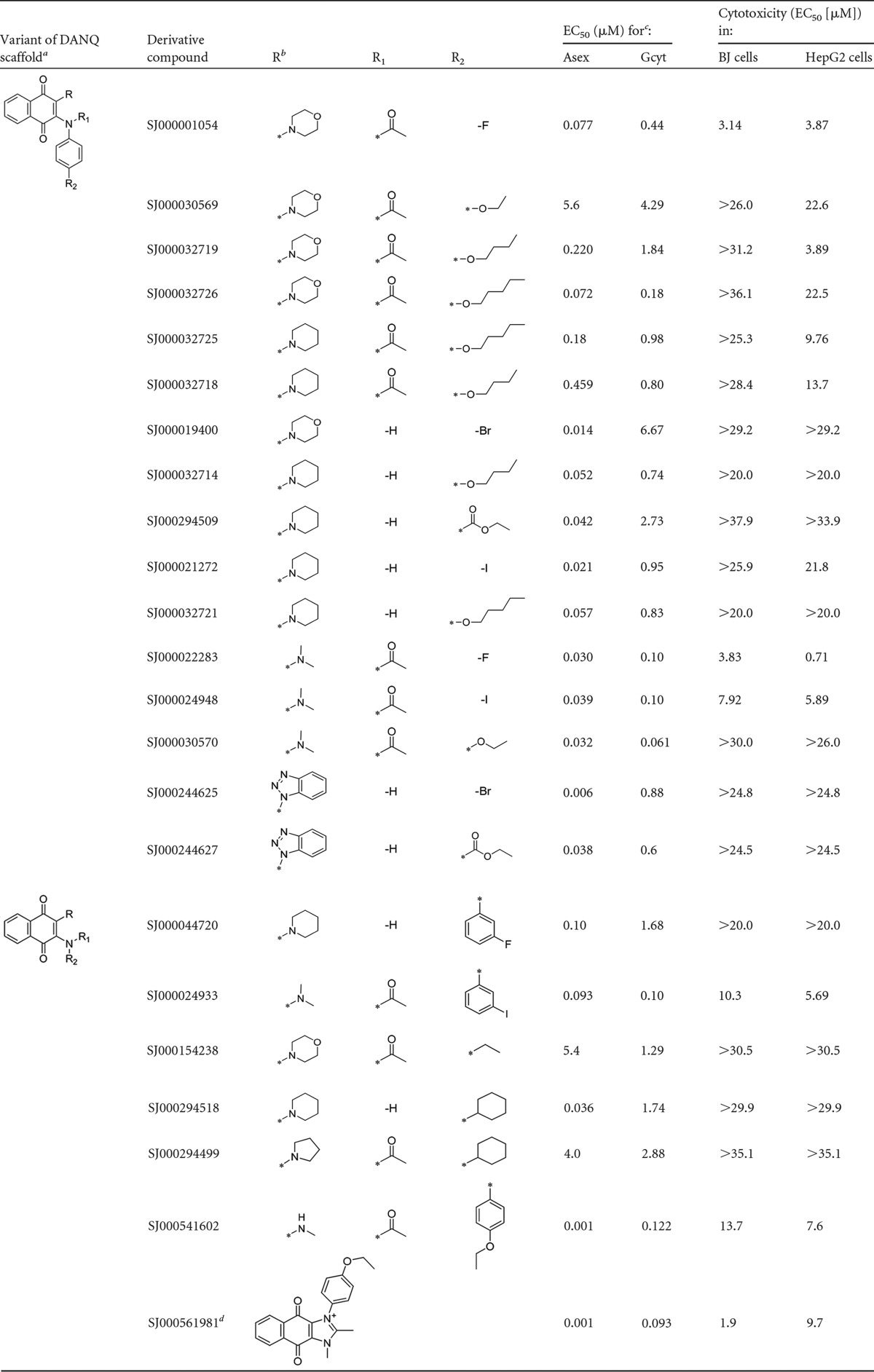
DANQ, 1,4-diaminonaphthoquinone. Variant 1, analogs with anilines substituted at the para position; variant 2, analogs without para-substituted anilines.
R, R1, and R2 are DANQ scaffold side chains.
Asex, asexual-stage parasites; Gcyt, gametocytes.
Cyclized imidazolium analog of SJ000541602.
FIG 3.
DANQ structure-activity analysis. The gametocytocidal EC50s of three series of DANQ derivatives were determined using the alamarBlue viability assay to allow the comparison of chemical structure and activity. (A) Tertiary amide/dimethylamine analogs. (B) Tertiary amide/morpholine analogs. (C) Secondary amine/piperadine analogs. (D) Comparison of SJ000030570 and photostable analogs. The acetyl group on the aniline nitrogen is indicated by a solid circle and the dimethylanime by a dashed circle.
All 21 DANQ derivatives were also assayed for cytotoxicity against 2 mammalian cell lines, BJ and HepG2 (Table 2). Four of the five most effective compounds (SJ000030570, SJ000024933, SJ000022283, SJ000024948, and SJ000032726) were >55-fold more potent against gametocytes than against BJ or HepG2 cells, with the most effective compound, SJ000030570, demonstrating 180- and 80-fold selectivity, respectively. These five compounds also had nanomolar activity against asexual parasites (Table 2), indicating a potential to be used both to treat patients and to block malaria transmission. However, there was poor correlation between the antigametocyte and anti-asexual-stage potency of the compounds, suggesting different modes of action in these two intraerythrocytic parasite stages (Table 2).
Structure-activity relationships of DANQ derivatives.
Analysis of the structures of gametocytocidal DANQ derivatives shows two structural variations of the DANQ scaffold that display gametocytocidal activity (Fig. 3; Table 2). The compounds with the most potent activity are analogs of the asexual-stage lead compound SJ000030570, which are defined by an acetyl group on the aniline nitrogen and a dimethylamine moiety (Fig. 3A). For this series, antimalarial activity is retained with either electron-donating (SJ000030570) or electron-withdrawing (SJ000024933, SJ000022283, and SJ000024948) substitutions on the aniline ring, suggesting that the electronics of this ring are not important. The tertiary amide DANQ scaffold retained potency when the dimethylamine was replaced with a morpholine moiety (Fig. 3B). For the morpholine series, the hydrocarbon chain length of the aniline ether significantly influenced gametocytocidal activity: reduction from a five-carbon chain (SJ000032726) to a four-carbon chain (SJ000032719) decreased activity 10-fold for the morpholine analogs. Hydrocarbon chain length did not have a consistent effect on potency against asexual-stage parasites. A fluorine substitution in the para position on the aniline (SJ000001054) was found to retain gametocytocidal and asexual activity, while replacement of the aniline with an alkyl substituent negatively affected both gametocytocidal and asexual activity, as shown by the results for SJ000154238.
A second DANQ scaffold that is characterized by a nonacetylated secondary amine aniline nitrogen with a piperidine moiety as the second amine substituent also displayed activity against both sexual- and asexual-stage parasites (Fig. 3C). Following the previously described series, gametocytocidal compounds with long-chain alkyl ether substitutions in the para position of the aniline (SJ000032721 and SJ000032714) were identified. In addition, analogs containing electron-withdrawing substitutions (SJ000294509, SJ000021272, and SJ000044720) and cyclohexane substituted analog (SJ000294518) showed gametocytocidal activity. Another variation on the second series of the DANQ scaffold containing benzotriazole substitutions, represented by SJ000244625 and SJ000244627, also provided compounds with gametocytocidal and asexual potency (Table 2).
During the lead optimization of SJ000030570, it was observed that compounds containing dialkylamine moieties display an inherent sensitivity to light. In the case of dimethylamine analogs (Fig. 3A), prolonged exposure to light results in the photolysis of one of the methyl groups on the amine, resulting in the formation of the monomethylamine analog SJ000541602. Furthermore, this analog is capable of cyclizing to form the corresponding imidazolium analog SJ000561981. The resulting secondary amine and the corresponding imidazolium have been shown to be more photostable and to provide elevated levels of antimalarial activity against asexual stages of malaria. To test this directly, SJ000030570 and two photostable derivatives were reevaluated, taking precautions to decrease light exposure. In both the alamarBlue and MitoProbe DiIC1(5) assay, light-protected SJ000030570 (EC50 of 0.319 ± 0.050 μM and 0.235 ± 0.093 μM, respectively) was less active than derivatives SJ000561981 (EC50 of 0.093 ± 0.027 μM and 0.090 ± 0.064 μM, respectively) and SJ000541602 (EC50 of 0.122 ± 0.034 μM and 0.124 ± 0.041 μM, respectively) when tested against stage III to V gametocytes (see Fig. S3 in the supplemental material). Importantly, all three compounds completely inhibited exflagellation, the derivatives at concentrations of ≥0.1 μM and light-protected SJ000030570 at concentrations of ≥0.3 μM, confirming the biological activity of these compounds.
Validation of the activity data with light-protected SJ000030570 and its photostable analogs has validated the gametocytocidal activity of the DANQ series. However, the potential exists for the other active compounds, which contain a dialkylamine moiety, to be partially degraded at the time of analysis. Therefore, the reported activity for these compounds may be overshadowed by the presence of more potent degradation products. Conversely, the potency may be underestimated by the degradation of the active constituent. Moving forward, further analysis will be conducted on analogs from the photostable series.
IBI activity and cytotoxicity.
A second class of compounds currently being investigated for anti-asexual-stage activity also consistently inhibited late-stage gametocyte viability (Table 1; see also Fig. S2 in the supplemental material). Over half of the five 2-imino-benzo[d]imidazole derivatives (IBI) tested had >50% gametocytocidal activity, with EC50s ranging between 1 and 4 μM. The most effective compound (SJ000016864) had a similar EC50 against asexual parasites and was >44-fold less toxic to the BJ human fibroblast cell line. Additional compounds from this promising chemotype will have to be screened to better define the structure-activity relationship.
DISCUSSION
Fifteen compounds with gametocytocidal activity in the nanomolar range were identified in a screen of a total of 650 compounds, including 260 lead-like antimalarial compounds discovered in a whole-cell screen against the asexual stages of the parasite life cycle. These 15 gametocytocidal compounds were derived from just 2 scaffolds: 14 were DANQ derivatives and one was an IBI. The most effective compound, SJ000030570, was initially found to be >100-fold more effective against gametocytes than against the BJ human fibroblast cell line (EC50 > 11 μM). In fact, all 14 DANQ compounds had a therapeutic window >7 times greater than the window for BJ cells. DANQ is one of the 3 scaffolds selected from the St. Jude chemical library for lead optimization as new antimalarials and possesses the best gametocytocidal potency of the 3 lead compounds (30).
Each of these 3 antimalarial leads (DANQ, DHP, and dihydroisoquinoline [DHIQ]), as well as IBI, are hypothesized to have novel mechanisms of action because they are structurally distinct from previous antimalarials and do not inhibit or bind to known asexual targets, including DHODH, DHFR, cytochrome bc1, falcipain 2, or hemozoin (5). In contrast to the DANQs, the hydroxynaphthoquinone, atovaquone, inhibits cytochrome bc1 and does not reduce gametocyte viability even at 10 μM (36). The dual anti-asexual-stage and gametocytocidal activity of DANQ and IBI suggests that they interfere with pathways that are essential for both these intraerythrocytic stages, while DHP and DHIQ target critical asexual-stage-specific pathways. However, the lack of correlation between the asexual and gametocytocidal potency of the DANQ derivatives suggests that their modes of action may differ in these two intraerythrocytic parasite stages. The structural differences between the DANQ derivatives could directly influence the binding of the compound to a specific target or alter access of the compound to the parasite or host red blood cell. RBC permeability has been shown to be enhanced in asexual-stage-infected RBCs but not in gametocyte-infected RBCs (19, 20), and this difference could lead to differential uptake of distinct compounds. Marked phenotypic and transcriptomic differences exist between the two life cycle stages (37–41). For example, late-stage female gametocytes contain a large set of translationally repressed transcripts that are not expressed until the gametocyte is taken up in a blood meal by a mosquito (42). A number of P. falciparum genes also have stage-specific homologues, including diaminopeptidase (DPAP2) and plasmepsins (VI to IX) (39, 41). It is possible that homologues expressed at different stages could have subtly different affinities for compounds like the DANQ derivative series that result in different activity profiles. The presence of distinct targets in different parasite stages also suggests that both genes would have to acquire mutations for the parasite to become completely resistant to the compound.
The lack of gametocytocidal activity of the majority of the anti-asexual-stage compounds tested (237/260, 91%) further demonstrates the distinct sensitivities of late-stage gametocytes and asexual parasites and confirms the gametocyte specificity of the assay. As previously reported, pathways involved in hemoglobin digestion, hemozoin formation, DNA replication, apicoplast activity, and increased RBC permeability were shown not to be essential for gametocyte maturation (14, 17). Elucidating the mechanisms of action of these 237 novel anti-asexual-stage-specific compounds will further increase the understanding of pathways required for asexual growth but not gametocyte viability. In contrast, the targets of DANQ and IBI are expected to be required for the viability of both asexual- and sexual-stage parasites. Both stages develop within the confines of an erythrocyte, and in silico profiling of proteomic data into broadly defined functional classes indicates the presence of common pathways, including glutathione metabolism and protein expression and degradation (43). Additional screening of the remaining compounds from the St. Jude chemical library that lack asexual activity will be of interest to reveal gametocyte-specific compounds and their corresponding targets.
In summary, two scaffolds, DANQ and IBI, that effectively block both asexual growth and late-stage gametocyte viability have been identified. One of the DANQs, SJ000030570, has already been selected for antimalarial lead optimization (30), resulting in the identification of two photostable analogs (SJ000541602 and SJ000561981) that were also found to have potent gametocytocidal activity. In contrast, 625 other novel compounds were inactive against late-stage gametocytes (>50% viability). Differences between asexual- and sexual-stage parasites were also observed in the structure-activity analysis of DANQ derivatives, as well as the other six chemotypes that had measurable activity against both parasite stages. Whether these structural differences reflect stage-specific targets or access to the parasite remains to be determined. The results clearly demonstrate the need to test both asexual and sexual stages to identify compounds with the potential to inhibit the symptoms and spread of malaria.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children's Research Hospital (SJCRH), and Public Health Service grant AI101396 from the National Institute of Allergy and Infectious Diseases. T.Q.T. is a JSPS Research Fellow in Biomedical and Behavioral Research at NIH.
We thank S. Desai for use of the fluorescent plate reader, B. Grimberg for suggesting MitoProbe DilC1(5), and C. Magle for critical reading of the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01930-13.
REFERENCES
- 1.malERAConsultativeGroup. 2011. A research agenda for malaria eradication: drugs. PLoS Med 8:e1000402. doi: 10.1371/journal.pmed.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, Song YZ. 1994. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin Med J (Engl) 107:709–711. [PubMed] [Google Scholar]
- 3.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. 2008. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar J 7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrara VI, Zwang J, Ashley EA, Price RN, Stepniewska K, Barends M, Brockman A, Anderson T, McGready R, Phaiphun L, Proux S, van Vugt M, Hutagalung R, Lwin KM, Phyo AP, Preechapornkul P, Imwong M, Pukrittayakamee S, Singhasivanon P, White NJ, Nosten F. 2009. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One 4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 7.Meister S, Plouffe DM, Kuhen KL, Bonamy GM, Wu T, Barnes SW, Bopp SE, Borboa R, Bright AT, Che J, Cohen S, Dharia NV, Gagaring K, Gettayacamin M, Gordon P, Groessl T, Kato N, Lee MC, McNamara CW, Fidock DA, Nagle A, Nam TG, Richmond W, Roland J, Rottmann M, Zhou B, Froissard P, Glynne RJ, Mazier D, Sattabongkot J, Schultz PG, Tuntland T, Walker JR, Zhou Y, Chatterjee A, Diagana TT, Winzeler EA. 2011. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334:1372–1377. doi: 10.1126/science.1211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A 105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. 2014. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the malaria box. Antimicrob Agents Chemother 58:811–819. doi: 10.1128/AAC.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. 2014. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS One 9:e105817. doi: 10.1371/journal.pone.0105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J 12:408. doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucantoni L, Duffy S, Adjalley SH, Fidock DA, Avery VM. 2013. Identification of MMV malaria box inhibitors of plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob Agents Chemother 57:6050–6062. doi: 10.1128/AAC.00870-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. 2014. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother 58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson KC. 2008. New antimalarials targeting both asexual and gametocyte stages. Drugs Future 33:1033–1040. doi: 10.1358/dof.2008.33.12.1287737. [DOI] [Google Scholar]
- 15.Dechy-Cabaret O, Benoit-Vical F. 2012. Effects of antimalarial molecules on the gametocyte stage of Plasmodium falciparum: the debate. J Med Chem 55:10328–10344. doi: 10.1021/jm3005898. [DOI] [PubMed] [Google Scholar]
- 16.Alano P, Carter R. 1990. Sexual differentiation in malaria parasites. Annu Rev Microbiol 44:429–449. doi: 10.1146/annurev.mi.44.100190.002241. [DOI] [PubMed] [Google Scholar]
- 17.Butcher GA. 1997. Antimalarial drugs and the mosquito transmission of Plasmodium. Int J Parasitol 27:975–987. doi: 10.1016/S0020-7519(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 18.Czesny B, Goshu S, Cook JL, Williamson KC. 2009. The proteasome inhibitor epoxomicin has potent Plasmodium falciparum gametocytocidal activity. Antimicrob Agents Chemother 53:4080–4085. doi: 10.1128/AAC.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisk G, Kang M, Cohn JV, Desai SA. 2006. Specific inhibition of the plasmodial surface anion channel by dantrolene. Eukaryot Cell 5:1882–1893. doi: 10.1128/EC.00212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go ML, Liu M, Wilairat P, Rosenthal PJ, Saliba KJ, Kirk K. 2004. Antiplasmodial chalcones inhibit sorbitol-induced hemolysis of Plasmodium falciparum-infected erythrocytes. Antimicrob Agents Chemother 48:3241–3245. doi: 10.1128/AAC.48.9.3241-3245.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saul A, Graves P, Edser L. 1990. Refractoriness of erythrocytes infected with Plasmodium falciparum gametocytes to lysis by sorbitol. Int J Parasitol 20:1095–1097. doi: 10.1016/0020-7519(90)90056-S. [DOI] [PubMed] [Google Scholar]
- 22.Lisk G, Pain M, Sellers M, Gurnev PA, Pillai AD, Bezrukov SM, Desai SA. 2010. Altered plasmodial surface anion channel activity and in vitro resistance to permeating antimalarial compounds. Biochim Biophys Acta 1798:1679–1688. doi: 10.1016/j.bbamem.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pukrittayakamee S, Chotivanich K, Chantra A, Clemens R, Looareesuwan S, White NJ. 2004. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrob Agents Chemother 48:1329–1334. doi: 10.1128/AAC.48.4.1329-1334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh E, DeRisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol 9:e1001138. doi: 10.1371/journal.pbio.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gantt SM, Myung JM, Briones MR, Li WD, Corey EJ, Omura S, Nussenzweig V, Sinnis P. 1998. Proteasome inhibitors block development of Plasmodium spp. Antimicrob Agents Chemother 42:2731–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindenthal C, Weich N, Chia YS, Heussler V, Klinkert MQ. 2005. The proteasome inhibitor MLN-273 blocks exoerythrocytic and erythrocytic development of Plasmodium parasites. Parasitology 131(Pt 1):37–44. doi: 10.1017/S003118200500747X. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds JM, El Bissati K, Brandenburg J, Gunzl A, Mamoun CB. 2007. Antimalarial activity of the anticancer and proteasome inhibitor bortezomib and its analog ZL3B. BMC Clin Pharmacol 7:13. doi: 10.1186/1472-6904-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka TQ, Williamson KC. 2011. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol Biochem Parasitol 177:160–163. doi: 10.1016/j.molbiopara.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobbs CV, Tanaka TQ, Muratova O, Van Vliet J, Borkowsky W, Williamson KC, Duffy PE. 2013. HIV treatments have malaria gametocyte killing and transmission blocking activity. J Infect Dis 208:139–148. doi: 10.1093/infdis/jit132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guiguemde WA, Shelat AA, Garcia-Bustos JF, Diagana TT, Gamo FJ, Guy RK. 2012. Global phenotypic screening for antimalarials. Chem Biol 19:116–129. doi: 10.1016/j.chembiol.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 32.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Development Core Team. 2011. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 34.Ritz C, Streibig JC. 2005. Bioassay analysis using R. J Stat Softw 12(5). http://www.jstatsoft.org/v12/i05/paper. [Google Scholar]
- 35.Zhang G, Manaca MN, McNamara-Smith M, Mayor A, Nhabomba A, Berthoud TK, Khoo SK, Wiertsema S, Aguilar R, Barbosa A, Quinto L, Candelaria P, Schultz EN, Hayden CM, Goldblatt J, Guinovart C, Alonso PL, Lesouef PN, Dobano C. 2012. Interleukin-10 (IL-10) polymorphisms are associated with IL-10 production and clinical malaria in young children. Infect Immun 80:2316–2322. doi: 10.1128/IAI.00261-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun W, Tanaka TQ, Magle CT, Huang W, Southall N, Huang R, Dehdashti SJ, McKew JC, Williamson KC, Zheng W. 2014. Chemical signatures and new drug targets for gametocytocidal drug development. Sci Rep 4:3743. doi: 10.1038/srep03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvestrini F, Lasonder E, Olivieri A, Camarda G, van Schaijk B, Sanchez M, Younis Younis S, Sauerwein R, Alano P. 2010. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 9:1437–1448. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lasonder E, Ishihama Y, Andersen JS, Vermunt AMW, Pain A, Sauerwein RW, Eling WMC, Hall N, Waters AP, Stunnenberg HG, Mann M. 2002. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419:537–542. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- 39.Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, Carucci DJ, Baker DA, Winzeler EA. 2005. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol 143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Eksi S, Haile Y, Furuya T, Ma L, Su X, Williamson KC. 2005. Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol Biochem Parasitol 143:90–99. doi: 10.1016/j.molbiopara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 42.Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, Khan SM, Dimopoulos G, Janse CJ, Waters AP. 2006. Regulation of sexual development of Plasmodium by translational repression. Science 313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ. 2002. A proteomic view of the Plasmodium falciparum life cycle. Nature 419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.