Abstract
We estimated the efficacy of the current single administration of peramivir on the basis of peramivir pharmacokinetics in the upper respiratory tract (URT) and determined the predictive peramivir concentration-time curve to assess its efficacy against viruses with decreased susceptibility to neuraminidase inhibitors. Serum, nasal swab, or aspiration samples were collected from 28 patients treated with 10 mg/kg body weight peramivir. The sequential influenza viral RNA load and susceptibility after peramivir administration were measured using a quantitative real-time reverse transcription-PCR and neuraminidase inhibition assay. The peramivir concentrations in the serum and URT after a single administration at 10 mg/kg were measured, and the predictive blood and URT peramivir concentration-time curves were determined to assess various administration regimens against resistant variants. The peramivir concentration decreased to <0.1% of the maximum concentration of drug in serum (Cmax) at 24 h after administration. Rapid elimination of peramivir from the URT by 48 h after administration may contribute to an increase in the influenza A viral load after day 3 but not to a decrease in the influenza B viral load, despite the absence of a decrease in the susceptibility to peramivir. A longer maintenance of a high level of peramivir in the URT is expected by divided administration rather than once-daily administration. When no clinical improvement is observed in patients with normal susceptibility influenza A and B, peramivir readministration should be considered. In severe cases caused by resistant variants, better inhibitory effectiveness and less frequent adverse events are expected by divided administration rather than once-daily administration with an increased dosage.
INTRODUCTION
Influenza is an important cause of morbidity and mortality in children and adults and is associated with an annual excess number of hospitalizations among children aged <5 years (1). Because the efficacy of vaccination varies among seasons, depending on the evolution of major viral antigens (2), antiviral agents, e.g., the neuraminidase (NA) inhibitors (NAIs) oseltamivir, zanamivir, and laninamivir, have become a primary option to treat patients with influenza virus infection. Because the clinical efficacy of a single intravenous peramivir administration in children and adults is reportedly noninferior to that of oseltamivir therapy for 5 days (3, 4), peramivir is administered only once a day to patients with mild illness after hospitalization. Once-daily administration of peramivir is also approved in Japan for patients with severe complications such as viral pneumonia. However, the pharmacokinetics (PK)/pharmacodynamics (PD) of peramivir in the respiratory tract and sequential viral load after a single peramivir administration have not been studied in children.
Since the 2009-2010 influenza season, after seasonal H1N1 was replaced by the pandemic A H1N1 in 2009 (H1N1 pdm09), few oseltamivir-resistant H1N1 pdm09 viruses have been isolated, and those circulating in 2009-2010 represented <1% of viruses detected in the United States, with the majority being recovered from patients who received oseltamivir (5, 6). However, in December 2013, H1N1 pdm09 with an H275Y substitution in NA (H1N1 pdm09 H275Y) was identified in Japanese patients who had not been exposed to oseltamivir (7). This suggests that this variant will circulate worldwide and all H1N1 pdm09 cases will be resistant to oseltamivir in the near future, according to trends observed for seasonal H1N1 until the 2008-2009 influenza season (8). The most serious concern regarding this issue is that the option to treat patients with severe pneumonia caused by H1N1 pdm09, similar to the situation during the 2009-2010 influenza pandemic (9), is limited when this strain has an H275Y substitution and is resistant to oseltamivir (8). A therapeutic option for controlling H7N9 with an R292K substitution in NA (H7N9 R292K), which has a highly decreased susceptibility to NAIs and causes severe pneumonia (10, 11), is also required.
The inhaled NAIs zanamivir and laninamivir are not suitable for treating patients with severe pneumonia caused by H1N1 pdm09 H275Y or H7N9 R292K, even if these strains are susceptible to inhalation NAIs. Therefore, intravenous peramivir administration should be the primary option for treating these patients at present because of its extremely high serum concentrations exceeding the 50% inhibitory concentration (IC50) of resistant strains after intravenous administration. However, because these strains have decreased susceptibility to peramivir, the current regimen of peramivir administration should be reconsidered.
In this study, we measured the sequential viral load and peramivir concentration in the blood and upper respiratory tract (URT) after a single administration in children to assess the efficacy of the current regimen of peramivir administration. We also aimed to propose a suitable regimen for peramivir administration in patients infected with NAI-resistant viruses using the predicted peramivir concentration-time curve in the blood and URT.
MATERIALS AND METHODS
Patients and samples.
During the 2011-2012, 2012-2013, and 2013-2014 influenza seasons, 28 children diagnosed with influenza A or B via a rapid antigen test were hospitalized at the Soma General Hospital, Ohara General Hospital, Hoshi General Hospital, Iwase Hospital, South Aizu Hospital, or Fukushima Medical University Hospital because of dehydration or respiratory complications. A single 8- to 12-mg/kg body weight dose of peramivir was intravenously administered over 30 to 60 min after hospitalization. The day on which a patient was admitted to the hospital was defined as day 0. Serum and nasal swab samples were collected from patients before and 0.5 to 2 h after peramivir administration and on days 1, 2, and 5. During the 2013-2014 influenza season, nasal aspiration samples were collected at the same time as serum samples, if possible. When a patient was discharged on days 3 or 4, samples were collected on the day of discharge. Each sample was stored at −80°C and transferred to the Department of Pediatrics, Fukushima Medical University, for subsequent analyses. The time of illness onset was defined as the time when a patient had fever with a body temperature of >37.5°C, and the time from the onset of illness to the hospital visit was recorded. Body temperature was recorded every 8 h, and the time of fever decline was defined as that when a patient had a fever of <37.5°C for >24 h. This study protocol and amendments were approved by the independent ethics committees and institutional review boards of each institution, and informed consent was obtained from the patients' parents.
Quantitative real-time reverse transcription-PCR.
Viral RNAs were extracted using the QIAamp MinElute virus spin kit (Qiagen, Valencia, CA, USA), and cDNA for quantitative real-time reverse transcription (qRT)-PCR was synthesized using the Prime-Script RT reagent kit (TaKaRa Bio Inc., Shiga, Japan), according to the manufacturer's instructions. The primers and probes used to detect the M gene of influenza A and B viruses, to measure viral load, and to subtype influenza A (i.e., H1N1 pdm09 and H3N2) were synthesized as described previously (12–14). The PCR mix consisted of final concentrations of 1× Premix Ex Taq (TaKaRa Bio Inc.), 900 nM each primer, 100 nM the probe, and 2 μl of target cDNA, and the final volume was eventually made 20 μl with nuclease-free water. cDNA was amplified in 40 two-step cycles (5 s at 95°C for denaturation and 31 s at 60°C for annealing and extension) using an ABI-7300 instrument (Life Technologies Corp., Carlsbad, CA, USA) (15). Virus copy numbers were determined via comparison with a serially diluted plasmid standard with a known concentration. Copy numbers of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase were measured in each sample for normalization. The viral RNA load was measured in duplicates of all samples.
Virus isolation.
Viruses were isolated from the samples stored at −80°C for subsequent NA inhibition assays, as described previously (15). In brief, 100 μl of nasal aspiration samples was inoculated on MDCK cells in a 12-well plate, and the cells were washed after a 1-h incubation at room temperature. The MDCK cells were incubated in 1 ml Eagle's minimum essential medium (MEM) containing MEM vitamin solution (Life Technologies), 0.2% bovine serum albumin (fraction V) (Calbiochem, La Jolla, CA, USA), 2 μg/ml of trypsin (Sigma-Aldrich, St. Louis, MO, USA), 2 mg/ml of glucose, 0.72 mg/ml of l-glutamine, an appropriate volume of NaHCO3 (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and 20,000 U/ml of penicillin and 200 mg/ml of streptomycin (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) for 7 days at 37°C in 5% CO2-95% air. Phenol red-free medium was used for virus isolation to prevent the interference of phenol red with the chemiluminescence-based NA inhibition assay. The supernatant of the cultured MDCK cells that showed cytopathic effects during or at the end of the observation period was collected and stored at −80°C. To avoid virus selection because of repeated passage, virus passage was not performed more than twice.
Neuraminidase inhibition assay.
Susceptibility of the isolated viruses to peramivir was determined using the NA-XTD chemiluminescence-based NA inhibition assay kit (Life Technologies), according to the manufacturer's instructions. In brief, 25 μl of diluted virus was incubated for 20 min in duplicate with 25 μl of serially diluted peramivir, followed by a 30-min incubation with the chemical substrate, according to the manufacturer's instructions. The chemiluminescent signal intensity was measured on a Chameleon V plate reader (Hidex, Turku, Finland). Peramivir was provided by Shionogi Pharma Ltd. (Osaka, Japan). The IC50 was calculated using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA).
Determination of the peramivir concentration-time curve and simulation of peramivir pharmacokinetics/pharmacodynamics against neuraminidase inhibitor-resistant viruses.
The peramivir concentrations in serum and nasal aspiration samples were measured via validated liquid chromatography-tandem mass spectrometry at Sumika Chemical Analysis Service, Ltd. (Osaka, Japan), as described previously (3). The blood peramivir concentration-time curve for a single 10-mg/kg dose was determined using GraphPad Prism 6 software, and its stability and robustness were evaluated using a bootstrap resampling procedure in 5,000 data sets obtained from the original data. The peramivir concentration-time curve in the URT for a single 10-mg/kg dose, which is assumed to follow a one-compartment model, was determined. When the peramivir concentrations of nasal aspiration samples collected on day 1 and after day 2 were less than the lower limit of quantification (3 nM), the concentrations in those samples were defined as 1 nM and 0 nM, respectively, for statistical analysis. As described previously (16, 17), the urea concentrations in serum and nasal aspiration samples obtained at the same time were measured using the QuantiChrom urea assay kit (BioAssay Systems, Hayward CA, USA) to correct the dilution of peramivir in nasal aspiration samples, according to the manufacturer's instructions.
Based on the assumption that the maximum concentrations of drug in serum (Cmax) and the URT were positively correlated after administration of a single dose of peramivir and that the same concentration-time curve obtained on the basis of the single dose repeatedly appeared at subsequent administrations (18), predictive curves for the following peramivir regimens were plotted: a 5-mg/kg dose every 12 h for 5 days (regimen 1), a 10-mg/kg dose every 24 h for 5 days (regimen 2); a 10-mg/kg dose every 12 h for 5 days (regimen 3), and a 20-mg/kg dose every 24 h for 5 days (regimen 4). To determine the PK/PD of each regimen for inhibiting NA activity continuously from the initial peramivir administration to 120 h, we tested the efficacy of the following four hypothetical peramivir concentrations in the URT: 50 and 100 nM for H1N1 pdm09 H275Y and 200 and 300 nM for H7N9 R292K. These concentrations were selected because the IC50s of the drug for H1N1 pdm09 H275Y and H7N9 R292K are 22.35 to 35.28 (7) and 101.89 to 127.60 nM (11), respectively.
Statistical analyses.
The Steel-Dwass test, Fisher's exact test, the Mann-Whitney U test, and the Wilcoxon signed-rank test, which were performed using Ekuseru-Toukei 2012 Excel add-in statistical analysis software (Social Survey Research Information Co. Ltd., Tokyo, Japan), were used for data analyses. A P value of <0.05 was considered statistically significant.
RESULTS
Patients and samples.
Among the 28 patients enrolled, 19 and 9 were diagnosed with influenza A and B, respectively, using a rapid antigen test. Of 19 patients diagnosed with influenza A, 2 were negative for influenza A by PCR, whereas 6 and 11 were positive for H1N1 pdm09 and H3N2, respectively. Peramivir was administered to 27 of 28 patients enrolled in this study within 48 h after the onset of illness.
No renal dysfunction was observed in any patient, and the clinical backgrounds of the patients are shown in Table 1. In total, 92 serum samples from 28 patients and 26 nasal aspiration samples from 9 patients were available to determine the peramivir concentration-time curve in the serum and URT, respectively. Among 91 nasal swab or aspiration samples available for measuring the viral RNA load, 73 were available for virus isolation.
TABLE 1.
Characteristics of the 28 patients treated with peramivira
| Clinical characteristic | Results for patients with type A influenza |
Results for patients with type B (n = 9) | ||
|---|---|---|---|---|
| Total (n = 28)b | H1N1 pdm09 (n = 6) | H3N2 (n = 11) | ||
| Age (yr)c | 4.7 ± 4.1 (2.9, 0.6–14.8) | 4.0 ± 3.0 (4.0, 0.8–9.2) | 4.7 ± 5.0 (2.8, 0.6–14.8) | 6.0 ± 3.9 (6.6, 1.1–11.8) |
| No. of males | 17 | 3 | 6 | 6 |
| No. of vaccinationsd | 9 | 3 | 1 | 4 |
| Time after onset (h)c | 19.0 ± 20.4 (16.0, 3.0–85.5) | 32.1 ± 30.6 (22.8, 5.0–85.5) | 11.8 ± 6.8 (9.5, 3.0–22.0) | 20.3 ± 15.3 (19.3, 3.0–42.0) |
| Body temp (°C)c | 39.2 ± 0.7 (39.2, 37.7–40.3) | 39.5 ± 0.7 (39.8, 38.2–40.0) | 39.5 ± 0.5 (39.6, 39.0–40.3) | 38.9 ± 0.7 (38.6, 38.0–39.8) |
| Duration of fever (h)c | 26.9 ± 12.0 (27.0, 11.5–43.0) | 26.5 ± 14.6 (39.0, 11.5–43.0) | 27.1 ± 11.2 (27.0, 13.0–42.0) | 25.6 ± 22.7 (17.0, 5.0–66.0) |
| BUN (mg/dl)c | 12.3 ± 4.8 (11.6, 3.6–24.0) | 12.0 ± 6.7 (11.5, 3.6–24.0) | 12.5 ± 3.8 (11.6, 7.5–20.0) | 10.6 ± 2.0 (10.5, 8.0–14.0) |
| Crea (mg/dl)c | 0.3 ± 0.2 (0.3, 0.2–0.9) | 0.3 ± 0.1 (0.3, 0.3–0.4) | 0.4 ± 0.2 (0.3, 0.2–0.9) | 0.4 ± 0.1 (0.4, 0.2–0.6) |
There was no statistically significant difference in clinical characteristics between each group. The Steel-Dwass test was used to analyze age, hours after onset, body temperature, duration of fever, blood urea nitrogen (BUN), and creatinine (Crea); Fisher's exact test was used to analyze sex and vaccination for comparisons between each group.
Two patients with false-positive results on the rapid antigen test were included.
Data are mean ± SD (median, range).
Influenza vaccines were received prior to each influenza season.
Sequential viral load.
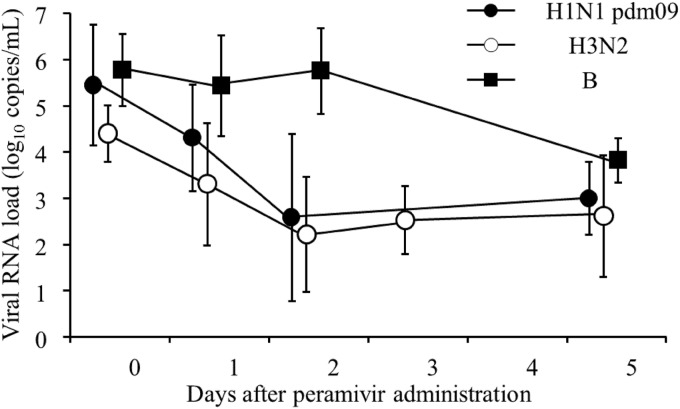
The means of the viral RNA loads in patients with influenza H1N1 pdm09, H3N2, and B upon hospital admission were 5.4 log10 copies/ml, 4.8 log10 copies/ml, and 5.7 log10 copies/ml, respectively. The percentages of residual viral RNA loads in patients with influenza H1N1 pdm09, H3N2, and B on day 2 were 0.5% ± 0.9% (mean ± standard deviation [SD]) (0.1%, 0% to 2.0% [median, range]), 3.8% ± 6.5% (0.5%, 0% to 19.8%), and 344.4% ± 624.8% (79.6%, 14.3% to 1,829.8%), respectively; a significant difference was observed in the percentages of the residual viral RNA loads between type A and type B (P < 0.05) (Fig. 1). After day 3, the influenza A viral RNA load increased in 9 of 12 patients (75%) whose nasal swab or nasal aspiration samples were collected after day 3 (see Fig. S1 in the supplemental material). Although these 9 patients were younger than the remaining 3 patients who did not exhibit an increased viral RNA load, no significant differences were observed between those patients (P = 0.19) (Table 2). No increase in the viral RNA load after day 3 was observed in patients with influenza B. Although there was a significant difference in the percentages of residual viral RNA loads on day 5 between influenza H1N1 pdm09 and B (P = 0.03), no significant difference was observed between H3N2 and B (H1N1 pdm09, 0.9% ± 1.3% [mean ± SD] [0.3%, 0.03% to 2.7% (median, range)]; H3N2, 10.9% ± 20.5% [1.4%, 0% to 47.4%]; and B, 6.6% ± 1.3% [3.9%, 3.0% to 13.0%]).
FIG 1.
Sequential viral RNA loads (log10 copies/ml) of influenza H1N1 pdm09, H3N2, or B viruses after peramivir administration. The error bars represent the standard deviation of the mean. Although the influenza viral RNA loads of both H1N1 pdm09 and H3N2 on day 2 were significantly decreased compared with those on day 0 (H1N1 pdm09, P = 0.043; H3N2, P = 0.005), no statistical differences in the viral RNA loads were observed between day 0 and day 5 for H1N1 pdm09 (P = 0.068), H3N2 (P = 0.180), and B (P = 0.109) viruses. The Wilcoxon signed-rank test was used for data analysis.
TABLE 2.
Characteristics of patients with or without an increase in influenza A viral RNA load after day 3a
| Clinical characteristic | Results for increasing group (n = 9) | Results for no increasing group (n = 3) |
|---|---|---|
| Subtype | ||
| H1N1 pdm09 | 3 | 1 |
| H3N2 | 6 | 2 |
| Age (yr)b | 2.1 ± 1.5 (1.3, 0.6–4.8) | 3.1 ± 0.3 (3.0, 2.8–3.5) |
| No. of males | 4 | 2 |
| No. of vaccinationsc | 2 | 1 |
| Time after onsetb | 14.3 ± 8.3 (18.0, 5.0–26.5) | 18.8 ± 25.3 (5.5, 3.0–48.0) |
| Body temp (°C)b | 39.7 ± 0.5 (39.8, 39.0–40.2) | 39.9 ± 0.4 (39.9, 39.6–40.3) |
| Duration of fever (h)b | 24.8 ± 11.9 (25.0, 11.5–43.0) | 38.7 ± 4.5 (40.5, 33.5–42.0) |
There was no statistically significant difference in clinical characteristics between the groups. The Mann-Whitney U test was used to analyze age, hours after onset, body temperature, and duration of fever; Fisher's exact test was used to analyze sex and vaccination for comparisons between each group.
Data are mean ± SD (median, range).
Influenza vaccines were received prior to each influenza season.
Virus isolation and susceptibility to peramivir.
Among the 73 nasal swab samples or nasal aspiration samples for virus isolation, 19, 29, and 25 were obtained from patients with H1N1 pdm09, H3N2, and B, respectively, and isolates were obtained from 7, 20, and 23 samples, respectively (see Table S1 in the supplemental material). The mean IC50s of peramivir on day 0 were 0.15, 0.18, and 1.05 nM for H1N1 pdm09, H3N2, and B, respectively (Table 3). No increases were observed in the mean IC50s of isolates that were recovered after treatment (Table 3).
TABLE 3.
Influenza virus susceptibility to peramivir
| Day after treatment | Peramivir IC50 (nM) fora: |
||
|---|---|---|---|
| H1N1 pdm09 | H3N2 | B | |
| Day 0 (n = 2/6/7)b | 0.15 ± 0.02 (0.15, 0.14–0.17) | 0.18 ± 0.04 (0.20, 0.12–0.22) | 1.05 ± 0.27 (1.00, 0.72–1.49) |
| Day 1 (n = 3/6/6) | 0.13 ± 0.05 (0.11, 0.10–0.19) | 0.20 ± 0.02 (0.20, 0.18–0.24) | 1.45 ± 0.43 (1.37, 0.97–1.22) |
| Day 2 (n = 1/6/6) | 0.24 | 0.16 ± 0.02 (0.16, 0.13–0.20) | 1.09 ± 0.20 (1.02, 0.83–1.36) |
| Day 3 (n = none/1/1) | None | 0.17 | 0.94 |
| Day 4 (n = none/1/0) | None | 0.22 | None |
| Day 5 (n = 1/0/3) | 0.10 | None | 1.04 ± 0.12 (0.99, 0.96–1.19) |
Data are mean ± SD (median, range).
Number of H1N1 pdm09/H3N2/B isolates.
Peramivir concentration over time in the serum and upper respiratory tract.
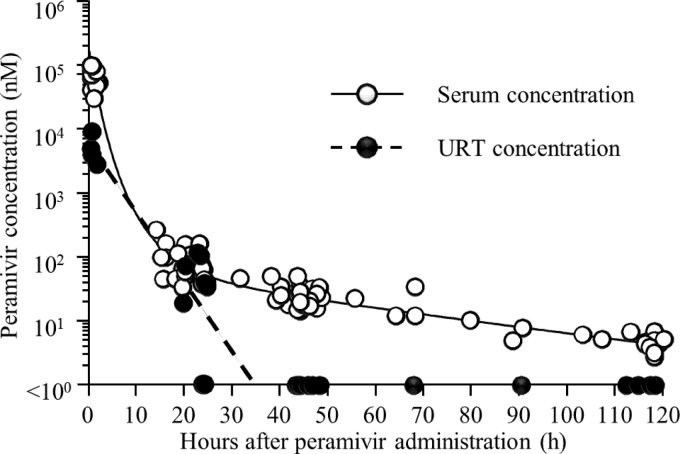
The serum peramivir concentration measured at 0.5 h after the initial dose was 88,883.6 ± 32,199.2 nM (mean ± SD) (88,000.6 nM, 40,803.0 to 165,039.1 nM [median, range]). The average time intervals from the first peramivir administration to serum sample collection at days 1, 2, 3, 4, and 5 were 20.6, 44.4, 69.9, 93.8, and 116.3 h, respectively, and the serum peramivir concentrations measured at these times were 83.2 ± 52.0 nM (66.1 nM, 33.5 to 265.2 nM), 25.1 ± 9.3 nM (22.6 nM, 14.4 to 49.0 nM), 17.1 ± 11.4 nM (12.0 nM, 10.1 to 34.1 nM), 6.2 ± 1.4 nM (6.1 nM, 4.9 to 7.7 nM), and 4.6 ± 1.2 nM (4.6 nM, 2.7 to 6.8 nM), respectively. The peramivir concentrations in the URT measured at 0.5 h after administration and on day 1 were 5,193.3 ± 2,758.4 nM (4,425.7 nM, 2,800.5 to 9,121.2 nM) and 48.3 ± 45.3 nM (36.5 nM, 1.0 to 117.4 nM), respectively; however, the drug was not detected after day 2. The blood and URT peramivir concentration-time curves are shown in Fig. 2. The formulae obtained for the blood and URT peramivir concentration-time curve, respectively, were as follows: Y = 7.772639 × exp(−0.1308 × t) − 3.672405 × exp(−0.001516 × t) + 20.629766 × exp(−0.001516 × t) − 12.70 and Y = −0.2511 × t + 8.6501 (r2 = 0.7713, P = 0.002) (Y, peramivir concentration [loge nM]; t, time after peramivir administration). The fitness of the predictive blood peramivir concentration-time curve was confirmed via a comparison between predicted and measured values (see Fig. S2 in the supplemental material). The predicted blood and URT half-lives of peramivir during the rapid decreasing period were calculated as 2.1 h and 2.8 h, respectively.
FIG 2.
Observed peramivir concentration (nM) in the serum and URT over time after a single intravenous administration of the drug and predictive blood and URT peramivir concentration-time curve. Peramivir in the URT was not detected after day 2, and the concentration in those samples was defined as 0 nM. URT, upper respiratory tract.
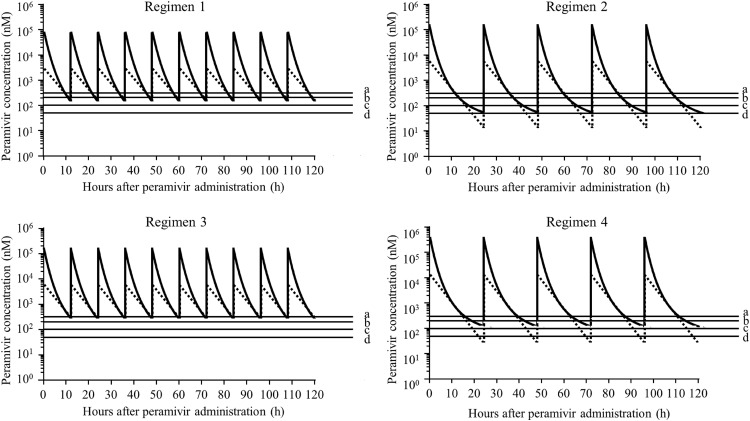
The predicted minimum blood and URT peramivir concentrations in the period between 0 and 120 h after the administration of the initial dose for regimens 1, 2, 3, and 4 were 134.9 and 152.8 nM, 54.0 and 13.8 nM, 263.1 and 280.6 nM, and 178.5 and 22.8 nM, respectively. (Fig. 3). The area under the concentration-time curve (AUC) (0 to 120 h) in the URT for regimens 1, 2, 3, and 4 was 109,104, 113,439, 216,254, and 223,946 nM · h, respectively (Table 4). The percentages of time above the proposed four peramivir concentrations in the URT in the period between 0 and 120 h after the initial doses for regimens 1, 2, 3, and 4 are shown in Table 4.
FIG 3.
Predictive blood (solid lines) and URT (broken lines) peramivir concentration-time curves for the following peramivir regimens: a 5-mg/kg dose every 12 h for 5 days (regimen 1) (top left panel), a 10-mg/kg dose every 24 h for 5 days (regimen 2) (top right panel), a 10-mg/kg dose every 12 h for 5 days (regimen 3) (bottom left panel), and a 20-mg/kg dose every 24 h for 5 days (regimen 4) (bottom right panel). Lines a, b, c, and d represent 300, 200, 100, and 50 nM, respectively. URT, upper respiratory tract.
TABLE 4.
Predictive peramivir PK/PD in the upper respiratory tract
| Administration regimena | AUCb (nM · h)c | Predicted % time (h) above ICd at required peramivir concn in the upper respiratory tract atc: |
|||
|---|---|---|---|---|---|
| 50 nM | 100 nM | 200 nM | 300 nM | ||
| 1 | 109,103 | 100 | 100 | 90.7 (108.9)e | 76.8 (92.2)e |
| 2 | 113,439 | 78.6 (94.3)e | 67.1 (80.5)e | 55.6 (66.7)e | 48.9 (58.7)e |
| 3 | 216,254 | 100 | 100 | 100 | 97.8 (117.4)e |
| 4 | 224,383 | 87.4 (104.9)e | 76.3 (91.5)e | 65.2 (78.2)e | 58.7 (70.4)e |
Regimen 1, 5 mg/kg every 12 h for 5 days; regimen 2, 10 mg/kg every 24 h for 5 days; regimen 3, 10 mg/kg every 12 h for 5 days; and regimen 4, 20 mg/kg every 24 h for 5 days.
AUC, area under the concentration-time curve.
From 0 to 120 h after the initial peramivir administration.
IC, inhibitory concentration.
Duration above the IC from 0 to 120 h after the initial administration of peramivir.
DISCUSSION
This study was the first report of peramivir PK in the URT in children. The mean duration of fever after a single administration of peramivir at 10 mg/kg was <30 h for influenza A and B, which was similar to that observed for oseltamivir or zanamivir administration for 5 days (19–21). However, in this study, the susceptibility of influenza A and B to peramivir did not decrease after peramivir administration: the influenza A viral RNA loads increased after day 3 in 9 of 12 patients, and the influenza B viral RNA loads did not decrease by day 2. This result may be attributed to the decline in drug efficacy to inhibit influenza A and B viral replication within 48 and 24 h after the initial peramivir administration, respectively, and the higher IC50 of type B than type A; the rapid peramivir elimination from the URT supports this contention. Therefore, when the progress of illness is attributable to an increase or lesser decrease in the viral load, even in the presence of susceptibility to peramivir, a second administration of peramivir must be considered at 48 and 24 h after the first administration in cases of influenza A and B, respectively.
Peramivir has hydrophobic and guanidine groups, which are responsible for inhibiting the NA activity of oseltamivir and zanamivir, respectively (22); thus, oseltamivir-resistant strains show decreased susceptibility to peramivir. However, because of the function of the guanidine group of peramivir and its extremely high concentration in the serum and respiratory tract exceeding the IC50s of resistant strains after intravenous administration, the clinical efficacy of such administration may be expected, even against oseltamivir-resistant viruses (H1N1 pdm09 H275Y or H7N9 R292K), when the drug is delivered within 48 h after the onset of illness.
The AUC/IC50 is the optimal PK/PD index for peramivir (23); however, the authors of that study did not consider the route of administration, i.e., gavage. In contrast, the time above the IC50 (time > IC50) and the AUC/IC50 are suggested as PK/PD indices that predict the inhibition of viral replication by intravenous zanamivir administration at a clinical 2.5-h half-life and an artificially prolonged 8-h half-life, respectively (24). Because the peramivir concentrations in both the serum and URT rapidly decreased after intravenous administration (Fig. 2) and the guanidine group is considered to be the main inhibitory mechanism (similar to zanamivir) against oseltamivir-resistant viruses (H1N1 pdm09 H275Y and H7N9 R292K), the time > IC50 was hypothesized to be the optimal PK/PD index for intravenous peramivir administration, similar to intravenous zanamivir administration, for the clinical 2.5-h half-life.
In this report, peramivir was readministered to two children 17.5 and 40.5 h after the first peramivir administration, and the serum peramivir concentrations after the second administration almost matched the predictive curve (data not shown). This finding indicates that the predictive curve determined here is appropriate as a simulation for other regimens, although the validity of the predictive curve was estimated in only two patients. The efficacy of a single peramivir administration at a 10-mg/kg dose within 48 h after the onset of illness was demonstrated in Japan (3, 4). The regimen 4 simulated here, at a 20-mg/kg dose, was theoretically expected to be effective against strains with highly decreased susceptibility to oseltamivir and peramivir. However, because the Cmax at 10 mg/kg in children is almost equal to that at a 600-mg/dose in adults, which is the approved maximum dose for a single administration in adults (3), and the Cmax at 20 mg/kg in children will be greater than that at a 600-mg/dose in adults, concerns of unexpected systemic side effects caused by the elevation of Cmax in the blood peramivir concentration must be considered, and a Cmax in children of less than that observed at 10 mg/kg is preferable. Moreover, it will be expected that Cmax will not be elevated even when peramivir is administered at 10 mg/kg every 12 h (regimen 3) because of its short half-life of 2.1 h in blood, and regimen 3 is expected to maintain a high concentration in the blood and URT for longer times than regimen 4 (20 mg/kg every 24 h), although the total dose administered is the same between the regimens (Table 4 and Fig. 3). Therefore, regimen 3 rather than regimen 4 is preferable for treating patients with severe illness caused by influenza viral strains with decreased susceptibility to the drug. Moreover, when a physician is considering an increase in the total administered dose but the peramivir concentration in the URT must be maintained at >50 nM, the administration of 5 mg/kg peramivir every 12 h (regimen 1) rather than 10 mg/kg every 24 h (regimen 2) should be considered based on the PK/PD index (Table 4). In summary, clinicians should consider dividing the dose to twice a day rather than increasing the dosage to maintain an effective concentration in the URT. However, because the emergence of a resistant variant during peramivir treatment after oseltamivir administration for several days has been demonstrated (25), our proposed regimens for maintaining a high concentration of peramivir should be applied to patients with a severe course of illness, and the emergence of resistant variants must be surveyed during and after treatment with peramivir. Informed consent should be obtained when peramivir is administered twice a day at 5 or 10 mg/kg every 12 h because this regimen has not been approved.
We proposed a regimen of peramivir administration against viruses with decreased susceptibility to NAIs. However, it should be emphasized that NAIs, including peramivir, are most effective when administered within 48 h after the onset of illness and that an early diagnosis followed by early antiviral therapy using NAIs is key to treating patients with influenza virus infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following physicians for sample collection: Hiroshi Mishima, Megumi Hoshina, Naohisa Ishibashi, Yusaku Abe, Yuichi Suzuki, Kazuhide Suyama, Ryo Maeda, Youich Tomita, and Kazuo Kato.
This work was not supported by any grants. The Department of Pediatrics, Fukushima Medical University, has received research funding through the research promotion division of the Fukushima Medical University from Astellas Pharma Inc., Otsuka Pharmaceutical Co., Ltd., Shiongi Co., Ltd., and Taisho Pharmaceutical Co., Ltd.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04263-14.
REFERENCES
- 1.Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. 2000. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 2.Katayose M, Hosoya M, Haneda T, Yamaguchi H, Kawasaki Y, Sato M, Wright PF. 2011. The effectiveness of trivalent inactivated influenza vaccine in children over six consecutive influenza seasons. Vaccine 29:1844–1849. doi: 10.1016/j.vaccine.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 3.Sugaya N, Kohno S, Ishibashi T, Wajima T, Takahashi T. 2012. Efficacy, safety, and pharmacokinetics of intravenous peramivir in children with 2009 pandemic H1N1 influenza A virus infection. Antimicrob Agents Chemother 56:369–377. doi: 10.1128/AAC.00132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohno S, Yen MY, Cheong HJ, Hirotsu N, Ishida T, Kadota J, Mizuguchi M, Kida H, Shimada J, S-021812 Clinical Study Group. 2011. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother 55:5267–5276. doi: 10.1128/AAC.00360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storms AD, Gubareva LV, Su S, Wheeling JT, Okomo-Adhiambo M, Pan CY, Reisdorf E, St George K, Myers R, Wotton JT, Robinson S, Leader B, Thompson M, Shannon M, Klimov A, Fry AM, US Antiviral Resistance Surveillance Working Group. 2012. Oseltamivir-resistant pandemic (H1N1) 2009 virus infections, United States, 2010-11. Emerg Infect Dis 18:308–311. doi: 10.3201/eid1802.111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okomo-Adhiambo M, Nguyen HT, Abd Elal A, Sleeman K, Fry AM, Gubareva LV. 2014. Drug susceptibility surveillance of influenza viruses circulating in the United States in 2011-2012: application of the WHO antiviral working group criteria. Influenza Other Respir Viruses 8:258–265. doi: 10.1111/irv.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takashita E, Ejima M, Itoh R, Miura M, Ohnishi A, Nishimura H, Odagiri T, Tashiro M. 2014. A community cluster of influenza A (H1N1) pdm09 virus exhibiting cross-resistance to oseltamivir and peramivir in Japan, November to December 2013. Euro Surveill. 19(1):pii=20666 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20666. [DOI] [PubMed] [Google Scholar]
- 8.Whitley RJ, Boucher CA, Lina B, Nguyen-Van-Tam JS, Osterhaus A, Schutten M, Monto AS. 2013. Global assessment of resistance to neuraminidase inhibitors, 2008-2011: the Influenza Resistance Information Study (IRIS). Clin Infect Dis 56:1197–1205. doi: 10.1093/cid/cis1220. [DOI] [PubMed] [Google Scholar]
- 9.Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, Kumar A, Lim M, Shindo N, Penn C, Nicholson KG. 2010. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 10.Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ, Xi XM, Gu Q, Zhou XM, Qu HP, Yan Z, Li FM, Zhao W, Gao ZC, Wang GF, Ruan LX, Wang WH, Ye J, Cao HF, Li XW, Zhang WH, Fang XC, He J, Liang WF, Xie J, Zeng M, Wu XZ, Li J, Xia Q, Jin ZC, Chen Q, Tang C, Zhang ZY, Hou BM, Feng ZX, Sheng JF, Zhong NS, Li LJ. 2013. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 11.Sleeman K, Guo Z, Barnes J, Shaw M, Stevens J, Gubareva LV. 2013. R292K substitution and drug susceptibility of influenza A(H7N9) viruses. Emerg Infect Dis 19:1521–1524. doi: 10.3201/eid1909.130724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward CL, Dempsey MH, Ring CJ, Kempson RE, Zhang L, Gor D, Snowden BW, Tisdale M. 2004. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J Clin Virol 29:179–188. doi: 10.1016/S1386-6532(03)00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenzel JJ, Panning M, Kaul KL, Mangold KA, Revell PA, Luna RA, Zepeda H, Perea L, Vazquez-Perez JA, Young S, Rodic-Polic B, Eickmann M, Drosten C, Jilg W, Reischl U. 2010. Analytical performance determination and clinical validation of the novel Roche RealTime Ready Influenza A/H1N1 Detection Set. J Clin Microbiol 48:3088–3094. doi: 10.1128/JCM.00785-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Infectious Diseases. 2014. Manual to detect influenza virus. (In Japanese.) National Institute of Infectious Diseases, Tokyo, Japan: http://www.nih.go.jp/niid/images/lab-manual/Influenza2014.pdf. [Google Scholar]
- 15.Sato M, Honzumi K, Sato T, Hashimoto K, Watanabe M, Miyazaki K, Kawasaki Y, Hosoya M. 2014. Sequential influenza B viral load and susceptibility in children treated with oseltamivir and zanamivir. Pediatr Infect Dis J 33:e168–e172. doi: 10.1097/INF.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 16.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538. [DOI] [PubMed] [Google Scholar]
- 17.Kaulbach HC, White MV, Igarashi Y, Hahn BK, Kaliner MA. 1993. Estimation of nasal epithelial lining fluid using urea as a marker. J Allergy Clin Immunol 92:457–465. doi: 10.1016/0091-6749(93)90125-Y. [DOI] [PubMed] [Google Scholar]
- 18.Pharmaceuticals and Medical Devices Agency. 2014. Interview form of peramivir. (In Japanese.) Pharmaceuticals and Medical Devices Agency, Tokyo, Japan: http://www.info.pmda.go.jp/go/pack/6250405A1032_1_02/. [Google Scholar]
- 19.Whitley RJ, Hayden FG, Reisinger KS, Young N, Dutkowski R, Ipe D, Mills RG, Ward P. 2001. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 20:127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hedrick JA, Barzilai A, Behre U, Henderson FW, Hammond J, Reilly L, Keene O. 2000. Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr Infect Dis J 19:410–417. doi: 10.1097/00006454-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Hosoya M, Kato K, Suzuki H. 2005. Viral shedding in children with influenza virus infections treated with neuraminidase inhibitors. Pediatr Infect Dis J 24:931–932. doi: 10.1097/01.inf.0000180976.81055.ce. [DOI] [PubMed] [Google Scholar]
- 22.Smith BJ, McKimm-Breshkin JL, McDonald M, Fernley RT, Varghese JN, Colman PM. 2002. Structural studies of the resistance of influenza virus neuraminidase to inhibitors. J Med Chem 45:2207–2212. doi: 10.1021/jm010528u. [DOI] [PubMed] [Google Scholar]
- 23.Drusano GL, Preston SL, Smee D, Bush K, Bailey K, Sidwell RW. 2001. Pharmacodynamic evaluation of RWJ-270201, a novel neuraminidase inhibitor, in a lethal murine model of influenza predicts efficacy for once-daily dosing. Antimicrob Agents Chemother 45:2115–2118. doi: 10.1128/AAC.45.7.2115-2118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown AN, Bulitta JB, McSharry JJ, Weng Q, Adams JR, Kulawy R, Drusano GL. 2011. Effect of half-life on the pharmacodynamic index of zanamivir against influenza virus delineated by a mathematical model. Antimicrob Agents Chemother 55:1747–1753. doi: 10.1128/AAC.01629-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renaud C, Pergam SA, Polyak C, Jain R, Kuypers J, Englund JA, Corey L, Boeckh MJ. 2010. Early emergence of an H275Y mutation in a hematopoietic cell transplant recipient treated with intravenous peramivir. Transpl Infect Dis 12:513–517. doi: 10.1111/j.1399-3062.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.