Abstract
Compounds that target the cellular factors essential for influenza virus replication represent an innovative approach to antiviral therapy. Sp2CBMTD is a genetically engineered multivalent protein that masks sialic acid-containing cellular receptors on the respiratory epithelium, which are recognized by influenza viruses. Here, we evaluated the antiviral potential of Sp2CBMTD against lethal infection in mice with an emerging A/Anhui/1/2013 (H7N9) influenza virus and addressed the mechanistic basis of its activity in vivo. Sp2CBMTD was administered to mice intranasally as a single or repeated dose (0.1, 1, 10, or 100 μg) before (day −7, −3, and/or −1) or after (6 or 24 h) H7N9 virus inoculation. A single Sp2CBMTD dose (10 or 100 μg) protected 80% to 100% of the mice when administered 7 days before the H7N9 lethal challenge. Repeated Sp2CBMTD administration conferred the highest protection, resulting in 100% survival of the mice even at the lowest dose tested (0.1 μg). When treatment began 24 h after exposure to the H7N9 virus, a single administration of 100 μg of Sp2CBMTD protected 40% of the mice from death. The administration of Sp2CBMTD induced the pulmonary expression of proinflammatory mediators (interleukin-6 [IL-6], IL-1β, RANTES, monocyte chemotactic protein-1 [MCP-1], macrophage inflammatory protein-1α [MIP-1α], and inducible protein [IP-10]) and recruited neutrophils to the respiratory tract before H7N9 virus infection, which resulted in less pronounced inflammation and rapid virus clearance from mouse lungs. Sp2CBMTD administration did not affect the virus-specific adaptive immune response, which was sufficient to protect against reinfection with a higher dose of homologous H7N9 virus or heterologous H5N1 virus. Thus, Sp2CBMTD was effective in preventing H7N9 infections in a lethal mouse model and holds promise as a prophylaxis option against zoonotic influenza viruses.
INTRODUCTION
Human infections with avian H7N9 influenza viruses remain a public health concern. Since the first cases in February 2013, H7N9 influenza viruses caused sporadic infection and reappeared in October 2013, with 453 laboratory-confirmed human cases reported to date (1). Overall, the case-fatality rate in humans is ∼30%; however, H7N9 influenza viruses have low pathogenicity in domestic poultry and thus can spread silently. Although sporadic family clusters of H7N9 infection have been reported (2–4), there is no evidence of sustained human-to-human transmission (5). There remains the potential risk of acquiring additional mutations by H7N9 viruses or human coinfections with H7N9 and seasonal influenza viruses, which might lead to the genesis of viruses with greater human infection and transmission characteristics. The possibility of enhanced transmissibility is underlined by the findings that avian H7N9 viruses retain some markers of mammalian adaptation (6), bind to both avian-type (α2,3-linked sialic acid [SA]) and human-type (α2,6-linked SA) cellular receptors (7, 8), and already possess limited transmissibility by the airborne route in ferrets, guinea pigs, and swine (9–12).
The control of H7N9 infection in humans relies on effective vaccines and antiviral therapy. The recently developed H7N9 vaccines show poor immunogenicity in humans (13), likely due to the lack of T-cell epitopes in the emerging H7N9 viruses (14). The absence of previous exposure of humans to this subtype of influenza virus (15) can be a challenge in selecting vaccine regimens against a novel H7N9 virus. The available antiviral options are limited to viral neuraminidase inhibitors (NAIs), but the emergence of antiviral resistance to NAIs during the treatment of H7N9 infections was reported in 6 patients, 3 of whom died (16, 17).
There is a pressing need to develop novel therapeutic agents that are efficacious against all influenza virus subtypes. Compounds that target cellular factors essential for virus replication provide an innovative approach for antiviral influenza therapy. Influenza viruses are expected to have a higher resistance barrier to this class of antivirals than to virus-targeted therapies, because host proteins are generally not subject to antiviral-mediated selective pressure. Currently, host-targeted influenza drugs are represented by an investigational drug, DAS181 (Fludase), which is undergoing clinical evaluation (18). DAS181 removes the terminal SA from cell surface sialoglycoconjugates, which are the primary receptors for influenza virus binding and entry into the host cell (19).
By using structural information, Connaris, Crocker, and Taylor (20) and Connaris et al. (21) recently developed multivalent proteins based on SA-recognizing carbohydrate-binding modules (mCBMs) derived from bacterial sialidases with high affinity to SA receptors (Vc2CBMTD and Sp2CBMTD) (20, 21). These novel mCBMs protected MDCK cells against the A/WSN/1933 (H1N1), A/Udorn/1972 (H3N2), A/Puerto Rico/8/1934 (H1N1), and B/Hong Kong/1973 influenza virus strains (50% effective concentrations [EC50s], 0.4 to 4.0 μM). Fluorescence imaging of mammalian cells revealed mCBMs targeting cell surface SA receptors and the abrogation of binding after sialidase treatment. mCBMs conferred protection in a mouse model against lethal doses of both the A/WSN/1933 (H1N1) and mouse-adapted A/California/04/2009 (H1N1pdm09) influenza viruses. Repeated-dose administration of hexameric mCBM (Sp2CBMTD) before viral challenge was the most beneficial regimen for complete protection and no weight loss (21). The biologics appear to stimulate inflammatory mediators that confer protective ability in mice (21). Human infections with zoonotic viruses circulating in natural reservoirs are introducing additional challenges for controlling infection because of the uncertainty about virus pathogenicity to humans and disease severity. Here, we examine whether Sp2CBMTD is effective against lethal infection in mice with the newly emerging A/Anhui/1/2013 (H7N9) influenza virus and study the potential mechanistic basis of its activity.
MATERIALS AND METHODS
Viruses, cells, and biologic.
Influenza A/Anhui/1/2013 (H7N9) and A/Turkey/15/2006 (H5N1) viruses were obtained through the World Health Organization network and propagated in embryonated chicken eggs for 48 h at 35°C. Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection and maintained as described previously (22). Sp2CBMTD was generated by PCR-based cloning techniques, using genes encoding the carbohydrate-binding module 40 (CBM40) domain from Streptococcus pneumoniae NanA sialidase and the trimerization domain from Pseudomonas aeruginosa pseudaminidase (21). The experiments with the H7N9 and H5N1 influenza viruses were conducted in an animal biosafety level 3+ containment facility approved by the U.S. Department of Agriculture.
Efficacy of Sp2CBMTD in mice.
Six-week-old female BALB/c mice (weight, 18 to 20 g; Jackson Laboratories, Bar Harbor, ME) were lightly anesthetized by isoflurane inhalation and inoculated intranasally with five 50% mouse lethal doses (MLD50) of A/Anhui/1/2013 (H7N9) influenza virus in 50 μl of phosphate-buffered saline (PBS). For the first study, 5 BALB/c mice per group were given a single dose of Sp2CBMTD (0.1, 1, 10, or 100 μg/mouse) intranasally on day 7, 3 or 1 before inoculation, or 6 or 24 h after H7N9 virus inoculation. For the repeated-dose regimens, Sp2CBMTD was administered either twice (days −3 and −1) or three times (days −7, −3, and −1) prior to H7N9 inoculation. The control mice received sterile PBS on days −7, −3, and −1. The survival of each mouse was monitored daily for 21 days postinfection (p.i.); any animal that showed signs of severe disease and a 25% weight loss was sacrificed. Three weeks after inoculation with the A/Anhui/1/2013 (H7N9) influenza virus, the surviving mice were reinfected with 25 MLD50 of homologous virus.
For the second study, the 10-μg dose of Sp2CBMTD was evaluated. Groups of 30 BALB/c mice each were given single 10-μg doses of Sp2CBMTD either once (day −7 or −3), twice (days −3 and −1), or three times (days −7, −3, and −1) before A/Anhui/1/2013 (H7N9) influenza virus inoculation. The survival of each mouse (10 mice/group) was monitored daily for 21 days p.i. The weight gain or loss was calculated for each mouse as a percentage of its weight before inoculation. Three mice from each group were sacrificed to determine the virus lung titers (on days 3, 6, and 9 p.i.) and levels of cytokine or chemokine responses (on days 0, 3, 6, and 9 p.i.) in the lung homogenate samples. The lungs were removed, thoroughly rinsed with sterile PBS, homogenized, and suspended in 1 ml of ice-cold PBS. Cellular debris was removed by centrifugation at 2,000 × g for 10 min, after which the supernatants were used for 50% tissue culture infectious dose (TCID50) assays in MDCK cells (incubation at 37°C for 3 days). For histopathological examination, an additional 2 mice from each group were sacrificed on days 0, 3, 6, and 9 p.i. Three weeks after the initial inoculation with A/Anhui/1/2013 (H7N9) influenza virus, the surviving mice received a second administration of Sp2CBMTD (10-μg dose) once (day −7 or −3), twice (days −3 and −1), or three times (days −7, −3, and −1) prior to being reinfected with 20 MLD50 of A/Turkey/15/2006 (H5N1) influenza virus. All studies were conducted under the applicable laws and guidelines and approved by the St. Jude Children's Research Hospital Animal Care and Use Committee.
Lung cytokine and chemokine analysis.
The concentrations of the 4 cytokines (gamma interferon [IFN-γ], interleukin-1β [IL-1β], interleukin-6 [IL-6], and tumor necrosis factor alpha [TNF-α]) and 4 chemokines (regulated upon activation, normal T cell expressed and secreted [RANTES], macrophage inflammatory protein-1α [MIP–1α], monocyte chemotactic protein-1 [MCP-1], and inducible protein [IP-10]) were measured using a mouse MCYTOMAG-70K-PMX Milliplex premixed kit (Millipore), according to the manufacturer's instructions. For each cytokine, the standard curve ranged from 3.2 to 10,000 pg/ml. The cytokines were measured in 25 μl of lung homogenate samples at days 0 (uninfected mice), 3, 6, and 9 p.i. The multiplex plates were read on the Luminex 100/200 analyzer using the xPonent data acquisition and analysis software.
Histopathology and immunohistochemistry.
The lungs of the mice in each experimental group (n = 2) of the second study were collected on days 0, 3, 6, and 9 p.i. after whole-body perfusion with 10% neutral-buffered formalin (NBF). The mouse lungs underwent inflation via tracheal infusion and were kept in 10% NBF for ≥7 days before embedding, sectioning, and staining for conventional histopathology with hematoxylin and eosin or with immunohistochemical (IHC) staining for influenza A virus (using nucleoprotein [NP]; U.S. Biological) and neutrophils [myeloperoxidase {MPO}]; Thermo Shandon).
The influenza A virus NP- and MPO-stained sections were blinded for pathology evaluation. The presence of antigens was quantified by capturing digital images of whole-lung sections using an Aperio ScanScope XT slide scanner (Aperio Technologies) and then manually outlining entire fields together with areas of noticeably decreased or absent NP and MPO staining. The percentage of the lung field with reduced staining coverage was calculated using the Aperio ImageScope software.
Serology.
Serum samples were obtained 21 days after H7N9 or H5N1 virus infection, treated with receptor-destroying enzyme (Denka Seiken Co.), heat inactivated at 56°C for 1 h, and tested for the presence of anti-hemagglutinin (HA) antibodies by the HA inhibition assay with 0.5% turkey red blood cells (Rockland Immunochemicals). The presence of anti-SpCBM antibodies in the serum samples was measured in an enzyme-linked immunosorbent assay (ELISA), using purified protein (1 μg/well) immobilized on 96-well plates (Corning) (21).
Statistical analysis.
The virus lung titers, concentrations of cytokines and chemokines, anti-SpCBM antibody titers, and the number of NP- and MPO-stained lung cells were compared by analysis of variance (ANOVA), followed by Bonferroni posttests, using the GraphPad Prism 5.0 software (La Jolla, CA). Kaplan-Meier survival curves were generated and compared using the Mantel-Cox log-rank test.
RESULTS
Efficacy of Sp2CBMTD on survival of mice lethally challenged with H7N9 influenza virus.
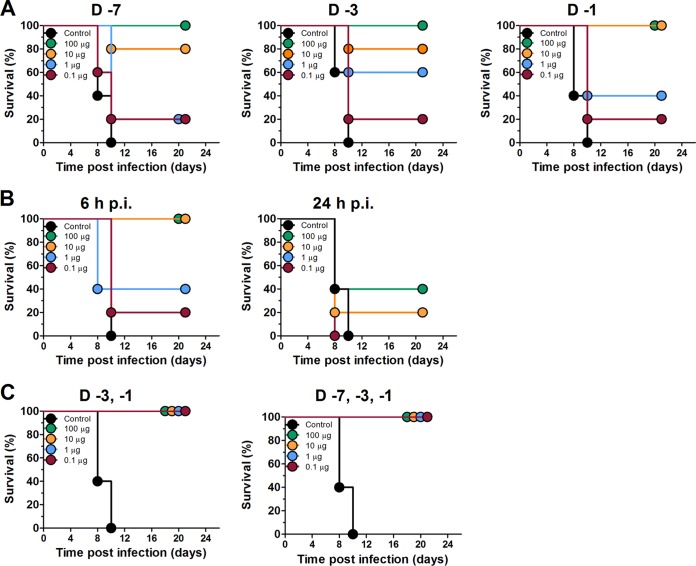
To determine whether Sp2CBMTD improved the survival of mice lethally challenged with A/Anhui/1/2013 (H7N9) influenza virus, the biologic was prophylactically administered as a single-dose regimen of 0.1, 1, 10, or 100 μg on day 7, 3 or 1 before viral challenge. The virus-inoculated PBS-treated control animals exhibited progressive weight loss, and all died between days 8 and 10 p.i. (Fig. 1). A single intranasal administration of Sp2CBMTD before the H7N9 virus challenge resulted in a dose-dependent protection of mice. The highest single-dose regimen of Sp2CBMTD (100 μg) provided the greatest protection, and 100% of the mice survived the infection when the biologic was administered on day 7, 3 or 1 before virus inoculation (Fig. 1A). The 10-μg single-dose regimen protected 80% of the mice when administered on day 7 or 3 before virus inoculation and 100% of the mice when administered on day 1 before inoculation. The protection of animals decreased when the 1-μg single-dose regimen was used: only 60% and 40% of the mice survived when the biologic was administered on day 3 or 1 before virus challenge (Fig. 1A), respectively. The lowest dose tested (0.1 μg) was the least effective, with only 20% of the mice being protected.
FIG 1.
Effect of single and repeated administration of Sp2CBMTD on survival of mice infected with a lethal dose of A/Anhui/1/2013 (H7N9) influenza virus. Groups of BALB/c mice (n = 5) were lightly anesthetized with isoflurane, and Sp2CBMTD was administered intranasally as a single-dose regimen (0.1, 1, 10, or 100 μg/mouse) at day 7, 3 or 1 before (A) or 6 or 24 h after (B) H7N9 virus inoculation. For the repeated-dose regimens, Sp2CBMTD (0.1, 1, 10, or 100 μg/mouse) was administered either twice (days −3 and −1) or three times (days −7, −3, and −1) prior to H7N9 inoculation, respectively (C). Each mouse was inoculated intranasally with 5 MLD50 of A/Anhui/1/2013 (H7N9) influenza virus and monitored daily for survival and weight loss. The control (untreated) animals received sterile PBS on days −7, −3, and −1.
Next, we assessed the effect of early postexposure treatment with Sp2CBMTD on the survival of mice. Sp2CBMTD given once at 6 h after the H7N9 virus challenge protected 100% of the mice at doses of 10 and 100 μg and 40% of the mice at a dose of 1 μg (Fig. 1B). The efficiency of protection decreased when the initiation of treatment was delayed by 24 h: only 40% of the mice treated with 100 μg and 20% of the mice treated with 10 μg survived H7N9 inoculation; the 0.1- and 1-μg single-dose regimens did not provide survival benefits.
The repeated administration of Sp2CBMTD given two or three times before lethal challenge of mice with A/Anhui/1/2013 (H7N9) influenza virus provided complete protection at all doses of the biologic tested (Fig. 1C). These results indicate that the repeated administration of Sp2CBMTD before H7N9 virus inoculation was the most effective for the survival of the mice and the minimization of weight loss (Table 1).
TABLE 1.
Efficacy of 10 μg of Sp2CBMTD against lethal A/Anhui/1/2013 (H7N9) influenza virus infection in BALB/c mice
| Sp2CBMTD administrationa | No. survived/total no. (%) | Mean ± SD survival daysb | % wt loss (avg ± SD) on p.i. dayc: |
Lung virus titer (mean ± SD) (log10 TCID50/ml) on p.i. dayd: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 12 | 3 | 6 | 9 | |||
| Single (day −7) | 6/10 (60) | 15.4 ± 5.9 | 10.5 ± 2.6 | 17.8 ± 3.7 | 24.9 ± 5.7 | 18.3 ± 8.3*** | 6.8 ± 0.5 | 6.2 ± 0.0 | 5.5 ± 0.2 |
| Single (day −3) | 8/10 (80) | 17.8 ± 4.6 | 6.0 ± 4.0* | 13.4 ± 4.3* | 20.0 ± 8.1 | 7.4 ± 6.1* | 6.7 ± 0.3 | 5.6 ± 0.4 | 5.1 ± 0.3 |
| Double (days −3 and −1) | 10/10 (100) | 20.0 ± 0.0 | 6.2 ± 1.9* | 10.3 ± 2.4** | 13.3 ± 5.1*** | 2.8 ± 4.5 | 6.8 ± 0.5 | 6.0 ± 0.3 | 2.9 ± 0.6*** |
| Triple (days −7, −3, and −1) | 10/10 (100) | 20.0 ± 0.0 | 4.0 ± 2.8** | 10.1 ± 3.7** | 12.9 ± 6.8*** | 0.9 ± 7.3 | 6.3 ± 0.1 | 5.8 ± 0.5 | 3.3 ± 0.0*** |
| Control | 0/10 (0) | 8.0 ± 1.0 | 13.2 ± 2.5 | 20.2 ± 4.5 | 26.6 ± 4.9 | NAe | 7.0 ± 0.0 | 6.0 ± 0.3 | 5.4 ± 0.3 |
Groups of 6- to 8-week-old BALB/c mice (n = 10) were given 50 μl of Sp2CBMTD (10 μg/mouse) intranasally as a single-, double-, or triple-dose regimen. Treatment with Sp2CBMTD was initiated at different time points between days 7 and 1 before inoculation with 5 MLD50 of A/Anhui/1/2013 (H7N9) influenza virus.
SD, standard deviation.
Loss of weight was calculated for each mouse as a percentage of its weight on day 0 p.i. (postinfection). *, P < 0.005; **, P < 0.001; and ***, P < 0.0001 compared to control animals, two-way ANOVA.
Data from 3 animals per group. The lower limit of virus detection was 0.75 log10 TCID50/ml.
NA, not applicable (all mice in the group died).
Effect of Sp2CBMTD on H7N9 influenza virus replication in mouse lungs.
In a second experiment, the mechanistic basis of the antiviral activity of Sp2CBMTD was examined using a 10-μg dose, which was prophylactically administered as a single-dose regimen either on day −7 or −3, a double-dose regimen on days −3 and −1, and a triple-dose regimen on days −7, −3, and −1. The kinetics of the H7N9 virus load was determined in the lungs of the infected mice. The virus titers in the lungs of Sp2CBMTD-treated mice with all biologic regimens tested did not differ from those of the controls on day 3 or 6 p.i. (Table 1). Notably, on day 9 p.i., repeated administrations of Sp2CBMTD (double- and triple-dose regimens) significantly reduced virus load in the lungs of infected mice (P < 0.0001), but both single-dose regimens tested did not reduce virus titers (Table 1).
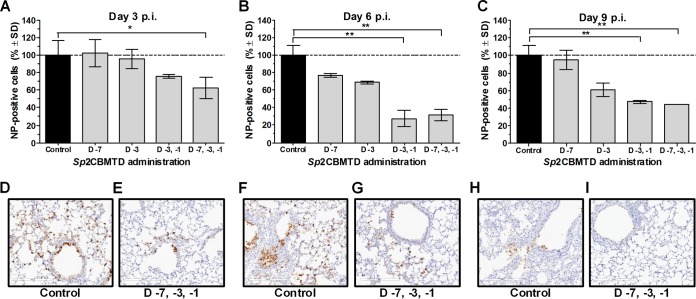
To compare A/Anhui/1/2013 (H7N9) influenza virus replication in the lower respiratory tract of infected mice given either PBS (control) or different regimens of the biologic, the virus-positive cells were quantified in lung sections on days 3, 6, and 9 p.i. (Fig. 2). In the control animals, NP-positive cells (a measure of influenza virus-infected cells) were detected throughout the whole lung sections, specifically in the epithelial cells of the bronchi, bronchioles, and peribronchiolar alveoli on days 3 and 6 p.i. (Fig. 2D and F), and the number of antigen-positive cells decreased on day 9 p.i. (Fig. 2H). The single administration of Sp2CBMTD did not significantly change the number of NP-positive cells in the infected mouse lungs compared with that in the control animals on day 3, 6, or 9 p.i. In contrast, double or triple administration of the biologic resulted in significant decreases (P < 0.01 and P < 0.05, respectively) in the number of NP-positive cells, with the cell number declining from 80% to 70% on day 3 p.i. (Fig. 2A) and to 50% on day 9 p.i. (Fig. 2C).
FIG 2.
Effect of Sp2CBMTD on the number of H7N9 influenza virus NP-positive cells in mouse lung sections. BALB/c mice were lightly anesthetized with isoflurane, and Sp2CBMTD (10 μg/mouse) was administered intranasally either once at day 7 or day 3, twice at days 3 and 1, or three times at days 7, 3 and 1 before H7N9 virus inoculation. Two mice in each experimental group were sacrificed on days 3, 6, and 9 p.i. The mouse lungs were removed, fixed in 10% neutral-buffered formalin, and stained for immunohistochemistry with anti-influenza A nucleoprotein (NP) antibody. One slide that included all lung lobes per mouse was used for evaluations. Quantitative analysis of the number of NP-positive cells on days 3 (A), 6 (B), and 9 p.i. (C) was done using the ImageScope viewing software. Specifically stained cells have dark brown color in the nucleus and cytoplasm. (D, F, and H) Images of the lung tissues of control animals showed H7N9 influenza antigen-positive staining in bronchiolar and respiratory epithelial cells on days 3 and 6 p.i. (D and F) compared with the few antigen-positive cells on day 9 p.i. (H). (E, G, and I) Images of the representative Sp2CBMTD-treated group (triple administration on days 7, 3 and 1 before H7N9 inoculation) showed a marked reduction in antigen-positive cells in the lungs of mice on days 3 and 6 p.i. (E and G) compared with that in the controls, and few positive cells on day 9 p.i. (I). The dashed line indicates H7N9 influenza antigen-positive cells determined in virus infected PBS-treated animals and was considered 100%. Magnification, ×20. *, P < 0.01; **, P < 0.05 compared with the results for the control group at each day p.i. studied, two-way ANOVA.
These findings indicate that repeated Sp2CBMTD administration controlled the spread of H7N9 virus in the lung tissues of the infected mice. The infectious virus titers were not affected by Sp2CBMTD treatment until day 9 p.i., suggesting that the immune response was responsible for virus clearance rather than a reduction in virus replication caused by limited access to SA receptors.
Effect of Sp2CBMTD on histological changes of lung tissues.
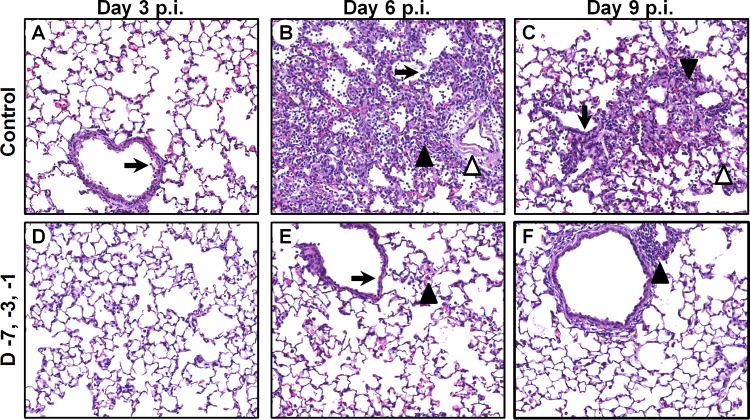
To determine the extent of the changes in the lungs, tissues obtained from different Sp2CBMTD regimen groups on days 0, 3, 6, and 9 p.i. were histologically examined (Fig. 3). The lungs of the H7N9-infected PBS-treated mice showed necrosis of epithelial cells in the bronchi on day 3 p.i. (Fig. 3A) and alveolar collapses and infiltration with inflammatory cells (neutrophils and macrophages), edema, and viral pneumonia on day 6 p.i. (Fig. 3B). Edema and infiltration with inflammatory cells continued on day 9 p.i., but the lesions were restricted to a few areas (Fig. 3C). In the Sp2CBMTD-treated mice that received a triple-dose regimen, there were no distinctive pathological changes on day 3 p.i., and epithelial necrosis and infiltration of the alveoli with inflammatory cells was minimal (Fig. 3D). The accumulation of inflammatory cells in the lung parenchyma and progression of virus spread were observed on day 6 p.i. and resolved on day 9 p.i. (Fig. 3E and F). These findings support that Sp2CBMTD administration decreases lung tissue damage, which is associated with lethal H7N9 virus infection.
FIG 3.
Histological changes in the lungs of mice treated with Sp2CBMTD and infected with 5 MLD50 of A/Anhui/1/2013(H7N9) influenza virus. Sp2CBMTD was administered as described in the Fig. 2 legend. Mouse lungs were fixed in 10% neutral-buffered formalin and stained with hematoxylin and eosin. The histological changes in the lungs of H7N9-infected PBS-treated mice are shown for days 3 (A), 6 (B), and 9 p.i. (C). The pathological changes in the lungs of mice given a triple-dose regimen of Sp2CBMTD are shown for days 3 (D), 6 (E), and 9 p.i. (F). Magnification, ×20. The black arrow indicates epithelial necrosis, the open arrowhead indicates edema, and the closed arrowhead indicates peribronchiolar alveoli infiltration with macrophages and neutrophils.
Effect of Sp2CBMTD on production of pulmonary cytokines and chemokines.
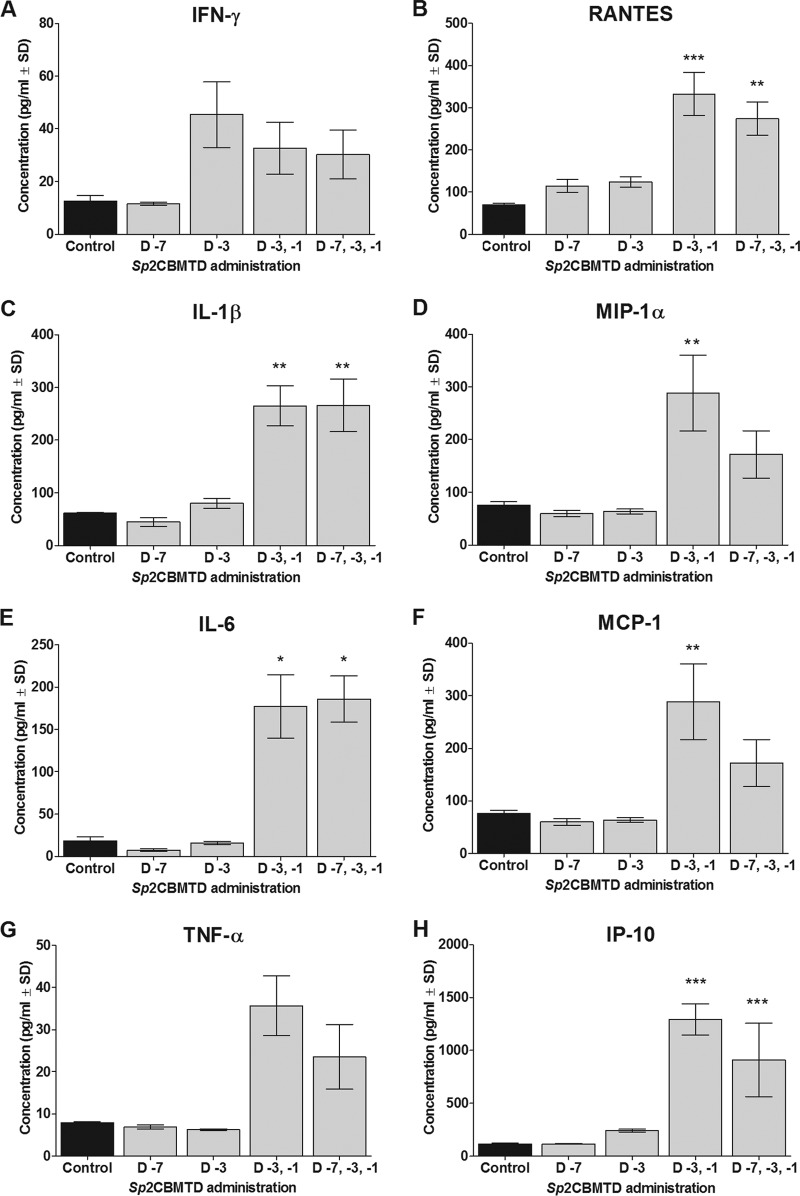
To determine the inflammatory and innate immune responses associated with Sp2CBMTD administration, the effect of Sp2CBMTD on the pulmonary expression of cytokines and chemokines was studied. Notably, Sp2CBMTD stimulated a proinflammatory response before H7N9 virus inoculation, and the levels of specific proinflammatory mediators increased in the Sp2CBMTD-treated animals by day 0 p.i., especially with repeated administrations (Fig. 4). The levels of IL-6 (P < 0.05), IL-1β, MIP-1α, MCP-1 (P < 0.01), RANTES (P < 0.01 or P < 0.001), and IP-10 (P < 0.001) were significantly higher in the mice that received 2 and/or 3 administrations of Sp2CBMTD than the levels of cytokines and chemokines secreted in the lung homogenates of the untreated control mice (Fig. 4). These effects may be due to activated alveolar macrophages in the lungs. The pulmonary expression of cytokines and chemokines varied between the experimental groups on days 3, 6, and 9 p.i., and, consistent with our previous findings on challenge with the A/WSN/1933 (H1N1) virus (21), there was no clear pattern of expression (data not shown). These results suggest that Sp2CBMTD might modulate the immune response by “priming” the host to better combat an oncoming influenza virus infection.
FIG 4.
Effect of Sp2CBMTD administration on pulmonary expression of cytokines and chemokines. Sp2CBMTD was administered as described in the Fig. 2 legend. Concentrations of IFN-γ (A), RANTES (B), IL-1β (C), MIP-1α (D), IL-6 (E), MCP-1 (F), TNF-α (G), and IP-10 (H) were assessed by the MCYTOMAG-70K-PMX Milliplex premixed kit in lung homogenates of BALB/c mice on the day of H7N9 virus infection. Each data point represents the mean concentration (pg/ml) from 3 independent animals. The error bars indicate the standard deviation (SD). The black bars represent H7N9 virus-inoculated PBS-treated mice (control). The gray bars represent mice treated with Sp2CBMTD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with results for the control group, two-way ANOVA.
Effect of Sp2CBMTD on neutrophil recruitment.
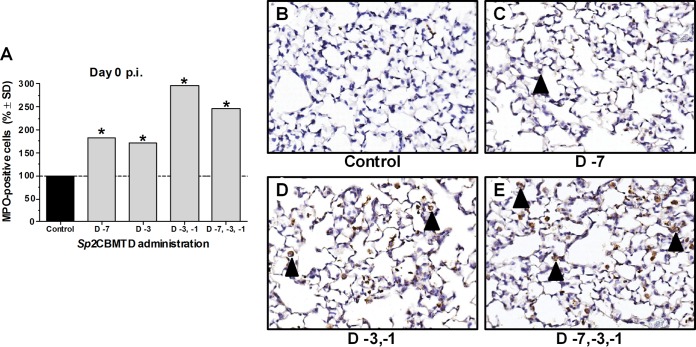
To confirm that the elevated levels of cytokines determined before virus inoculation were associated with an increase in the immune cell population in the lungs, the number of neutrophils was determined (Fig. 5). Before H7N9 virus challenge (day 0 p.i.), the neutrophil counts were significantly higher in the samples from Sp2CBMTD-treated mice than those from the controls (P < 0.0001), with the greatest increase seen in the samples obtained from mice given repeated administrations of Sp2CBMTD (Fig. 5). Our results indicate that the recruitment of immune cells to the virus replication site caused by Sp2CBMTD administration contributed to rapid recovery and survival from lethal H7N9 virus infection.
FIG 5.
Infiltration of mouse lung tissues with neutrophils after administration of Sp2CBMTD. Sp2CBMTD was administered as described in the Fig. 2 legend. Mouse lungs were obtained on day 0 p.i., fixed in 10% neutral-buffered formalin, and stained with myeloperoxidase (MPO), a specific marker for neutrophils. The MPO-stained sections were blinded for pathology evaluation. The presence of antigens was quantified by capturing digital images of whole-lung sections using an Aperio ScanScope XT slide scanner (Aperio Technologies) and then manually outlining entire fields together with areas of noticeably decreased or absent MPO staining. The percentage of the lung field with reduced staining coverage was calculated using the Aperio ImageScope software and expressed as a relative value over virus-infected PBS-treated (control) animals (A). Representative MPO-stained lung images of the control animals (B) and animals given single-dose (C), double-dose (D), or triple-dose (E) regimens of Sp2CBMTD before H7N9 virus infection. Magnification, ×20. The black arrowhead indicates neutrophils. *, P < 0.0001 compared with results for the control group, one-way ANOVA.
Reinfection of mice with H7N9 influenza virus.
To examine whether Sp2CBMTD treatment interferes with the induction of adaptive immunity, the serum titers of anti-HA antibodies against A/Anhui/1/2013 (H7N9) influenza virus were determined. All surviving mice had moderate titers of anti-HA antibodies (40 to 160), regardless of the regimen used (Table 2). The anti-HA antibody titers were sufficient to protect 100% of the surviving animals against a 25-MLD50 dose of homologous H7N9 virus reinfection (data not shown).
TABLE 2.
Titers of anti-HA and anti-SpCBM antibodies in mouse sera
| Sp2CBMTD administrationa | Range of HI titers after infection with influenza virusb: |
Concn (mean ± SD) (mg/ml) of anti-SpCBM antibodies after Sp2CBMTD administrationc |
||||||
|---|---|---|---|---|---|---|---|---|
| A/Anhui/1/2013 (H7N9) | A/Turkey/15/2006 (H5N1) | First administration |
Second administration |
|||||
| IgG | IgM | IgA | IgG | IgM | IgA | |||
| Single (day −7) | 80 | 40–80 | 174.7 ± 7.0 (26.5)*** | 5.3 ± 3.0 (132.5) | 4.5 ± 3.3 (1.4) | 257.7 ± 0.2 (1.5)*** | 17.7 ± 0.2 (3.3)** | 30.2 ± 6.1 (6.7)*** |
| Single (day −3) | 80–160 | 20–40 | 143.1 ± 7.4 (21.7)*** | 3.8 ± 1.2 (95.0) | 5.1 ± 4.6 (1.5) | 238.6 ± 2.9 (1.7)*** | 16.6 ± 1.1 (4.4)* | 42.7 ± 9.1 (8.4)*** |
| Double (days −3 and −1) | 40–80 | 40–80 | 214.9 ± 9.0 (32.6)*** | 11.2 ± 1.9 (280.0) | 3.9 ± 2.8 (1.2) | 273.8 ± 1.4 (1.3)*** | 19.2 ± 0.7 (1.7)** | 59.4 ± 2.6 (15.2)*** |
| Triple (days −7, −3, and −1) | 80–160 | 40–80 | 211.6 ± 16.1 (32.1)*** | 12.7 ± 2.2 (317.5) | 7.9 ± 5.5 (2.4) | 269.9 ± 1.6 (1.3)*** | 18.0 ± 0.4 (1.4)** | 85.1 ± 13.5 (10.8)*** |
| Naive mice | NAd | NA | 6.6 ± 3.0 | 0.04 ± 0.1 | 3.3 ± 1.8 | 5.6 ± 0.4 | 0.1 ± 0.0 | 3.1 ± 1.8 |
Sp2CBMTD (10 μg/mouse) was administered as described in the Table 1 legend. Three weeks after the initial H7N9 virus inoculation and the first use of Sp2CBMTD, the biologic was used the second time and administered to the animals at the same regimens as during the first use. The animals were reinfected with 20 MLD50 of the highly pathogenic A/Turkey/15/2006 (H5N1) influenza virus (28 days p.i. with the H7N9 virus). The naive mice did not receive Sp2CBMTD and were not infected with the influenza virus. The days are the days before A/Anhui/1/2013 (H7N9) influenza virus inoculation.
HI titers against A/Anhui/1/2013 (H7N9) influenza virus were determined 3 weeks after the initial H7N9 virus inoculation (expressed as reciprocal values, e.g., 40 versus 1:40) using 0.5% turkey red blood cells. The HI titers against A/Turkey/15/2006 (H5N1) influenza virus were determined 3 weeks after the initial H5N1 virus inoculation (or 48 days after H7N9 virus inoculation).
Values are means ± standard deviation (SD) from 4 mice per group. The fold change in the levels of anti-SpCBM antibodies relative to the mean concentrations of naive animals is shown in parentheses for the first administration data and relative to the first administration in parentheses for the second administration data. *, P < 0.01; **, P < 0.001; and ***, P < 0.0001 compared to the concentration after the first administration of Sp2CBMTD, two-way ANOVA.
NA, not applicable (naive mice did not possess anti-HA antibodies against A/Anhui/1/2013 (H7N9) and A/Turkey/15/2006 (H5N1) influenza viruses).
Induction of anti-SpCBM antibodies after repeated administration and reinfection with H5N1 influenza virus.
The development of anti-biologic antibodies potentially abrogates protection when the biologic is used repeatedly. We assessed the levels of anti-SpCBM antibodies in mouse sera after two uses of the biologic (Table 2). Compared to the naive mice, the most prominent increase after the first use of Sp2CBMTD was observed for IgM, which presents the pool of acute antibodies, and the least increase was shown for IgA. After the second use of Sp2CBMTD, the levels of IgG and IgM increased 1.3- to 1.7-fold and 1.4- to 4.4-fold in all treatment groups, respectively (Table 2). To address the question of whether repeated Sp2CBMTD use can affect protection against influenza virus infection, we reinfected mice with the highly pathogenic H5N1 virus. Importantly, the animals in the groups that received the biologic twice were completely protected from lethal challenge with H5N1 virus (data not shown). All naive untreated mice died between days 8 and 10 after the H5N1 virus challenge, although 50% of the naive animals that received a double administration of Sp2CBMTD (10 μg) on days −3 and −1 were protected from lethal infection (data not shown).
DISCUSSION
In a previously developed mouse model of influenza H7N9 virus-induced acute respiratory distress syndrome (23), we demonstrated the antiviral efficacy of the novel host-targeted biologic Sp2CBMTD in preventing lethal infection with the newly emerging human pathogen. The highest efficacy and 100% protection of the mice were achieved by repeated administration of Sp2CBMTD before the H7N9 viral challenge, although 20% to 100% of the animals were protected with a single dose of Sp2CBMTD given after the viral challenge, depending on the timing and dose. The repeated administration of Sp2CBMTD induced some key proinflammatory cytokines and recruited immune cells to the lung epithelia before H7N9 virus infection, which resulted in less pronounced inflammation and rapid virus clearance from mouse lungs.
Human infection caused by avian influenza viruses raises concerns about the optimal therapeutics for controlling zoonotic infections and highlights the need to develop novel anti-influenza drugs. The targets for novel drugs have broadened in recent years, focusing on not only influenza virus-specific proteins but also the host factors essential for virus replication. The major advantages of host-targeted drugs are their broad spectrum of activity and efficacy against different influenza virus subtypes and the low risk of emergence of drug-resistant variants (24). An attractive strategy for drug development is to inhibit influenza virus entry into the host cell. Therefore, Sp2CBMTD, which was designed to mask SA-containing host cell receptors, is a promising candidate. Unlike the investigational antiviral biologic DAS181, Sp2CBMTD does not remove cellular receptors; instead it masks them and prevents viral attachment (21). Glycan array screening shows that SpCBM recognizes glycans containing terminal α2,3- or α2,6-linked SA (20), emphasizing the feasibility of a biologic that can bind to receptors in the upper and lower respiratory tract of humans. The high affinity of Sp2CBMTD, which was achieved through multivalency, allows it to mask SA receptors for an extended time period. Sp2CBMTD has been detected in mouse lungs up to 7 days after a single administration (21), which allows its administration in advance of a viral infection, thus making it a valuable component of preventive measures. Recent studies on the antiviral activity of DAS181 in H7N9-infected mice showed that a once-daily treatment for 6 days substantially reduced morbidity and conferred a high level of protection in mice, especially when given within 48 h of the lethal challenge (25). Despite the target (cell surface sialoglycoconjugates) being identical for the two drugs, the regimens are different in their duration of treatment and dose. In our study, Sp2CBMTD was therapeutically administered as a single dose at either 6 h or at 24 h p.i., while DAS181 was administered continuously for 6 days. However, note that the doses of DAS181 used were lower than those for Sp2CBMTD to examine protection against lethal challenge with H7N9 virus.
Our data suggest that the mechanism of antiviral action of Sp2CBMTD is complex and is driven by 2 major factors: (i) preventing virus binding to the SA-containing cellular receptors and (ii) modulating the host immune response. Although the masking of SA receptors was not addressed in detail in this study, our previous study demonstrated that Sp2CBMTD targeted cell surfaces along the respiratory tract and was present in mouse lungs, where it bound strongly to the alveolar epithelial cell surfaces of lung tissues (21). The multifunctional role of Sp2CBMTD in protecting against influenza virus infection is demonstrated by the stimulation of an innate immune response and the recruitment of immune cells to the site of influenza virus infection, thus reducing the severity of the immunopathology induced by the H7N9 virus. The difference in the infectious virus titers and the number of virus-positive cells in the lungs of the infected mice also points to differences between host tolerance in the biologic-treated and control groups, a concept that is well established in plant biology but has only been recently introduced to the field of animal infectious diseases (26). Thus, our findings provide further support for the key role of the stimulated immune response in protection in vivo and provide the first experimental proof of the stimulation of proinflammatory mediators in murine lungs by Sp2CBMTD before virus challenge. It is possible that Sp2CBMTD binds to a yet-unknown receptor(s) in addition to SA (possibly via sialylated receptors or other pattern recognition receptors involved in innate immunity) and thus acquired the ability to modulate the immune response. Importantly, either one or both mechanisms of action depend on the timing and amount of the biologic given.
Furthermore, sera collected from Sp2CBMTD-treated mice that survived H7N9 virus infection were positive for specific anti-HA antibodies, therefore, the development of an adaptive immune response had occurred. The level of immune response was sufficient to protect against H7N9 virus reinfection with a higher dose of the homologous virus.
A major concern about the long-term use of this novel therapeutic is the development of specific antibodies against it. Our results demonstrated that although serum levels of IgG, IgM, and IgA anti-SpCBM antibodies increased after a second administration of Sp2CBMTD 3 weeks after the first one, the protection was not affected, and all animals survived the heterologous challenge with the highly pathogenic H5N1 influenza virus. All the internal gene segments of newly emerging H7N9 influenza viruses and highly pathogenic H5N1 are similar and closely related to those from avian H9N2 viruses (16, 27). Therefore, the cross-reactive CD4+ T-cell and CD8+ cytotoxic T-lymphocyte immune responses established by the initial H7N9 virus infection may have contributed to protection against H5N1 reinfection. Further studies are required to determine the efficacy of Sp2CBMTD against different HA clades of highly pathogenic H5N1 influenza viruses. Our results confirm that repeated use of Sp2CBMTD is possible even within a short time period. If the time period between Sp2CBMTD administrations is longer, the anti-SpCBM antibodies might be eliminated, and their possible effect on the level of protection may decrease. To reduce the possible concern of immunogenicity, methods to deimmunize the biologic can be employed. As our biologic is based on a bacterial (nonhuman) protein sequence, methods to alter their sequence to reduce immunogenicity would include T-cell epitope screening to identify peptides that stimulate CD4+ T cells so that they can be modified to produce a less immunogenic molecule (28).
Sp2CBMTD represents a new class of host-directed therapeutics for influenza infections that shows promise for the prophylaxis of disease caused by potentially pandemic strains of influenza. Previous studies suggest that this biologic is effective against pandemic H1N1pdm09 viruses (21). Taken together, these data support the notion that Sp2CBMTD is a promising prophylactic option against emerging influenza viruses. Future studies can address the effectiveness of Sp2CBMTD against influenza viruses resistant to FDA-approved antivirals and analyze whether other routes or regimens for Sp2CBMTD administration are more effective than those used in our study. Moreover, the therapeutic potential of Sp2CBMTD against influenza virus infection warrants further investigation, as only a single-dose regimen was tested in this study, and repeated-dose regimens of the biologic may be more beneficial. Other respiratory pathogens, such as parainfluenza viruses, some coronaviruses, and S. pneumoniae also use SA receptors for pathogenesis, which may indicate that a broader application may be implemented for Sp2CBMTD in the future. Our findings in mice confirmed that a regimen of repeated low doses resulted in the highest survival rates and minimized tissue damage in mouse lungs. The immunomodulatory properties of Sp2CBMTD require further investigation, but the findings of this study advocate an even broader applicability of this approach for preventing respiratory disease caused by pathogens that do not use SA receptors.
ACKNOWLEDGMENTS
We thank Paul Thomas for great advice, Vani Shanker for editing the manuscript, and the Animal Resource Center and Pathology Veterinary Core, St. Jude Children's Research Hospital, for animal care and technical assistance. We also thank Yuelong Shu for providing the influenza A/Anhui/1/2013 (H7N9) virus and Neziha Yilmaz for providing the influenza A/Turkey/15/2006 (H5N1) virus.
This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN266200700005C and HHSN272201400006C, by the American Lebanese Syrian Associated Charities (ALSAC), and by the UK Medical Research Council Biomedical Catalyst grant MR/L012847/1.
H.C. and G.L.T. are coinventors on a patent related to the multivalent CBM technology (patent no. WO2010029312A1). E.A.G., T.B., B.M.M., L.Y., M.A.T., and R.G.W. do not have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). Although this study did not utilize corporate funding, E.A.G. and R.G.W. are currently performing a different research study funded by Roche TCRC, Inc., New York, NY, USA.
REFERENCES
- 1.World Health Organization. 2014. Number of confirmed human cases of avian influenza A(H7N9) reported to WHO. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/10u_ReportWebH7N9Number.pdf. [Google Scholar]
- 2.Qi X, Qian YH, Bao CJ, Guo XL, Cui LB, Tang FY, Ji H, Huang Y, Cai PQ, Lu B, Xu K, Shi C, Zhu FC, Zhou MH, Wang H. 2013. Probable person to person transmission of novel avian influenza A (H7N9) virus in eastern China, 2013: epidemiological investigation. BMJ 347:f4752. doi: 10.1136/bmj.f4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T, Bi Z, Wang X, Li Z, Ding S, Bi Z, Wang L, Pei Y, Song S, Zhang S, Wang J, Sun D, Pang B, Sun L, Jiang X, Lei J, Yuan Q, Kou Z, Yang B, Shu Y, Yang L, Li X, Lu K, Liu J, Zhang T, Xu A. 2014. One family cluster of avian influenza A(H7N9) virus infection in Shandong, China. BMC Infect Dis 14:98. doi: 10.1186/1471-2334-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao XC, Li KB, Chen ZQ, Di B, Yang ZC, Yuan J, Luo HB, Ye SL, Liu H, Lu JY, Nie Z, Tang XP, Wang M, Zheng BJ. 2014. Transmission of avian influenza A(H7N9) virus from father to child: a report of limited person-to-person transmission, Guangzhou, China, January 2014. Euro Surveill 19:pii=20837 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20837. [DOI] [PubMed] [Google Scholar]
- 5.Hu J, Zhu Y, Zhao B, Li J, Liu L, Gu K, Zhang W, Su H, Teng Z, Tang S, Yuan Z, Feng Z, Wu F. 2014. Limited human-to-human transmission of avian influenza A(H7N9) virus, Shanghai, China, March to April 2013. Euro Surveill 19:pii=20838 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20838. [DOI] [PubMed] [Google Scholar]
- 6.van Riel D, Leijten LM, de Graaf M, Siegers JY, Short KR, Spronken MI, Schrauwen EJ, Fouchier RA, Osterhaus AD, Osterhaus AD, Kuiken T. 2013. Novel avian-origin influenza A (H7N9) virus attaches to epithelium in both upper and lower respiratory tract of humans. Am J Pathol 183:1137–1143. doi: 10.1016/j.ajpath.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Wang D, Gao R, Zhao B, Song J, Qi X, Zhang Y, Shi Y, Yang L, Zhu W, Bai T, Qin K, Lan Y, Zou S, Guo J, Dong J, Dong L, Zhang Y, Wei H, Li X, Lu J, Liu L, Zhao X, Li X, Huang W, Wen L, Bo H, Xin L, Chen Y, Xu C, Pei Y, Yang Y, Zhang X, Wang S, Feng Z, Han J, Yang W, Gao GF, Wu G, Li D, Wang Y, Shu Y. 2013. Biological features of novel avian influenza A (H7N9) virus. Nature 499:500–503. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
- 8.Chan MC, Chan RW, Chan LL, Mok CK, Hui KP, Fong JH, Tao KP, Poon LL, Nicholls JM, Guan Y, Peiris JS. 2013. Tropism and innate host responses of a novel avian influenza A H7N9 virus: an analysis of ex-vivo and in-vitro cultures of the human respiratory tract. Lancet Respir Med 1:534–542. doi: 10.1016/S2213-2600(13)70138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J, Katz JM, Tumpey TM. 2013. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature 501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, Takashita E, McBride R, Noda T, Hatta M, Imai H, Zhao D, Kishida N, Shirakura M, de Vries RP, Shichinohe S, Okamatsu M, Tamura T, Tomita Y, Fujimoto N, Goto K, Katsura H, Kawakami E, Ishikawa I, Watanabe S, Ito M, Sakai-Tagawa Y, Sugita Y, Uraki R, Yamaji R, Eisfeld AJ, Zhong G, Fan S, Ping J, Maher EA, Hanson A, Uchida Y, Saito T, Ozawa M, Neumann G, Kida H, Odagiri T, Paulson JC, Hasegawa H, Tashiro M, Kawaoka Y. 2013. Characterization of H7N9 influenza A viruses isolated from humans. Nature 501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Bao L, Deng W, Dong L, Zhu H, Chen T, Lv Q, Li F, Yuan J, Xiang Z, Gao K, Xu Y, Huang L, Li Y, Liu J, Yao Y, Yu P, Li X, Huang W, Zhao X, Lan Y, Guo J, Yong W, Wei Q, Chen H, Zhang L, Qin C. 2014. Novel avian-origin human influenza A(H7N9) can be transmitted between ferrets via respiratory droplets. J Infect Dis 209:551–556. doi: 10.1093/infdis/jit474. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H. 2013. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341:410–414. doi: 10.1126/science.1240532. [DOI] [PubMed] [Google Scholar]
- 13.Osterholm MT, Ballering KS, Kelley NS. 2013. Major challenges in providing an effective and timely pandemic vaccine for influenza A(H7N9). JAMA 309:2557–2558. doi: 10.1001/jama.2013.6589. [DOI] [PubMed] [Google Scholar]
- 14.De Groot AS, Ardito M, Terry F, Levitz L, Ross T, Moise L, Martin W. 2013. Low immunogenicity predicted for emerging avian-origin H7N9: implication for influenza vaccine design. Hum Vaccin Immunother. 9:950–956. doi: 10.4161/hv.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, Xiang N, Chen E, Tang F, Wang D, Meng L, Hong Z, Tu W, Cao Y, Li L, Ding F, Liu B, Wang M, Xie R, Gao R, Li X, Bai T, Zou S, He J, Hu J, Xu Y, Chai C, Wang S, Gao Y, Jin L, Zhang Y, Luo H, Yu H, He J, Li Q, Wang X, Gao L, Pang X, Liu G, Yan Y, Yuan H, Shu Y, Yang W, Wang Y, Wu F, Uyeki TM, Feng Z. 2014. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med 370:520–532. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Lu S, Song Z, Wang W, Hao P, Li J, Zhang X, Yen HL, Shi B, Li T, Guan W, Xu L, Liu Y, Wang S, Zhang X, Tian D, Zhu Z, He J, Huang K, Chen H, Zheng L, Li X, Ping J, Kang B, Xi X, Zha L, Li Y, Zhang Z, Peiris M, Yuan Z. 2013. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet 381:2273–2279. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. 2014. Single dose escalating study of DAS181 in adults. http://www.clinicaltrials.gov/ct2/show/NCT00527865?term=Fludase&rank=2.
- 19.Malakhov MP, Aschenbrenner LM, Smee DF, Wandersee MK, Sidwell RW, Gubareva LV, Mishin VP, Hayden FG, Kim DH, Ing A, Campbell ER, Yu M, Fang F. 2006. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother 50:1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connaris H, Crocker PR, Taylor GL. 2009. Enhancing the receptor affinity of the sialic acid-binding domain of Vibrio cholerae sialidase through multivalency. J Biol Chem 284:7339–7351. doi: 10.1074/jbc.M807398200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connaris H, Govorkova EA, Ligertwood Y, Dutia BM, Yang L, Tauber S, Taylor MA, Alias N, Hagan R, Nash AA, Webster RG, Taylor GL. 2014. Prevention of influenza by targeting host receptors using engineered proteins. Proc Natl Acad Sci U S A 111:6401–6406. doi: 10.1073/pnas.1404205111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilyushina NA, Bovin NV, Webster RG, Govorkova EA. 2006. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res 70:121–131. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Baranovich T, Burnham AJ, Marathe BM, Armstrong J, Guan Y, Shu Y, Peiris JM, Webby RJ, Webster RG, Govorkova EA. 2014. The neuraminidase inhibitor oseltamivir is effective against A/Anhui/1/2013 (H7N9) influenza virus in a mouse model of acute respiratory distress syndrome. J Infect Dis 209:1343–1353. doi: 10.1093/infdis/jit554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster RG, Govorkova EA. 2014. Continuing challenges in influenza. Ann N Y Acad Sci 1323:115–139. doi: 10.1111/nyas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marjuki H, Mishin VP, Chesnokov AP, De La Cruz JA, Fry AM, Villanueva J, Gubareva LV. 2014. An investigational antiviral drug, DAS181, effectively inhibits replication of zoonotic influenza A virus subtype H7N9 and protects mice from lethality. J Infect Dis 210:435–440. doi: 10.1093/infdis/jiu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan Y, Shortridge KF, Krauss S, Webster RG. 1999. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci U S A 96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Groot AS, Terry F, Cousens L, Martin W. 2013. Beyond humanization and-de-immunization: tolerization as a method for reducing the immunogenicity of biologics. Expert Rev Clin Pharmacol 6:651–662. doi: 10.1586/17512433.2013.835698. [DOI] [PMC free article] [PubMed] [Google Scholar]