Abstract
The dwindling repertoire of antibiotics to treat methicillin-resistant Staphylococcus aureus (MRSA) calls for novel treatment options. Quorum-quenching agents offer an alternative or an adjuvant to antibiotic therapy. Three biaryl hydroxyketone compounds discovered previously (F1, F12, and F19; G. Yu, D. Kuo, M. Shoham, and R. Viswanathan, ACS Comb Sci 16:85–91, 2014) were tested for efficacy in MRSA-infected animal models. Topical therapy of compounds F1 and F12 in a MRSA murine wound infection model promotes wound healing compared to the untreated control. Compounds F1, F12, and F19 afford significant survival benefits in a MRSA insect larva model. Combination therapy of these quorum-quenching agents with cephalothin or nafcillin, antibiotics to which MRSA is resistant in monotherapy, revealed additional survival benefits. The quorum-quenching agents sensitize MRSA to the antibiotic by a synergistic mode of action that also is observed in vitro. An adjuvant of 1 μg/ml F1, F12, or F19 reduces the MIC of nafcillin and cephalothin about 50-fold to values comparable to those for vancomycin, the antibiotic often prescribed for MRSA infections. These findings suggest that it is possible to resurrect obsolete antibiotic therapies in combination with these novel quorum-quenching agents.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a widespread bacterial pathogen, causing various infections, ranging from skin and soft tissue infections to serious invasive infections, such as pneumonia, endocarditis, bacteremia, and sepsis (1, 2). Rising antibiotic resistance and diminishing investment by the pharmaceutical industry in the development of new antibiotics have created an urgent need for novel anti-MRSA agents (3). Quorum-quenching agents provide an alternative and an adjuvant to conventional antibiotic therapy (4, 5). The mechanism of action of quorum-quenching agents is fundamentally different from that for antibiotics. Quorum-quenching agents are neither bactericidal nor bacteriostatic. They inhibit the production of disease-causing toxins by the pathogen, thereby disarming the pathogen of its capacity to inactivate host defense factors. An intact host immune system has a better chance to clear a bacterial infection. A quorum-quenching agent tips the balance of bacterial virulence factors and host defense factors in favor of the host.
Virulence factor production in Staphylococcus aureus is regulated by a quorum-sensing mechanism predominantly under the control of the agr operon (6, 7). In previous work, we have identified small-molecule biaryl hydroxyketone compounds that target the response regulator AgrA and inhibit its interaction with promoter P3, curtailing the production of toxins and virulence factors (8). In a follow-up study, a combinatorial library of 148 compounds was synthesized based on the most efficacious hit compound (9). A member of this biaryl hydroxyketone library, named F12, was the most efficacious of the synthesized compounds, demonstrating 98% in vitro MRSA rabbit erythrocyte hemolysis inhibition at a concentration of 1 μg/ml. In this work, we examine the in vivo efficacy of compounds F1, F12, and F19 (structures shown in Fig. 1) in a murine MRSA wound infection model and in an MRSA insect larva model. In the murine MRSA wound infection model, topical application of F12 twice a day at 20 mg/kg of body weight for 8 days promoted wound healing by 72.3% without affecting the bacterial load. Compound F12 at 20 mg/kg increased the survival of MRSA-inoculated insect larvae from 12 h in untreated controls to 42 h. Combination therapy of F12 with the β-lactam antibiotic cephalothin, to which MRSA is resistant in monotherapy, further increased survival to 84 h, indicating a synergism between the quorum-quenching compound and the antibiotic.
FIG 1.
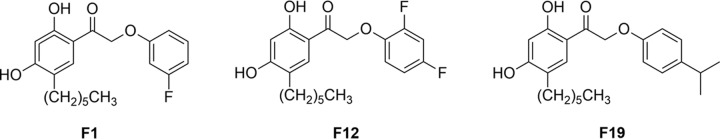
Chemical structure of biaryl hydroxyketone compounds F1, F12, and F19.
MATERIALS AND METHODS
Reagents, microorganisms, and animals.
Reagents and culture media were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA), except for nafcillin and cephalothin (MP Biomedicals LLC, Solon, OH, USA). All solutions were made with sterile ultrapure deionized water. Phosphate-buffered saline (1× PBS), 0.1 M NaHCO3 (5% dimethyl sulfoxide [DMSO]), and BBL Trypticase soy (TS) broth were sterilized by autoclaving at 121°C for 25 min.
MRSA strain USA300 is a clinical isolate from a patient from Metro Health Medical Center, Cleveland, Ohio.
Macrophage cytotoxicity assay.
Assays were performed using the Promega CytoTox 96 nonradioactive cytotoxicity assay kit (G1780) according to the manufacturer's instructions. A target cell dilution of 50,000 cells/ml was used across all trials and measured using a hemacytometer. The concentration of fetal calf serum in the DMEM/high-glucose growth media was 4.5%. The serum was replaced with Hanks' balanced salt solution (HBSS) during the assay. The cells survive in this medium during the 50-min incubation period.
MIC determination.
The standard agar diffusion protocol was followed using Whatman glass microfiber filter discs (25-mm diameter; catalog no. 1821-025) on 100-mm-diameter LB agar petri dishes.
Insect larva animal model.
Batches of wax moth larvae (Galleria mellonella) (Vanderhorst Wholesale Inc., St. Marys, OH, USA) in their final instar stage were stored in the dark at 4°C and used within 3 days of receipt. Larvae were selected from batches for an approximate weight of 250 mg, and this value was used to calculate treatment doses. Only larvae in the weight range of 235 to 265 mg were used. One hundred larvae were used for each data point. Two negative-control groups were used. One underwent no manipulation whatsoever, and another uninfected control group was injected only with 0.1 M NaHCO3 (5% DMSO) to account for the impact of physical trauma. Treated larvae and the control groups were stored in petri dishes in the dark at 37°C. Larvae were inspected every 6 h and monitored over a period of 5 days. Larvae were considered dead if they did not move in response to touching their heads.
Selection of S. aureus load in insect larva experiments.
Groups of larvae were inoculated with live S. aureus USA300 at 1 × 107 (optical density at 600 nm [OD600] of 0.510 ± 0.01) and 2 × 107 CFU (OD600 of 0.986 ± 0.01) using four different volumes: 5 μl, 10 μl, 15 μl, and 20 μl. An inoculation bolus of 2 × 107 CFU in 10 μl was selected as the standard based on a survival half-life of longer than 6 h and complete mortality by 12 h and a lack of bloating or leakage due to the injection. Injections were carried out with a 25-μl Hamilton syringe.
Combination therapy of a quorum-quenching agent and a β-lactam antibiotic in insect larvae.
Larvae infected with 10 μl of 2 × 107 CFU live S. aureus were treated every 6 h (including t = 0) with a 10-μl injection of a combination of F1, F12, or F19 at 20 mg/kg and cephalothin at 30 mg/kg or nafcillin at 45 mg/kg.
Statistical analysis.
Statistical analysis was performed using IBM SPSS Statistics version 21.0 for Windows. For statistical testing and the preparation of figures, data from duplicate experiments were pooled to give n = 100. These pooled survival data were plotted using the Kaplan-Meier method, and comparisons were made between groups using the log-rank test. In all cases, P ≤ 0.05 was considered significant and Holm's correction was applied to account for multiple comparisons. In all comparisons to the negative control, it was the 10-μl uninfected blank control group that was used.
Murine wound infection model. (i) Animal care.
All procedures in the protocol were in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (10), and the Office of Laboratory Animal Welfare. Mice were used upon review and approval of an addendum to our existing protocol by the Institutional Animal Care and Use Committee.
(ii) Procurement and housing of animals.
Murine wound infection model experiments were performed at the Animal Resource Center (ARC) at Case Western Reserve University on female albino CD-1 mice (Charles River, Wilmington, DE) with a body weight of ∼30 g. Animals were allowed to acclimate for 5 days prior to use. Environmental controls for the animal room were set to maintain a temperature of 16 to 22°C, a relative humidity of 30 to 70%, and a 12-h light/12-h dark cycle.
(iii) Preparation of standard inoculum.
MRSA strain USA300 was used as the infecting bacteria. Several petri dishes were plated with MRSA USA300 on brain heart infusion (BHI; Difco Laboratories) and incubated at 37°C for 1 to 2 days. Colonies were transferred to BHI broth and placed in a shaking water bath at 37°C overnight. MRSA cells were harvested by centrifugation and sterile normal saline (0.85% NaCl) washes. A challenge inoculum of 1 × 107 was prepared using a spectrophotometer. To check the inoculum count, 10-fold dilutions of MRSA working suspension were plated onto BHI media. The plates were incubated at 37°C for 2 days and the colony counts determined.
(iv) Evaluation of efficacy of small-molecule quorum-quenching agents in murine wound model of S. aureus infection.
Mice were anesthetized by administering ketamine, xylazine, and acepromazine (3:3:1, vol/vol/vol) intraperitoneally. A 3- by 3-cm midline back area was delineated, shaved, and depilated. The dorsal area was prepared for wounding using a Betadine scrub and wiping with 70% alcohol. A stainless steel wire ring (16-mm diameter and 19 gauge) was secured to the skin with wound clips 2 to 5 mm to the left of the midline. After splint placement, a 6-mm full-thickness excisional wound was created with a punch biopsy tool in the center of each splint. The infected area was inoculated with 10 μl of 1 × 107 CFU of S. aureus. After infection of the wounds, a separate sterile wound dressing (Tegaderm) was placed over the infected area.
A pilot study was performed using 3 animals per group (two inocula, 106 and 107 CFU). The purpose of this study was to ensure the virulence of MRSA strain USA300 and confirm its ability to cause infection in this model. After 7 days of observation the infected area was evaluated.
Infected mice were randomized into the following groups: infected and treated with F1 (20 mg/kg), infected and treated with F12 (20 mg/kg), infected and treated with vehicle, uninfected control, uninfected and treated with F1 (20 mg/kg), uninfected and treated with F12 (20 mg/kg), and an infected and untreated control group.
Treatments were administered topically, 10 μl twice a day for 7 days. Wounds were measured daily with electronic calipers along diameters parallel and perpendicular to the mouse axial skeleton. Animals were treated and the Tegaderm replaced.
Mice were sacrificed 1 day after the last day of treatment (day 8), and the infected area was removed aseptically and weighed. Tissue was homogenized and serially diluted in saline. The homogenates were cultured for 48 h on BHI plates to determine the CFU; tissue burden is expressed as log CFU/g of tissue.
At the end of the study, all surviving animals were sacrificed by CO2 asphyxiation and disposed to an animal resource center for incineration.
A statistical analysis of data from the microbial tissue burden and measurements was performed. Significance was determined using a t test to evaluate wound measurements and an analysis of variance (ANOVA) with a Bonferroni post hoc test to evaluate tissue burden. The treated groups were compared to determine compound activity.
RESULTS
Cytotoxicity in a mouse macrophage cell line.
The cytotoxicity of compounds F1, F12, and F19 was evaluated by leakage of lactate dehydrogenase (LDH) from murine macrophage cell line J774.2 using a commercially available kit. These compounds cause no significant leakage of LDH up to a concentration of 200 μM, as shown in Fig. 2.
FIG 2.
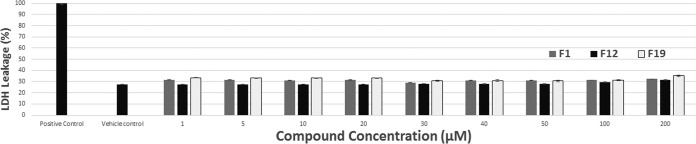
Cytotoxicity measurements of compounds F1, F12, and F19 on mouse macrophage cell line J774.2. Toxicity was assessed by measuring lactate dehydrogenase (LDH) leakage from cells at 490 nm. There is no statistically significant LDH leakage beyond that of the vehicle control for any of the three compounds up to a concentration of 200 μM. The positive control is 1× lysis control solution from the Promega CytoTox 96 assay kit, and the vehicle control is 1% DMSO, 1% ethanol, 1% Kolliphor.
Healing of MRSA-infected wounds in a murine model.
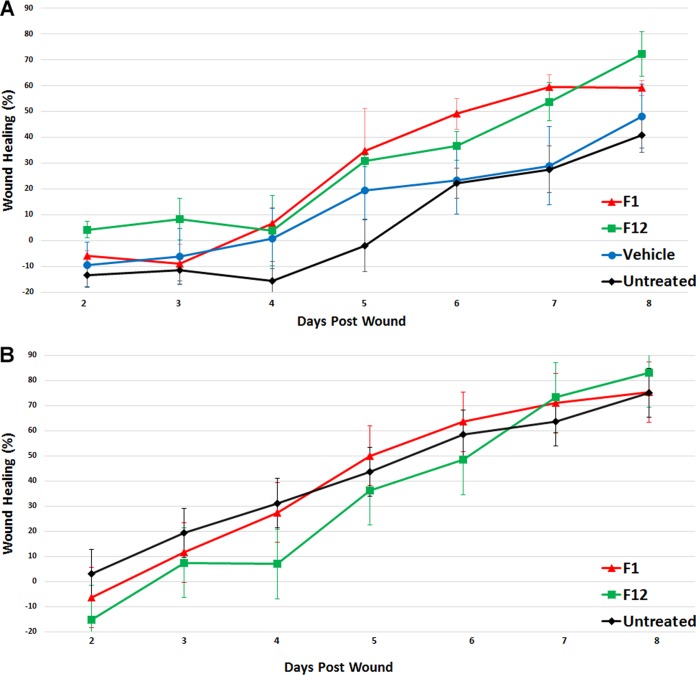
Compounds F1 and F12, previously referred to as 4f-1 and 4f-12 (9), were evaluated for their ability to prevent the pathology of MRSA-infected wounds in a murine model. Figure 3A shows wound healing expressed as an average percentage of wound size compared to the wound status on the first day postinoculation. As expected, the untreated and vehicle controls demonstrated the least amount of healing, with average percent healing of 40.3 and 48.1%, respectively, on day 8. Groups of animals treated with 20 mg/kg of F1 or F12 demonstrated an average of 59.1 and 72.3% wound healing, respectively. The difference between the F1- and F12-treated groups and the infected untreated group was significant, with P values of 0.047 and 0.040, respectively.
FIG 3.
Average percent healing of wounds in mice compared to wound status on the first day postinoculation. Wounds were treated with compound F1 or F12 at 20 mg/kg 1 h after inoculation. Treatment was repeated twice daily for 7 days. (A) Wounds infected with 10 μl of 1 × 107 MRSA USA300. (B) Uninfected wounds.
Figure 3B shows wound healing in uninfected mice expressed as an average percentage of wound size compared to the first day after wounding. On day 8 the F1- and F12-treated groups displayed healing similar to that of the untreated control, with average percent healing of 75.4, 83.1, and 75.0%, respectively. There was no significant difference between these groups (P > 0.05).
Tissue burden was assessed 1 day after the last treatment. The tissue burdens for the F1-treated, F12-treated, vehicle-treated, and infected untreated controls were 7.94 ± 0.73, 7.81 ± 0.96, 8.30 ± 0.52, and 8.97 ± 0.38 CFU, respectively. There was no significant difference between these groups (P > 0.05).
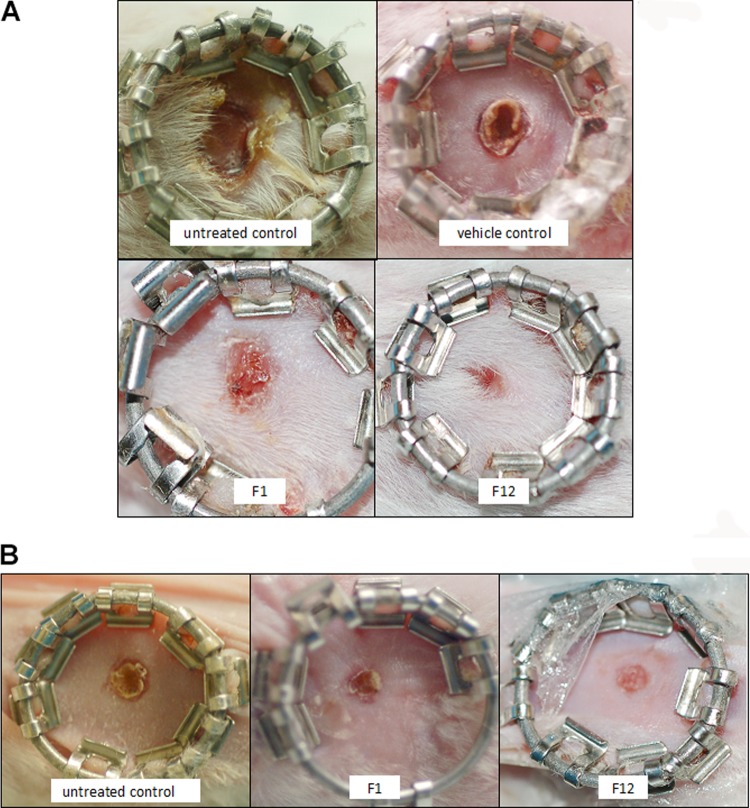
Thus, compounds F1 and F12 afforded protection from the pathology of MRSA in infected wounds compared to the untreated control (P < 0.05), but these compounds did not decrease the bacterial load. Photographs of the wounds are shown in Fig. 4.
FIG 4.
Photographs of wounds in mice 8 days after the onset of the procedure. Wounds were treated with compound F1 or F12 at 20 mg/kg 1 h after inoculation. Treatment was repeated twice daily for 7 days. (A) Wounds infected with 10 μl of 1 × 107 MRSA USA300. (B) Uninfected wounds.
Survival benefit of MRSA-infected insect larvae in the presence of quorum-quenching compounds.
Galleria mellonella larvae infected with 10 μl of 2 × 107 CFU live S. aureus USA300 were given a single treatment of a quorum-quenching compound at 20 mg/kg from the combinatorial library of 148 synthesized small molecules (9) immediately upon inoculation. The treatment was repeated every 6 h up to an elapsed time of 84 h. The most efficacious compounds, F1, F12, and F19, increased survival from 12 h (untreated control) to 60, 42, and 66 h, respectively (Table 1).
TABLE 1.
Survival benefit of MRSA-infected larvae by quorum-quenching compounds and conventional antibiotics in mono- and combination therapy
| Treatment | Elapsed time to 0% survival (h) |
|---|---|
| Untreated | 12 |
| Cephalothin (30 mg/kg) | 12 |
| Nafcillin (45 mg/kg) | 12 |
| F1 (20 mg/kg) | 60 |
| F1 (20 mg/kg) plus cephalothin (30 mg/kg) | 72 |
| F1 (20 mg/kg) plus nafcillin (45 mg/kg) | 78 |
| F12 (20 mg/kg) | 42 |
| F12 (20 mg/kg) plus cephalothin (30 mg/kg) | 84 |
| F12 (20 mg/kg) plus nafcillin (45 mg/kg) | 42 |
| F19 (20 mg/kg) | 66 |
| F19 (20 mg/kg) plus cephalothin (30 mg/kg) | 84 |
| F19 (20 mg/kg) plus nafcillin (45 mg/kg) | 84 |
The efficacy of these compounds was further investigated by combination experiments with conventional antibiotics.
Combination of quorum-quenching therapy with β-lactam antibiotics.
The β-lactam antibiotics cephalothin and nafcillin, to which MRSA is resistant, were selected for combination therapy in insect larvae in order to evaluate the potential of quorum-quenching agents to sensitize MRSA to conventional antibiotics.
Larvae infected with 10 μl of 2 × 107 CFU live S. aureus USA300 were immediately treated with 10 μl of nafcillin or cephalothin with doses of 10 to 90 mg/kg in intervals of 10 mg/kg. As shown in Fig. 5, cephalothin displayed no survival benefit at doses of 40 mg/kg or lower. Likewise, nafcillin afforded no survival benefit at 60 mg/kg (data not shown) or lower. Toxicity was assessed by administering repeat doses of nafcillin at 45 mg/kg and cephalothin at 30 mg/kg every 6 h to uninfected larvae. No adverse effects on the larvae were observed at these dosages. Therefore, these dosages were selected for combination experiments with quorum-quenching agents in the presence of MRSA.
FIG 5.
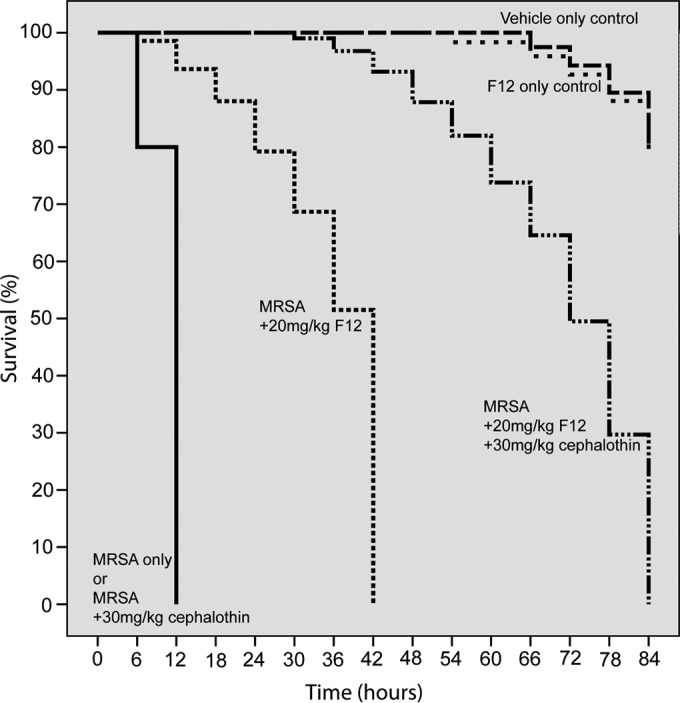
Survival curves of MRSA-infected insect larvae in the presence of compound F12 and cephalothin. Caterpillars were injected with 2 × 107 CFU of MRSA strain USA300. Untreated infected larvae died within 12 h. The mortality rate of uninfected and untreated larvae is 20% at the conclusion of the experiment, the same rate as that of F12-treated uninfected larvae (curves in the upper right corner). Treatments were initiated right after inoculation and repeated every 6 h at the doses indicated. Cephalothin is a cephalosporin β-lactam antibiotic to which MRSA USA300 is resistant, as shown by the solid black curve. The administration of compound F12 at 20 mg/kg increased the survival from 12 to 42 h (broken line). The combination of F12 (20 mg/kg) with cephalothin (30 mg/kg) further increased survival to 84 h (solid broken line). This result indicates a synergism between F12 and an antibiotic, indicating resensitization of MRSA to the antibiotic in the presence of the quorum-quenching compound. Similar results were obtained with compounds F1 and F19.
In combination therapy experiments, larvae were infected with 10 μl of 2 × 107 CFU live S. aureus USA300 and treated at time 0 and every 6 h thereafter with a 10-μl injection of a cocktail of F1, F12, or F19 at 20 mg/kg along with cephalothin at 30 mg/kg or nafcillin at 45 mg/kg. Synergism between any of the three quorum-quenching compounds and any of the two antibiotics tested was observed in all but one combination therapy experiment, as shown in Table 1. F19 afforded the longest survival, to 84 h, in combination with either cephalothin or nafcillin. Representative survival curves demonstrating synergism are shown in Fig. 5.
Quorum-quenching compounds reduce MIC values of nafcillin and cephalothin.
Addition of 1 μg/ml F1, F12, or F19 reduces the MIC of nafcillin and cephalothin from 60 and 40 to 1 μg/ml, as shown in Table 2. This is an indication that these compounds sensitize MRSA to β-lactam antibiotics to a level equivalent to that of current antibiotic treatments against MRSA. The mechanism of this synergy is unknown.
TABLE 2.
MICs of β-lactam antibiotics toward MRSA USA300 in the presence of quorum-quenching compounds
| Compound | MIC (μg/ml) |
|---|---|
| F1 | >50 |
| F12 | >50 |
| F19 | >50 |
| Vancomycin | 3 |
| Nafcillin | 60 |
| Cephalothin | 40 |
| 1 μg/ml F1 plus cephalothin | 1 |
| 1 μg/ml F12 plus cephalothin | 1 |
| 1 μg/ml F19 plus cephalothin | 1 |
| 1 μg/ml F1 plus nafcillin | 1 |
| 1 μg/ml F12 plus nafcillin | 6 |
| 1 μg/ml F19 plus nafcillin | 1 |
Taken together, the in vitro and in vivo data indicate the ability of tested quorum-quenching compounds to sensitize MRSA to β-lactam antibiotics. Thus, these agents have the potential to be used as adjuvants in conventional antibiotic therapy.
DISCUSSION
The dwindling repertoire of antibiotics available to treat drug-resistant infections of S. aureus calls for the discovery of novel treatments. Quorum-quenching agents offer an alternative to antibiotics (4, 5). Quorum-quenching therapy has the potential to eliminate the disease, not the bacteria. However, such a treatment has advantages and disadvantages. Since quorum-quenching agents are not bactericidal, there is reason to anticipate less pressure on the pathogen to develop resistance. A cell able to produce toxins in the presence of the quorum-quenching agent will have an advantage in combatting host defense factors but not in other environments (11). Moreover, the vast majority of bacterial cells will have reduced capacity to harm the host immune system in the presence of a quorum-quenching agent. The encounter between virulent S. aureus and host defense factors can be described as a tug of war. The immune system is designed to take out pathogens, and S. aureus secretes virulence factors, which curtail the function of immune system cells, such as macrophages and neutrophils. If the bacteria are particularly virulent or the patient is immunocompromised, an infection occurs. Administration of a quorum-quenching agent tips the balance in favor of the host by inhibiting the expression of virulence factors, thereby effectively boosting the immune system. An intact immune system has a better chance to clear the infection. Thus, skin and soft-tissue infections, which comprise the majority of S. aureus infections, might be successfully treated with a quorum-quenching agent. Recently a quorum-sensing inhibitor termed savirin, different in structure from the compounds described here, was reported to be efficacious against S. aureus skin infections without showing signs of resistance (12). However, once the infection has become invasive, quorum-quenching therapy might not be enough. Another concern in the clinical use of quorum-quenching agents is that the infection might recur once treatment is stopped since the bacteria are not eliminated, as demonstrated in this study in the murine wound infection model. Treatment with F12 by and large healed the MRSA-infected wound, but the bacterial load was unchanged from the inoculation at the onset of the experiment. This is somewhat surprising, as inhibition of quorum-sensing mechanisms are supposed to secure an intact host response, which in principle should eliminate the pathogen. Thus, combination therapy with an antibiotic might be necessary to prevent a recurring wound infection.
Since MRSA is resistant to most antibiotics, we wanted to examine whether a quorum-quenching agent sensitizes MRSA to an antibiotic to which it is resistant in monotherapy. Two such antibiotics, nafcillin, a first-generation β-lactam antibiotic, and cephalothin, a cephalosporin antibiotic, were selected for the combination experiments. These antibiotics were used in combination with compound F1, F12, or F19 in MRSA-infected insect larvae. An intriguing synergism was observed in survival studies. Combination therapy of F12 with cephalosporin doubled the survival rate of infected larvae from 42 h with F12 alone to 84 h. Monotherapy with cephalothin afforded no survival benefit at all.
Enhancement of the activity of antibiotics in animal models also has been reported by quorum-quenching peptides (13–15). However, small-molecule compounds have an advantage over peptides in that they can be administered orally. Silver enhances antibiotic activity against Gram-negative bacteria (16). Compounds lacking antibacterial activity of their own also have been reported to sensitize drug-resistant bacteria to conventional antibiotics. Some metabolites in combination with aminoglycosides are able to eradicate persister cells (17). RecA inhibitors and vitamin E potentiate the activity of certain antibiotics (18, 19). Thus, the use of antibiotic adjuvants is not a new idea, but so far, only the combination of β-lactamase inhibitors and β-lactam antibiotics has made it into the clinic.
Synergism between discovered quorum-quenching compounds and β-lactam antibiotics also was observed in vitro. The addition of 1 μg/ml F1, F12, or F19 reduced the MIC of nafcillin and cephalothin about fiftyfold to 1 μg/ml, indicating the potential of these compounds to be used as adjuvants in conventional antibiotic therapy.
If such a synergism can be demonstrated in higher organisms, it will offer a new treatment option for MRSA infections. It might be possible to bring back into the clinic “old and obsolete” antibiotics in combination with quorum-quenching agents. This would not only present a new treatment option but also might lower health care costs, since these early antibiotics are off patent.
ACKNOWLEDGMENTS
Grant support was provided to M.S. by grant-in-aid 09GRNT2380131 from the American Heart Association Great Rivers Affiliate, by a grant from the Steris Foundation, and by an award from the Council to Advance Human Health (CAHH) at Case Western Reserve University.
We thank Jae Hyung Jung and Brittney Bunn for assistance in the expression and purification of AgrA. Robert Bonomo is thanked for providing us with a clinical isolate of MRSA USA300, for suggesting nafcillin and cephalothin for combination experiments with conventional antibiotics, and for thoughtful discussions. William Harte is thanked for invaluable advice and continuous encouragement.
REFERENCES
- 1.Gordon RJ, Lowy FD. 2008. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto M. 2012. MRSA virulence and spread. Cell Microbiol 14:1513–1521. doi: 10.1111/j.1462-5822.2012.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Infectious Diseases Society of America (IDSA), Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. 2011. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis 52:S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoham M. 2011. Antivirulence agents against MRSA. Future Med Chem 3:775–777. doi: 10.4155/fmc.11.43. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Kaufmann GF. 2013. Quo vadis quorum quenching? Curr Opin Pharm 13:688–698. doi: 10.1016/j.coph.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Novick RP, Projan SJ, Kornblum J, Ross HF, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. 1995. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet 248:13. [DOI] [PubMed] [Google Scholar]
- 7.Date SV, Modrusan Z, Lawrence M, Morisaki JH, Toy K, Shah IM, Kim J, Park S, Xu M, Basuino L, Chan L, Zeitschel D, Chambers HF, Tan M-W, Brown EJ, Diep BA, Hazenbos WLW. 2013. Global gene expression of methicillin-resistant Staphylococcus aureus USA300 during human and mouse infection. J Infect Dis 209:1542–1550. doi: 10.1093/infdis/jit668. [DOI] [PubMed] [Google Scholar]
- 8.Khodaverdian V, Pesho M, Truitt B, Bollinger L, Patel P, Nithianantham S, Yu G, Delaney E, Jankowsky E, Shoham M. 2013. Discovery of antivirulence agents against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:3645–3652. doi: 10.1128/AAC.00269-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu G, Kuo D, Shoham M, Viswanathan R. 2014. Combinatorial synthesis and in vitro evaluation of a biaryl hydroxyketone library as antivirulence agents against MRSA. ACS Comb Sci 16:85–91. doi: 10.1021/co400142t. [DOI] [PubMed] [Google Scholar]
- 10.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 11.Shopsin B, Eaton C, Wasserman GA, Mathema B, Adhikari RP, Agolory S, Altman DR, Holzman RS, Kreiswirth BN, Novick RP. 2010. Mutations in agr do not persist in natural populations of methicillin-resistant Staphylococcus aureus. J Infect Dis 202:1593–1599. doi: 10.1086/656915. [DOI] [PubMed] [Google Scholar]
- 12.Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, Gresham HD. 2014. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog 10:e1004174. doi: 10.1371/journal.ppat.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonetti O, Cirioni O, Ghiselli R, Orlando F, Silvestri C, Mazzocato S, Kamysz W, Kamysz E, Provinciali M, Giacometti A, Guerrieri M, Offidani A. 2014. In vitro activity and in vivo animal model efficacy of IB-367 alone and in combination with imipenem and colistin against Gram-negative bacteria. Peptides 55:17–22. doi: 10.1016/j.peptides.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Simonetti O, Cirioni O, Mocchegiani F, Cacciatore I, Silvestri C, Baldassarre L, Orlando F, Castelli P, Provinciali M, Vivarelli M, Fornasari E, Giacometti A, Offidani A. 2013. The efficacy of the quorum sensing inhibitor FS8 and tigecycline in preventing prosthesis biofilm in an animal model of staphylococcal infection. Int J Mol Sci 14:16321–16332. doi: 10.3390/ijms140816321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirioni O, Mocchegiani F, Cacciatore I, Vecchiet J, Silvestri C, Baldassarre L, Ucciferri C, Orsetti E, Castelli P, Provinciali M, Vivarelli M, Fornasari E, Giacometti A. 2013. Quorum sensing inhibitor FS3-coated vascular graft enhances daptomycin efficacy in a rat model of staphylococcal infection. Peptides 40:77–81. doi: 10.1016/j.peptides.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. 2013. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med 5:190ra181. doi: 10.1126/scitranslmed.3006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson EJR, Janzen WP, Kireev D, Singleton SF. 2012. High-throughput screening for RecA inhibitors using a transcreener adenosine 5′-o-diphosphate assay. Assay Drug Dev Technol 10:260–268. doi: 10.1089/adt.2011.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierpaoli E, Cirioni O, Barucca A, Orlando F, Silvestri C, Giacometti A, Provinciali M. 2011. Vitamin E supplementation in old mice induces antimicrobial activity and improves the efficacy of daptomycin in an animal model of wounds infected with methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 66:2184–2185. doi: 10.1093/jac/dkr254. [DOI] [PubMed] [Google Scholar]