Abstract
New therapeutic strategies are needed to combat the emergence of infections due to multidrug-resistant Neisseria gonorrhoeae. In this study, fosfomycin (FOS) was tested against 89 N. gonorrhoeae isolates using the Etest method, showing MIC50/MIC90s of only 8/16 μg/ml (range, ≤1 to 32 μg/ml). FOS in combination with ceftriaxone (CRO) or azithromycin (AZT) was then evaluated using the checkerboard method for eight strains, including N. gonorrhoeae F89 (CRO-resistant) and AZT-HLR (high-level AZT-resistant). All combinations that included FOS gave indifferent effects (fractional inhibitory concentration [FIC] index values, 1.2 to 2.3 for FOS plus CRO, 1.8 to 3.2 for FOS plus AZT). Time-kill experiments for FOS, CRO, AZT, and their combinations (at 0.5×, 1×, 2×, and 4× the MIC) were performed against N. gonorrhoeae strain ATCC 49226, one N. gonorrhoeae multiantigen sequence typing (NG-MAST) sequence type 1407 (ST1407) strain, F89, and AZT-HLR. For all strains, at 24 h, the results indicated that (i) FOS was bactericidal at 2× the MIC, but after >24 h, there was regrowth of bacteria; (ii) CRO was bactericidal at 0.5× the MIC; (iii) AZT was bactericidal at 4× the MIC; (iv) CRO plus AZT was less bactericidal than was CRO alone; (v) FOS plus AZT was bactericidal at 2× the MIC; and (vi) CRO plus AZT and FOS plus CRO were both bactericidal at 0.5× the MIC, but FOS plus CRO had more rapid effects. FOS is appealing for use in the management of N. gonorrhoeae infections because of its single and oral formulation. However, our results suggest it be used in combination with CRO. After the appropriate clinical trials are conducted, this strategy could be implemented for the treatment of infections due to isolates possessing resistance to CRO and/or AZT.
INTRODUCTION
Infections due to Neisseria gonorrhoeae are estimated to be the most common of the curable bacterial sexually transmitted infections (STIs), the number of which is identical to the number of Chlamydia trachomatis infections (106 million cases in 2008), and their incidence and prevalence have continued to increase worldwide. This fact represents a serious global health problem because gonococcal infections are frequently asymptomatic and can silently progress and result in severe complications and sequelae, such as pelvic inflammatory disease, ectopic pregnancy, and infertility (1, 2).
Due to the development of resistance to all previously introduced antimicrobials (e.g., penicillin, tetracyclines, quinolones, and cefixime) (3, 4), a combination of ceftriaxone (CRO) plus azithromycin (AZT) is now recommended in the United States and Europe to treat gonococcal infections (5, 6). However, multidrug-resistant (MDR) and extensively drug-resistant (XDR) N. gonorrhoeae isolates that are also highly resistant to CRO or AZT are emerging (7–11), and clinical cases of treatment failure are reported when CRO and AZT are used separately (10, 12–17). Taking into account this overall threatening situation, new antimicrobials are under development, but these drugs will require time to become available for treatment (4).
Fosfomycin (FOS) is an old antibiotic that inhibits the first step of peptidoglycan synthesis and shows potent bactericidal activities against common Gram-negative and Gram-positive organisms. This drug is an appealing choice for the management of gonococcal infections because of its single and oral formulation (3 g of FOS trometamol), low toxicity, and very high peak concentration levels in different body sites. For instance, the maximum concentrations of drug in serum (Cmaxs) of 22 to 32 μg/ml in serum and 700 μg/ml in urine are reached in ∼2 h (18–20); also, intraprostatic and bladder mucosal concentrations of 5 μg/g and 17 μg/g have been recorded after ∼10 and ∼6 h, respectively (21, 22). However, data regarding the in vitro activity of FOS against N. gonorrhoeae isolates are very scarce (23, 24).
In this study, we evaluated the in vitro activity of FOS alone and in combination with CRO or AZT against a collection of both susceptible and highly resistant MDR strains of N. gonorrhoeae, and we compared the efficacies of these treatments to those of the current standard therapeutic options.
MATERIALS AND METHODS
Clinical isolates and reference strains.
We analyzed 89 N. gonorrhoeae isolates collected from January 1998 to June 2014 at the Laboratory of Clinical Microbiology of the Institute for Infectious Diseases, University of Bern (Switzerland). Species confirmation was performed using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics). The isolates found during 1998 to 2001 (n = 26) and 2009 to 2012 (n = 34) have been described with regard to (i) their MICs for eight antimicrobials (penicillin, cefixime, CRO, AZT, ciprofloxacin, tetracycline, gentamicin, and spectinomycin) using the Etest method (bioMérieux), (ii) their sequence types (STs) by N. gonorrhoeae multiantigen sequence typing (NG-MAST), and (iii) a molecular analysis of their antimicrobial resistance genes (25). In the present work, the remaining 29 N. gonorrhoeae isolates underwent the same characterization. The isolates were defined as MDR as previously described (9), and susceptibility and resistance to antimicrobials were categorized using the 2014 European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria (26). For all in vitro tests (see below), N. gonorrhoeae ATCC 49226 was used as a control; the MDR AZT-HLR and XDR F89 N. gonorrhoeae strains were also included because of their high-level resistances to AZT and CRO, respectively (Table 1) (7, 8).
TABLE 1.
Susceptibility test results for five N. gonorrhoeae isolates, the ATCC 49226 control, and two strains (AZT-HLR and F89) highly resistant to azithromycin and ceftriaxone, respectively
| Isolate (yr of detection): ST by NG-MAST, main molecular characteristics (7, 8, 25)a | MIC (μg/ml) by Etest/susceptibilityb |
MIC (μg/ml) by microdilutionb,c |
MBC (μg/ml)c,d |
Mean FICIe,f |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | GEN | SPE | TET | PEN | CFX | CRO | AZT | FOS | CRO | AZT | FOS | CRO | AZT | FOS | AZT + CRO | FOS + AZT | FOS + CRO | |
| AE-7570 (2011): ST1407, PBP1 (L421P), PBP2 (XXXIV), PorB (G120K, A121N), mtrR promoter (deletion A) | 12/R | 6/NA | 8/S | 1/I | 0.38/I | 0.125/S | 0.023/S | 0.38/I | 6/NA | 0.008/S | 1/R | 8/NA | 0.008 | 2 | 16 | 1.6 | 1.9 | 1.9 |
| AE-4615 (2011): ST8707, TEM-1, PBP1 (L421P), PBP2 (XIX), PorB (A121S), MtrR (A39T), Tet(M) | 1.5/R | 4/NA | 2/S | 24/R | 12/R | ≤0.016/S | 0.004/S | 0.064/S | 3/NA | 0.004/S | 0.25/S | 4/NA | 0.004 | 0.5 | 4 | 1.7 | 3.2 | 1.7 |
| AE-6772 (2012): ST8827, TEM-1, PBP1 (L421P), PBP2 (XIX), PorB (A120K, A121D), MtrR (A39T), Tet(M) | 2/R | 6/NA | 6/S | 16/R | 3/R | ≤0.016/S | ≤0.002/S | 0.023/S | 4/NA | 0.002/S | 0.064/S | 8/NA | 0.002 | 0.064 | 8 | 1.3 | 1.8 | 2.3 |
| AE-2655 (2012): ST8826, PBP1 (L421P), PBP2 (XXXIV), PorB (G120K, A121N), mtrR promoter (deletion A) | 24/R | 6/NA | 3/S | 0.25/S | 0.38/I | 0.094/S | 0.023/S | 0.125/S | 8/NA | 0.008/S | 0.5/I | 16/NA | 0.008 | 1 | 16 | 2.2 | 2.3 | 1.5 |
| AE-9562 (2012): ST437, PBP1 (L421P), PBP2 (V), PorB (G120K, A121D), mtrR promoter (deletion A) | 24/R | 8/NA | 3/S | 0.5/I | 0.25/I | 0.023/S | 0.023/S | 0.19/S | 4/NA | 0.008/S | 0.5/I | 8/NA | 0.008 | 1 | 8 | 2.4 | 2.3 | 1.4 |
| ATCC 49226 | 0.006/S | 12/NA | 12/S | 1.5/I | 0.75/I | 0.023/S | 0.016/S | 0.25/S | 32/NA | 0.008/S | 1/R | 64/NA | 0.008 | 1 | 64 | 1.6 | 2.0 | 1.2 |
| AZT-HRL (2011): ST285, 23S rRNA (3 alleles with mutation A2059G) | ≥32/R | 12/NA | 12/S | 4/R | 2/R | 0.047/S | 0.064/S | ≥256/Rf | 12/NA | 0.016/S | 512/R | 8/NA | 0.008 | 1,024 | 8 | 1.5 | 2.0 | 1.5 |
| F89 (2010): ST1407, PBP2 (XXXIV and A501P) | ≥32/R | 8/NA | 12/S | 3/R | 0.38/I | 2/R | 1.5/R | 0.25/S | 12/NA | 0.5/R | 1/R | 32/NA | 1 | 2 | 32 | 2.4 | 2.6 | 1.3 |
PBP1, penicillin-binding protein 1.
CIP, ciprofloxacin; GEN, gentamicin; SPE, spectinomycin; TET, tetracycline; PEN, penicillin; CFX, cefixime; CRO, ceftriaxone; AZT, azithromycin; FOS, fosfomycin; R, resistant; NA, not available; S, susceptible; I, intermediate. The MIC interpretation according to the 2014 EUCAST criteria (26) was determined as follows: for PEN, S at ≤0.06 μg/ml and R at ≥2 μg/ml; for CFX and CRO, S at ≤0.125 μg/ml and R at ≥0.25 μg/ml; for AZT, S at ≤0.25 μg/ml and R at ≥1 μg/ml; for CIP, S at ≤0.025 μg/ml and R at ≥0.125 μg/ml; for GEN, not available; for SPE, S at ≤64 μg/ml and R at ≥128 μg/ml; and for TET, S at ≤0.25 μg/ml and R at ≥2 μg/ml.
All tests in broth microdilution were performed with FB broth supplemented with 1% IsoVitaleX and G6P (25 μg/ml).
MBC, minimum bactericidal concentration.
FICI, fractional inhibitory concentration index. The mean FICI was obtained by calculating the average from at least three independent checkerboard tests. It was interpreted as synergistic, additive, indifferent, or antagonist effect (<0.5, 0.5 to 1, >1 to 4, and >4, respectively) (7).
For the time-kill experiments, the MIC was 256 μg/ml.
Susceptibility to fosfomycin.
The MICs of FOS were obtained using the Etest method on GC agar plates (bioMérieux), according to the manufacturer's instructions. The MICs were adjusted to whole MIC doubling concentrations (e.g., from 12 to 16 μg/ml). Since no susceptibility criteria for N. gonorrhoeae have been established, the FOS MICs were interpreted according to both the EUCAST and Clinical and Laboratory Standards Institute (CLSI) cutoffs (i.e., susceptible at MICs of ≤32 μg/ml and ≤64 μg/ml, respectively) set for Enterobacteriaceae (26, 27).
Broth microdilution tests.
For eight representative N. gonorrhoeae strains (including the three control strains; see Table 1), the MICs for CRO, AZT, and FOS were also determined by a broth microdilution (BMD) method (96-well plates; Sarstedt) using fastidious broth (FB) (Remel) supplemented with 1% IsoVitaleX (Oxoid) and glucose-6-phosphate (G6P) (25 μg/ml) (27, 28). The powder for each antibiotic and G6P were purchased from Sigma. The panels were incubated with shaking for 24 h at 35°C in a humid atmosphere containing 5% CO2.
After MIC determination, the minimum bactericidal concentration (MBC) for each antibiotic was obtained by culturing of 30 μl of the broth from the endpoint well and from the log2 dilution above the MIC onto GC agar plates. The MBC was defined as the lowest concentration of antibiotic able to kill ≥99.9% of the initial bacterial inoculum (29).
Checkerboard assay.
The activity of FOS in combination with CRO or AZT (drug B in the formula below) was analyzed using the checkerboard broth dilution method (96-well plates) to determine the fractional inhibitory concentration index (FICI). The strains, media, and conditions were the same as those used in the BMD tests (see above). The FICI was determined by the following formula: FICI = (MICFOScomb/MICFOSalone) + (MICdrug Bcomb/MICdrug Balone) (comb, combination) (30). The following combinations were tested: AZT plus CRO, FOS plus AZT, and FOS plus CRO. At least three independent experiments were performed for each strain and each antibiotic combination. The calculated FICIs were interpreted as representing a synergistic, additive, indifferent, or antagonistic effect (FICIs, <0.5, 0.5 to 1, >1 to 4, and >4, respectively) (7).
Time-kill curve analyses.
Time-kill curve analyses were performed for four representative N. gonorrhoeae strains (ATCC 49226, clinical isolate AE-7570, AZT-HLR, and F89). Briefly, fresh overnight N. gonorrhoeae colonies from GC agar plates were used to prepare a 0.5 McFarland inoculum in NaCl (0.85%). Next, 5 μl was transferred into 5 ml of FB supplemented with 1% IsoVitaleX and G6P (25 μg/ml) in 50-ml Falcon tubes (TPP) that were incubated with shaking at 35°C in a humid atmosphere containing 5% CO2.
The strains were tested against the following antibiotic(s): AZT, FOS, CRO, AZT plus CRO, FOS plus AZT, and FOS plus CRO. All experiments were performed at least three times at 0.5×, 1×, 2×, and 4× the MIC obtained with the Etest method (Table 1). Experiments without any antibiotic were also done (growth control curves). The CFU count was determined at specific intervals (0, 2, 4, 6, 8, 24, 30, and 48 h) by plating 100-μl aliquots from serial 10-fold dilutions; the limit of detection was set at ≤10 CFU/ml (31). The drug carryover effect on the viability count was evaluated as previously described (32). Bactericidal activity was defined as a ≥3-log10 decrease in CFU/ml compared to the growth control curve (29).
RESULTS AND DISCUSSION
In this study, we assessed the in vitro activity of fosfomycin (FOS) to elucidate whether FOS can be implemented for the treatment of gonococcal infections, especially those due to the MDR and XDR strains with resistance to azithromycin (AZT) and/or ceftriaxone (CRO) (7, 8, 26, 29, 30). For this purpose, the MIC distribution of FOS was studied testing a collection of well-characterized and contemporary N. gonorrhoeae isolates (25), and the activity of FOS alone and in combination with AZT or CRO was evaluated for several representative strains using both checkerboard and time-kill methodologies.
Susceptibility to FOS.
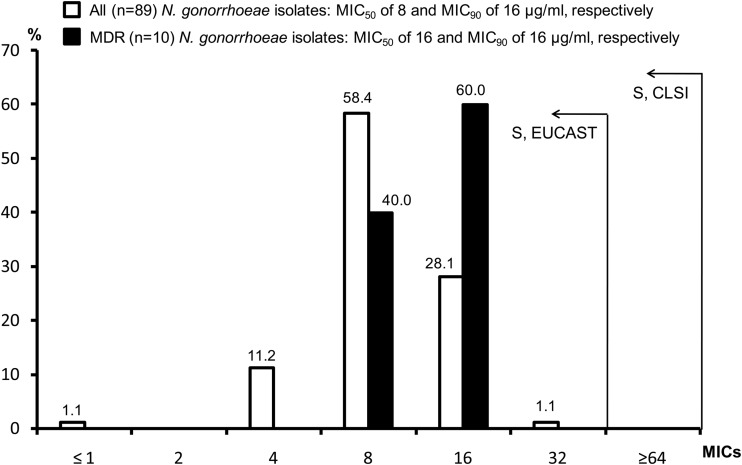
As shown in Fig. 1, FOS had an overall MIC90 of 16 μg/ml (range, ≤1 to 32 μg/ml). All 89 clinical isolates (of which 10 were MDR) had MICs of ≤32 μg/ml, a value that is lower than the susceptibility cutoff set by EUCAST for the oral treatment of infections due to Enterobacteriaceae and by CLSI for the management of urinary tract infections (UTIs) due to Escherichia coli or Enterococcus faecalis (26, 27). Our MIC results were also consistent with those recently obtained by Barbee et al. (23), who analyzed a smaller collection of 28 N. gonorrhoeae isolates and four control strains. Accordingly, FOS appears to be active against N. gonorrhoeae in vitro and might also be effective in vivo for the treatment of STIs due to N. gonorrhoeae. However, for other Gram-negative pathogens (e.g., E. coli), FOS can rapidly lead to resistance when used as monotherapy (19, 33). To overcome this problem, the drug has been used in combination, showing good clinical outcomes even for non-UTIs (18, 34). Consequently, in the present study, we performed further in vitro experiments (see below) to explore the activity of FOS in combination with the standard antigonococcal antibiotics CRO and AZT.
FIG 1.
MIC distributions of fosfomycin (FOS) obtained using the Etest on GC agar (MICs were adjusted to the whole MIC doubling concentrations, e.g., from 12 to 16 μg/ml). Eighty-nine clinical isolates (of which 10 had an MDR phenotype) were evaluated. Notably, the MICs of the three control strains (F89, AZT-HLR, and ATCC 49226) were not included in the distribution. According to the CLSI or the EUCAST criteria set for Enterobacteriaceae (26, 27), all N. gonorrhoeae isolates were susceptible (S) to FOS in vitro.
MIC and MBC values in broth microdilution.
As shown in Table 1, the MICs obtained with Etest and BMD showed mainly minor differences. These were a maximum of one to two 2-fold MIC dilutions for some isolates and were not considered to significantly affect any results. Further studies also using the standard agar dilution method might address these discrepancies in MICs.
For the majority of isolates, the MBCs for AZT were one 2-fold dilution higher than the MICs obtained in BMD, whereas those for CRO and FOS were mainly consistent with the MICs (Table 1). These results for FOS were quite surprising, because we were expecting the MBCs to be higher than the MICs. This is based on the fact that the drug can rapidly select for spontaneous resistant mutants (19), which our current time-kill experiments also indicated by the regrowth of FOS-resistant N. gonorrhoeae strains after the initial killing activity (regrowth at up to 2× the MIC but not at 4× the MIC; see below). The frequency of spontaneous FOS-resistant mutants might have been too low to be detected when plating only 30 μl of broth. Moreover, the regrowth of resistant N. gonorrhoeae observed during the time-kill experiments usually appeared after 30 h, whereas the microdilution MIC/MBC test results were obtained in ≤24 h. Subsequent studies addressing the specific mechanism conferring resistance to FOS and its frequency of occurrence in N. gonorrhoeae are needed.
Assessing the FIC index using the checkerboard.
The mean FICIs were obtained with the microdilution checkerboard method to evaluate whether the combination of FOS and AZT or FOS and CRO had synergistic effects against N. gonorrhoeae isolates, as has been frequently observed for other combinations of FOS against classic MDR Gram-negative pathogens (34). We implemented this methodology because (i) it is more standardized than that using two Etest strips, (ii) it is significantly more rapid and less labor-consuming than the agar dilution method (23, 29), and (iii) the results can be more adequately linked with those of time-kill experiments due to the same growth conditions.
Overall, our analysis showed that none of the eight strains tested with the AZT plus CRO, FOS plus CRO, or FOS plus AZT combinations demonstrated synergistic, additive, or antagonistic properties but only indifferent effects (FICI ranges: AZT plus CRO, 1.3 to 2.4; FOS plus CRO, 1.2 to 2.3; and FOS plus AZT, 1.8 to 3.2) (Table 1). Furuya et al. (35) obtained analogous results for AZT plus CRO using the same methodology. Other authors have also obtained consistent results using two Etest strips or agar dilution (FICI ranges: AZT plus CRO, 1.5 to 2; FOS plus CRO, 1 to 1.5) (23, 30). These overall figures indicate that synergy testing by Etest strips might be used to rapidly and easily obtain the FIC indexes for antibiotic combinations against MDR N. gonorrhoeae. However, larger studies comparing the three methodologies are required.
Time-kill results for AZT, CRO, and AZT plus CRO.
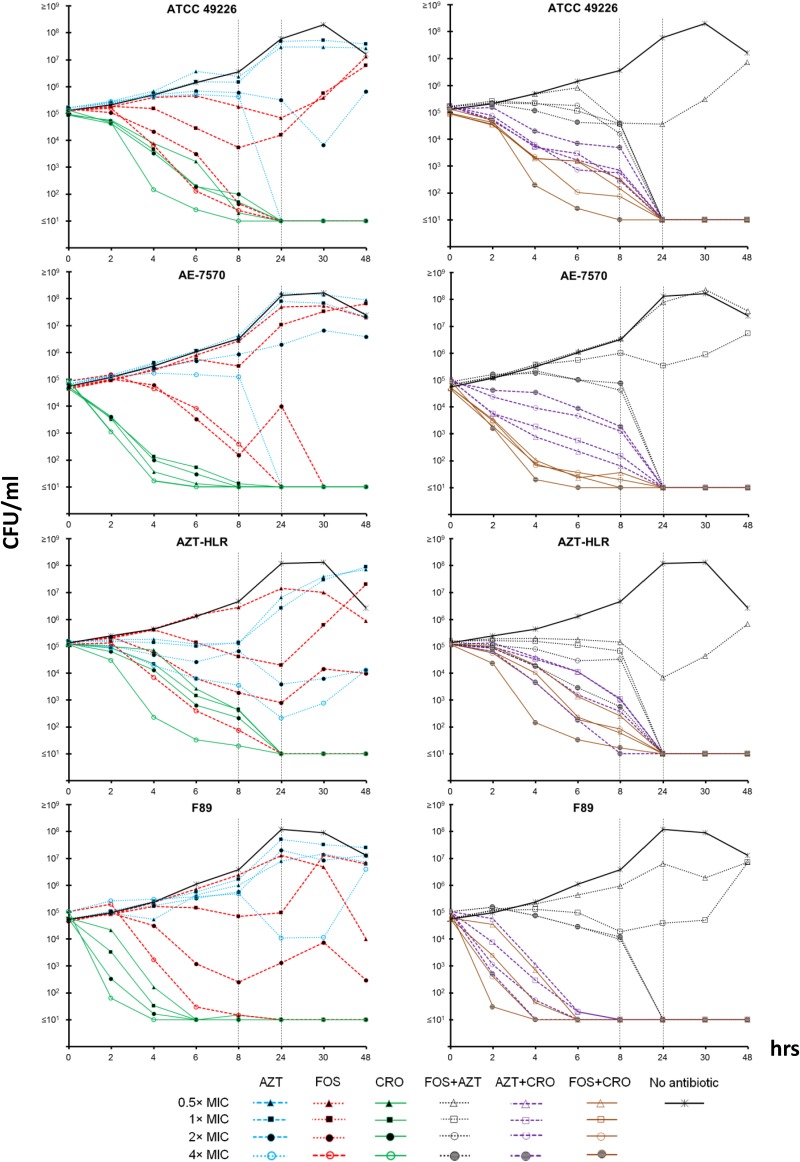
As shown in Fig. 2, at 0.5×, 1×, and 2× the MIC, AZT alone was unable to inhibit bacterial growth of the four tested strains. Only at 4× the MIC were bactericidal effects observed, but for the AZT-HLR and F89 strains, regrowth was recorded after 1 day of incubation. The initial bactericidal effects observed within the first 24 h for AZT-HLR at 2× and 4× the MIC (i.e., 512 and 1,024 μg/ml, respectively) were surprising, because this strain was the only one highly resistant to the antibiotic. These data are difficult to explain, but one could hypothesize they are due to nonspecific physical and/or chemical antibacterial effects of the very high AZT concentrations used.
FIG 2.
Time-kill curve analyses for azithromycin (AZT), fosfomycin (FOS), and ceftriaxone (CRO) and their combinations. For all strains, at 24 h, the results indicated that (i) FOS was bactericidal at 2× the MIC, but at >24 h, there was regrowth of bacteria; (ii) CRO was bactericidal at 0.5× the MIC (including for strain F89); (iii) AZT was bactericidal only at 4× the MIC; (iv) CRO plus AZT was less bactericidal than CRO alone; (v) FOS plus AZT was bactericidal at 2× the MIC; (vi) CRO plus AZT and FOS plus CRO were both bactericidal at 0.5× the MIC, but FOS plus CRO had more rapid effects.
CRO was rapidly (within 4 to 6 h) bactericidal already at 0.5× the MIC (including for the resistant strain F89 of ST1407). However, for isolates with high MICs for CRO and that infect/colonize anatomical sites where N. gonorrhoeae is difficult to eradicate (e.g., the tonsillopharyngeal tissue), CRO may still fail clinically, as has rarely been reported (e.g., for isolates of genogroup 1407) (17). As previously observed (36), the MICs for F89 obtained by Etest (1.5 μg/ml) and by BMD (0.5 μg/ml) can differ notably, emphasizing that the strain might be particularly susceptible to different growth conditions and/or have suboptimal biological fitness. This might explain our results of CRO being bactericidal at only 0.5× the MIC for F89.
The standard combination of AZT plus CRO was bactericidal at all concentrations and for all tested strains. However, its overall killing effects were slower than those of CRO alone when tested at the same concentrations. This paradoxical phenomenon might be due to the bacteriostatic effect of AZT that inhibits the required exponential growth of gonococcus for the adequate mechanism of action of CRO (37, 38). This type of antagonistic effect was not recorded by calculating the FICIs, but it was instead clearly indicated from the killing curves obtained for ATCC 49226 and the clinical N. gonorrhoeae strain AE-7570 of ST1407: the bactericidal action was more rapid for AZT plus CRO at 0.5× the MICs than at 4× the MIC (Fig. 2).
Time-kill results for FOS, FOS plus AZT, and FOS plus CRO.
For all tested strains, at 24 h, FOS was bactericidal at 2× the MIC (i.e., between 8 and 64 μg/ml for the four tested strains), but regrowth was constantly observed during the second day of incubation (Fig. 2). This effect was due to the spontaneous emergence of resistant strains, as verified with the Etest for strains regrowing between 24 and 48 h (i.e., all with MICs of ≥64 μg/ml; data not shown) (26, 27). In contrast, at 4× the MIC, no regrowth of N. gonorrhoeae strains was recorded at the end of the 48 h of incubation; moreover, in the first 6 to 8 h, FOS showed potent bactericidal effects. Such antibiotic concentrations (i.e., between 24 and 128 μg/ml for the four tested strains) are significantly lower than the peak concentration of FOS in urine and in the same range of that in serum (18–20, 27); it should also be noted that the MIC90 for N. gonorrhoeae isolates was only 16 μg/ml. Therefore, the present time-kill data for FOS at 4× the MIC might be representative of what can occur in vivo, especially for urethral infections. Even so, the emergence of resistance during therapy argues against FOS as a monotherapy, despite the fact that in a small early study, an intramuscular dose of 2 g in each buttock (4 g in total) was shown to be relatively successful (86% cure rate in 43 patients) for gonococcal urethritis (39). In this context, we also note that after a single intramuscular dose of 2 g, the serum Cmax after ∼2 h is 37.6 ± 4.5 μg/ml (40), a value significantly higher than the MIC90 of the tested N. gonorrhoeae isolates. Unfortunately, further conclusions about the hypothetical use of intramuscular FOS cannot be made due to the lack of tissue pharmacokinetic data (e.g., concentration in urethra, tonsillopharynx, rectum, or even synovial fluid) after intramuscular administration. This lack of data about the tissue distribution of FOS for the key tissues involved in gonococcal infections is also true for the oral formulation of the drug.
FOS plus AZT was evaluated, because in a real clinical scenario, coinfections of N. gonorrhoeae and C. trachomatis can be common, and AZT is one of the antibiotics in the currently recommended dual-antibiotic-therapy regimens (1, 41). At 24 h, the combination of FOS plus AZT was bactericidal at 2× the MIC, and no regrowth of bacteria was recorded (Fig. 2). As for AZT alone, a very good bactericidal effect of FOS plus AZT (even at 1× the MIC) was observed for isolate AZT-HLR. The addition of AZT did not antagonize the effect of FOS, as was indicated for CRO. Although both FOS and CRO inhibit cell wall synthesis, FOS is a concentration-dependent antibiotic (18–20, 27), whereas CRO is a time-dependent drug that is probably more affected by the decline in exponential growth determined by AZT and other bacteriostatic antibiotics (37, 38, 42).
As shown in Fig. 2, FOS in combination with CRO demonstrated strong and rapid bactericidal action in the first 4 to 6 h of incubation for all tested strains. This effect was also substantially more rapid than that with the standard combination of AZT plus CRO, even when the concentration of FOS plus CRO was lower than that of AZT plus CRO. For instance, for the clinical isolate AE-7570, the combination of FOS plus CRO at 0.5× the MIC was significantly more bactericidal than AZT plus CRO at 4× the MIC. These data indicate that CRO can reach the same bactericidal performance at lower concentrations if combined with FOS rather than AZT. However, this aspect needs to be evaluated further (e.g., in an animal model) because it might have important clinical implications, especially regarding the dose and route of administration of CRO.
Conclusions.
This is the first study that extensively analyzed the in vitro activity of FOS alone and in combination with AZT and CRO against clinical N. gonorrhoeae isolates and MDR/XDR strains (e.g., F89 and AZT-HLR) that may represent a future challenge in the treatment of gonococcal infections (7, 9, 43). Our results confirm that CRO is the current mainstay of gonorrhea treatment. However, FOS may be a potential substitute of AZT to be given with CRO. The high oral bioavailability, excellent safety, and good track record of FOS in the treatment of UTIs further support this. In particular, oral FOS plus CRO could be implemented for the treatment of STIs due to the emerging MDR/XDR N. gonorrhoeae isolates possessing resistance to CRO (e.g., strain F89) and AZT (e.g., strain AZT-HLR) (7, 8). However, the following additional studies are crucial in the preparation for the potential future implementation of FOS in the treatment of gonorrhea: (i) testing of activity in vitro against a wider variety of N. gonorrhoeae strains representing the current clones in circulation worldwide, (ii) the characterization of mechanisms of FOS resistance in N. gonorrhoeae, (iii) the provision of pharmacokinetic and pharmacodynamic data for the tissues involved in gonococcal infections, and finally, (iv) randomized controlled clinical trials in patients with genital and extragenital (especially pharyngeal) gonorrhea, while evaluating optimal dosing and efficacy.
ACKNOWLEDGMENTS
This work was supported by grant 84800363 2013-05 from the Bern University Hospital and University of Bern (to C.H.), the Institute of Infectious Diseases (IFIK) of the University of Bern (to A.E.), and the Department of Infectious Diseases, Bern University Hospital and University of Bern (to H.F.).
We thank Yuvia N. Guilarte, João Pires, Agnese Lupo, Parham Sendi, and Valentina Donà for their technical advice.
REFERENCES
- 1.World Health Organization (WHO). 2012. Global incidence and prevalence of selected curable sexually transmitted infections–2008. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf?ua=1. [Google Scholar]
- 2.World Health Organization (WHO). 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2012/9789241503501_eng.pdf?ua=1. [Google Scholar]
- 3.Whiley DM, Goire N, Lahra MM, Donovan B, Limnios AE, Nissen MD, Sloots TP. 2012. The ticking time bomb: escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. J Antimicrob Chemother 67:2059–2061. doi: 10.1093/jac/dks188. [DOI] [PubMed] [Google Scholar]
- 4.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Diseases Control and Prevention (CDC). 2012. Update to CDC's Sexually Transmitted Diseases Treatment Guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61:590–594. [PubMed] [Google Scholar]
- 6.Bignell C, Unemo M, European STI Guidelines Editorial Board. 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 7.Unemo M, Golparian D, Hellmark B. 2014. First three Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Sweden: a threat to currently available dual-antimicrobial regimens for treatment of gonorrhea? Antimicrob Agents Chemother 58:624–625. doi: 10.1128/AAC.02093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapsall JW, Ndowa F, Lewis DA, Unemo M. 2009. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther 7:821–834. doi: 10.1586/eri.09.63. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolan GA, Sparling PF, Wasserheit JN. 2012. The emerging threat of untreatable gonococcal infection. N Engl J Med 366:485–487. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 12.Morita-Ishihara T, Unemo M, Furubayashi K, Kawahata T, Shimuta K, Nakayama S, Ohnishi M. 2014. Treatment failure with 2 g of azithromycin (extended-release formulation) in gonorrhoea in Japan caused by the international multidrug-resistant ST1407 strain of Neisseria gonorrhoeae. J Antimicrob Chemother 69:2086–2090. doi: 10.1093/jac/dku118. [DOI] [PubMed] [Google Scholar]
- 13.Unemo M, Golparian D, Hestner A. 2011. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010 Euro Surveill 16:pii=19792 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19792. [PubMed] [Google Scholar]
- 14.Read PJ, Limnios EA, McNulty A, Whiley D, Lahra MM. 2013. One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500mg ceftriaxone in Sydney, Australia. Sex Health 10:460–462. doi: 10.1071/SH13077. [DOI] [PubMed] [Google Scholar]
- 15.Chen YM, Stevens K, Tideman R, Zaia A, Tomita T, Fairley CK, Lahra M, Whiley D, Hogg G. 2013. Failure of 500 mg of ceftriaxone to eradicate pharyngeal gonorrhoea, Australia. J Antimicrob Chemother 68:1445–1447. doi: 10.1093/jac/dkt017. [DOI] [PubMed] [Google Scholar]
- 16.Unemo M, Golparian D, Potocnik M, Jeverica S. 2012. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011 Euro Surveill 17:pii=20200 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20200. [PubMed] [Google Scholar]
- 17.Golparian D, Ohlsson A, Janson H, Lidbrink P, Richtner T, Ekelund O, Fredlund H, Unemo M. 2014. Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014 Euro Surveill 19:pii=20862 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20862. [DOI] [PubMed] [Google Scholar]
- 18.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis 46:1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 19.Keating GM. 2013. Fosfomycin trometamol: a review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs 73:1951–1966. doi: 10.1007/s40265-013-0143-y. [DOI] [PubMed] [Google Scholar]
- 20.Mazzei T, Cassetta MI, Fallani S, Arrigucci S, Novelli A. 2006. Pharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infections. Int J Antimicrob Agents 28(Suppl 1):S35–S41. doi: 10.1016/j.ijantimicag.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Gardiner BJ, Mahony AA, Ellis AG, Lawrentschuk N, Bolton DM, Zeglinski PT, Frauman AG, Grayson ML. 2014. Is fosfomycin a potential treatment alternative for multidrug-resistant Gram-negative prostatitis? Clin Infect Dis 58:e101–e105. doi: 10.1093/cid/cit704. [DOI] [PubMed] [Google Scholar]
- 22.Scaglione F, Cicchetti F, Demartini G, Arcidiacono M. 1994. Fosfomycin distribution in the lower urinary tract after administration of fosfomycin trometamol salt. Int J Clin Pharmacol Res 14:107–109. [PubMed] [Google Scholar]
- 23.Barbee LA, Soge OO, Holmes KK, Golden MR. 2014. In vitro synergy testing of novel antimicrobial combination therapies against Neisseria gonorrhoeae. J Antimicrob Chemother 69:1572–1578. doi: 10.1093/jac/dkt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickgiesser N, Kuntz P. 1984. The activity of rosoxacin, fosfomycin, cefotiam, and spectinomycin on β-lactamase producing Neisseria gonorrhoeae. Br J Vener Dis 60:154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endimiani A, Guilarte YN, Tinguely R, Hirzberger L, Selvini S, Lupo A, Hauser C, Furrer H. 2014. Characterization of Neisseria gonorrhoeae isolates detected in Switzerland (1998–2012): emergence of multidrug-resistant clones less susceptible to cephalosporins. BMC Infect Dis 14:106. doi: 10.1186/1471-2334-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.EUCAST. 2014. Clinical breakpoints, version 4.0, 2014. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden. [Google Scholar]
- 27.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100–S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Takei M, Yamaguchi Y, Fukuda H, Yasuda M, Deguchi T. 2005. Cultivation of Neisseria gonorrhoeae in liquid media and determination of its in vitro susceptibilities to quinolones. J Clin Microbiol 43:4321–4327. doi: 10.1128/JCM.43.9.4321-4327.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahon CR, Manuselis G. 2000. Textbook of diagnostic microbiology, 2nd ed W. B. Saunders Company, Philadelphia, PA. [Google Scholar]
- 30.Pereira R, Cole MJ, Ison CA. 2013. Combination therapy for gonorrhoea: in vitro synergy testing. J Antimicrob Chemother 68:640–643. doi: 10.1093/jac/dks449. [DOI] [PubMed] [Google Scholar]
- 31.Scott S. 2011. Accuracy of plate counts. J Validation Technol 17:42–46. [Google Scholar]
- 32.Bajaksouzian S, Visalli MA, Jacobs MR, Appelbaum PC. 1997. Activities of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with amikacin, against acinetobacters as determined by checkerboard and time-kill studies. Antimicrob Agents Chemother 41:1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karageorgopoulos DE, Wang R, Yu XH, Falagas ME. 2012. Fosfomycin: evaluation of the published evidence on the emergence of antimicrobial resistance in Gram-negative pathogens. J Antimicrob Chemother 67:255–268. doi: 10.1093/jac/dkr466. [DOI] [PubMed] [Google Scholar]
- 34.Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. 2012. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur J Clin Microbiol Infect Dis 31:695–701. doi: 10.1007/s10096-011-1360-5. [DOI] [PubMed] [Google Scholar]
- 35.Furuya R, Koga Y, Irie S, Ikeda F, Kanayama A, Kobayashi I, Tanaka M. 2013. In vitro activities of antimicrobial combinations against clinical isolates of Neisseria gonorrhoeae. J Infect Chemother 19:1218–1220. doi: 10.1007/s10156-013-0597-6. [DOI] [PubMed] [Google Scholar]
- 36.Gose S, Kong CJ, Lee Y, Samuel MC, Bauer HM, Dixon P, Soge OO, Lei J, Pandori M. 2013. Comparison of Neisseria gonorrhoeae MICs obtained by Etest and agar dilution for ceftriaxone, cefpodoxime, cefixime and azithromycin. J Microbiol Methods 95:379–380. doi: 10.1016/j.mimet.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Ocampo PS, Lazar V, Papp B, Arnoldini M, Abel Zur Wiesch P, Busa-Fekete R, Fekete G, Pal C, Ackermann M, Bonhoeffer S. 2014. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob Agents Chemother 58:4573–4582. doi: 10.1128/AAC.02463-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asmar BI, Prainito M, Dajani AS. 1988. Antagonistic effect of chloramphenicol in combination with cefotaxime or ceftriaxone. Antimicrob Agents Chemother 32:1375–1378. doi: 10.1128/AAC.32.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Gracía J. 1977. Treatment of acute and subacute gonococcal urethritis with fosfomycin. Chemotherapy 23(Suppl 1):S293–S300. [DOI] [PubMed] [Google Scholar]
- 40.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Ison CA, Town K, Obi C, Chisholm S, Hughes G, Livermore DM, Lowndes CM, GRASP Collaborative Group. 2013. Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007–2011. Lancet Infect Dis 13:762–768. doi: 10.1016/S1473-3099(13)70143-9. [DOI] [PubMed] [Google Scholar]
- 42.McDowell L, Kim BN, Paterson DL. 2010. Ceftriaxone, p 351–389. In Grayson ML, Crowe SM, McCarthy JS, Mills J, Mouton JW, Norrby SR, Patterson DL, Pfaller MA (ed), Kucers' the use of antibiotics, 6th ed CRC Press, Boca Raton, FL. [Google Scholar]
- 43.Unemo M, Nicholas RA. 2012. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 7:1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]