Abstract
Reducing the transmission of the malarial parasite by Anopheles mosquitoes using drugs or vaccines remains a main focus in the efforts to control malaria. Iron chelators have been studied as potential antimalarial drugs due to their activities against different stages of the parasite. The iron chelator FBS0701 affects the development of Plasmodium falciparum early gametocytes and lowers blood-stage parasitemia. Here, we tested the effect of FBS0701 on stage V gametocyte infectivity for mosquitoes. The incubation of stage V gametocytes for up to 3 days with increasing concentrations of FBS0701 resulted in a significant dose-related reduction in mosquito infectivity, as measured by the numbers of oocysts per mosquito. The reduction in mosquito infectivity was due to the inhibition of male and female gametocyte activation. The preincubation of FBS0701 with ferric chloride restored gametocyte infectivity, showing that the inhibitory effect of FBS0701 was quenched by iron. Deferoxamine, another iron chelator, also reduced gametocyte infectivity but to a lesser extent. Finally, the simultaneous administration of drug and gametocytes to mosquitoes without previous incubation did not significantly reduce the numbers of oocysts. These results show the importance of gametocyte iron metabolism as a potential target for new transmission-blocking strategies.
INTRODUCTION
As part of their life cycle, Plasmodium parasites must develop inside the Anopheles mosquito vector in order to be transmitted to a human host. After being ingested by a female mosquito, Plasmodium gametocytes, the sexual forms of the parasite, undergo activation into male and female gametes. The fertilization of the gametes produces zygotes that transform into motile ookinetes. The ookinetes traverse the mosquito midgut epithelium and form oocysts (1). One of the main barriers to malaria control is the detection and treatment of gametocyte carriers, as the widely used chloroquine and other clinical blood-stage quinolines are inactive against gametocytes. People treated for the asexual stages of malaria may retain circulating viable gametocytes for up to a month. In addition, nontreated asymptomatic malaria patients can have circulating gametocytes for many months (2). Current efforts to control malaria transmission include the improvement of methods to detect gametocyte carriers as well as the development of transmission-blocking drugs and vaccines that aim to interrupt parasite development in the vector mosquito.
Some antimalarial drugs have shown to affect gametocyte development or fertility; however, these drugs are currently not in use due to the development of resistance by the parasite or to toxicity in humans. For example, chloroquine, mefloquine, and quinine affect early Plasmodium falciparum gametocytes by disrupting hemoglobin degradation (3, 4). Likewise, atovaquone inhibits cytochrome b, which is important for the maintenance of the mitochondrial membrane potential (3). However, none of these drugs kill stage IV/V gametocytes (5, 6). Artemisinin-based combination therapy (ACT) reduces the amount of gametocytes circulating in the blood by targeting early gametocytes, although transmission can still occur after treatment (7, 8). Primaquine, the only drug currently used to target stage V gametocytes, and other 8-aminoquinolines can kill stage V P. falciparum gametocytes (8). However, the active metabolite of primaquine has safety issues in people with glucose-6-phosphate dehydrogenase deficiency, leading to hemolytic anemia (9). In addition, a new drug screening study against Plasmodium showed that 3 new agents in development, NPC1161B, OZ277, and methylene blue, reduce gametocyte viability by ≥50% (10).
Iron, an important element in the metabolism of living organisms, has been considered for use as a target in malaria treatment for the past few decades. Importantly, Plasmodium development is inhibited either by iron chelation or dietary iron deficiency in mice and humans (11, 12). Plasmodium growth in culture is also inhibited by iron chelators, with a greater effect occurring in the trophozoite stage (13, 14). In addition, iron chelators inhibit liver-stage malaria, and iron supplementation increases the number of merozoites released from the liver (12). Deferoxamine (DFO) requires intravenous administration, and other oral iron chelators show minimal clinical efficacy against Plasmodium organisms. Iron requirements may increase during Plasmodium gametocytogenesis. The tricarboxylic acid cycle becomes more active, which produces succinyl coenzyme A (CoA) for heme biosynthesis instead of the full oxidation of glucose (15–17). P. falciparum cytochrome b, which binds two heme molecules, has an increased expression of several-fold in the sexual stages compared to that in the asexual stages (18). In addition, the mRNAs of three (δ-aminolevulinate synthetase, porphobilinogen deaminase, and uroporphyrinogen decarboxylase) of the six genes involved in heme biosynthesis are upregulated during gametocytogenesis (19).
FBS0701 is a new iron chelator, for which a phase 2 clinical trial was recently completed to treat transfusional iron overload (20). FBS0701 can be administered as a single daily dose because of its favorable absorption and pharmacokinetic properties compared to those of the iron chelators DFO and deferiprone (21). FBS0701 was recently shown to inhibit early gametocyte stages; however, it has not been tested against late-stage gametocytes (22). Although iron chelation is a strategy for targeting malaria with some scientific basis, there is a need for the development of a more efficacious, biologically available, and safer chelator. The metabolism of Plasmodium gametocytes, and especially their iron requirements, have not been explored in detail. In this study, we tested the activity of FBS0701 as a potential P. falciparum transmission-blocking drug.
MATERIALS AND METHODS
Ethics statement.
Anonymous human blood samples used for parasite cultures and mosquito feeding were obtained under institutional review board (IRB) protocol NA 00019050 approved by the Johns Hopkins School of Medicine.
Biological materials.
P. falciparum strain NF54W (from the Walter Reed National Military Medical Center) infectious gametocyte cultures were provided by the Johns Hopkins Malaria Research Institute Parasite Core Facility and were diluted to 0.1% gametocytemia before feeding to the mosquitoes using an artificial membrane feeder. Anopheles gambiae (Keele) (23) female mosquitoes were reared in the Johns Hopkins Malaria Research Institute insectary.
Gametocyte drug treatment.
The drug assays were performed in 6-well plates by adding dilutions of FBS0701 (from 100 μM to 3 μM) and deferoxamine (DFO) (Sigma, St. Louis, MO) at 12.5 μM and 25 μM to P. falciparum NF54W gametocytes from cultures that had been initiated at the asexual stage 15 days earlier. The cultures were maintained in complete RPMI 1640 (Corning Cellgro, Manassas, VA) with glutamine (along with HEPES, 10% human serum, and 50 mg/ml hypoxanthine) without antibiotics at 2.5% parasitemia and 4% hematocrit in a gassed jar (5% O2, 5% CO2, and 90% N2) and kept in an incubator at 37°C for 24, 48, or 72 h. The control gametocytes were incubated with complete RPMI medium without antibiotics. The medium was replaced daily with new FBS0701 diluted in RPMI, without the addition of erythrocytes.
The same methodology was applied to incubate cultures with ferric chloride at 2.5 μM, 12.5 μM, and 50 μM alone or in combination with FBS0701 at 25 μM for 24 h.
Membrane feeding assays.
The cultures were centrifuged at 108 × g for 4 min. The pellets were resuspended with human serum to 50% hematocrit and with erythrocytes to 0.1% gametocytemia. The gametocytes were fed to 6-day-old sucrose-starved female A. gambiae mosquitoes through a membrane glass feeder. The fed mosquitoes were sorted and maintained at 27°C for 7 days until midgut dissection. The midguts were stained with 0.1% mercurochrome, and the number of oocysts in each mosquito was determined in order to evaluate the effect of the drug on the parasites.
Viability assays.
Two assays were used in the study. For the first assay, gametocytes from cultures that had been initiated at the asexual stage 15 days earlier were incubated with 25 μM FBS0701 for 48 h, as described above. The cultures were incubated with propidium iodide (PI) to a final concentration of 10 μg/ml (Sigma, St. Louis, MO) for 10 min at 37°C. The stained samples were pelleted by a quick spin at maximum speed, and the pellets were resuspended with 37°C 1× phosphate-buffered saline (PBS) and placed immediately on a slide to be observed on a fluorescence microscope. Viable gametocytes do not stain with the membrane-impermeable PI, while dead gametocytes become permeable and stain with PI. A total of 100 gametocytes were analyzed for each sample.
The second assay was performed as described previously (24). Briefly, approximately 1 million N-acetylglucosamine-treated Percoll-enriched gametocytes from cultures that had been initiated at the asexual stage 15 days earlier were incubated on a 96-well plate in triplicate with 5 μl of FBS0701 or pyrvinium pamoate (positive control) at final concentrations of 25 μM and 10 μM, respectively. Incubation with 5 μl of RPMI was used as the negative control. The treated gametocytes were cultured for 48 h at 37°C in a gassed jar without changing the medium, as described previously. Eleven microliters of 10× exflagellation medium (RPMI 1640, 200 mM HEPES, 40 mM sodium bicarbonate, 100 mM glucose [pH 8]) was added, and the gametocytes were incubated at room temperature for 30 min. Next, 11 μl of 10× CyQUANT (background suppressor permeable to dead cells; Life Technologies, Grand Island, NY, USA) and SYBR green I (dye for the detection of live cells; Life Technologies, Eugene, OR, USA) diluted in 1× PBS were added, and the gametocytes were incubated at room temperature for 1 h. Fluorescence was determined at 535 nm following an excitation at 485 nm in a plate reader (HTS7000; PerkinElmer). Because of female predominance in gametocyte cultures, the inhibition of male exflagellation contributes up to 20% of the signal decrease with drug inhibition.
Female gametocyte activation assay.
P. falciparum gametocytes were incubated with 25 μM FBS0701 for 48 h, as described above. The gametocytes were activated in gametocyte activation medium (RPMI 1640 medium containing 25 mM HEPES, 2 mM glutamine, 50 mg of hypoxanthine, 2 g of sodium bicarbonate, 100 M xanthurenic acid, and 20% human serum) for 4 h. Activated female gametocytes were identified with anti-Pfs25 monoclonal antibody (MAb) 4B7 from mouse ascites at a 1:500 dilution (25). Pfs25 is a gamete surface protein expressed in activated female gametes only (26). The binding of anti-PFS25 antibody was detected with an Alexa Fluor 488 goat anti-mouse IgG antibody (Invitrogen). Red blood cells (RBCs) were stained with a rabbit anti-band 3 antibody (Abcam), and the binding of the anti-band 3 antibody was detected by incubation with an Alexa Fluor 594 goat anti-rabbit IgG antibody (Invitrogen). The activation of female gametocytes was determined as a percentage of Pfs25-positive cells (female gametes) of the total RBCs. The results were normalized to the blank control.
Exflagellation assays.
For the exflagellation assays, 10 μl of the gametocyte culture from each treatment was pelleted and resuspended in 10 μl of gametocyte activation medium (RPMI 1640 medium containing 25 mM HEPES, 2 mM glutamine, 50 mg of hypoxanthine, 2 g of sodium bicarbonate, 100 μM xanthurenic acid, and 20% human serum). The exflagellation reaction mixtures were incubated for 15 min at room temperature and then loaded into an improved Neubauer chamber (Reichert, Buffalo, NY) for an estimation of the numbers of exflagellations and RBCs per μl of culture. The results were reported as the number of exflagellations per 106 RBCs.
Statistics.
The following equations/measures were used for calculations: percent inhibition = [(median no. of oocysts in control mosquitoes − median no. of oocysts in experimental mosquitoes)/median no. of oocysts in control mosquitoes] × 100; prevalence = percent infected mosquitoes; and percent reduction in prevalence = ([prevalence of control mosquitoes − prevalence of experimental mosquitoes]/prevalence of control mosquitoes) × 100. For the SYBR green I/CyQUANT assay, percent inhibition was calculated as 100 − ([mean fluorescence of FBS0701 − mean fluorescence of the positive control]/[mean fluorescence of the negative control − mean fluorescence of the positive control]) × 100.
The significance of the differences between the mean number of oocysts and fluorescence units after FBS0701 treatments compared to those of the blank control were determined by one-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test.
RESULTS
Effect of FBS0701 on P. falciparum gametocyte infectivity.
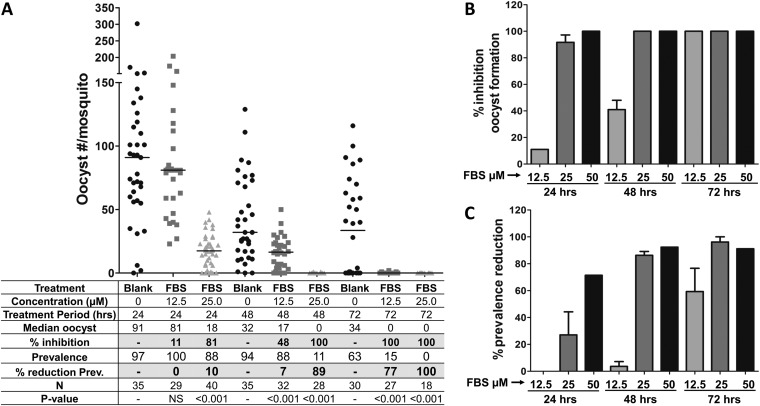
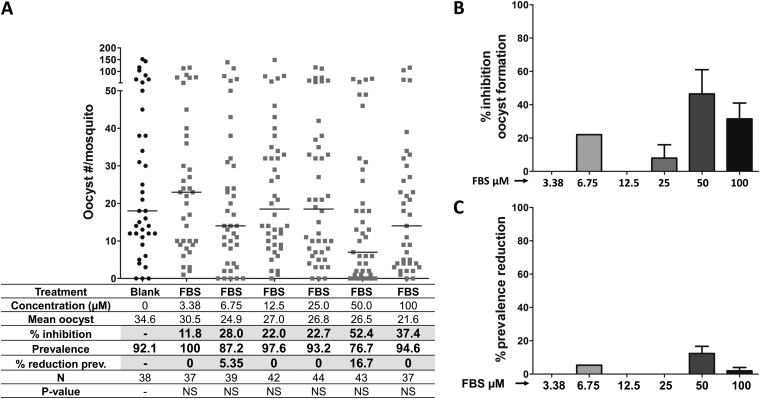
To analyze the effect of FBS0701 on P. falciparum gametocyte infectivity in mosquitoes, stage V gametocytes were incubated with different concentrations of FBS0701 for 24, 48, and 72 h and then fed to A. gambiae mosquitoes. To measure gametocyte transmission viability, the numbers of oocysts were determined on mosquito midguts dissected at 7 days postinfection (p.i.). Thin blood smears of the cultures were made before membrane feeding to mosquitoes to confirm the presence of gametocytes (see Fig. S1 in the supplemental material) The numbers of gametocytes were similar after the different doses and durations of FBS0701. However, gametocyte incubation with FBS0701 induced a dose-dependent reduction in the number of oocysts (Fig. 1A and B; see also Table S1 in the supplemental material). Incubation with 12.5 μM for 24 h resulted in no significant reduction in oocyst formation; however, incubation for 48 and 72 h resulted in a significant reduction of oocyst numbers, up to 41% and 100%, respectively (Fig. 1A and B; see also Table S1). Gametocyte incubation with 25 and 50 μM resulted in a significant reduction in oocyst formation (up to 100%) at all incubation times (Fig. 1A and B; see also Table S1). A drastic reduction in infection prevalence (% infected mosquitoes) was observed for 50 μM FBS0701 at 24 h of incubation (71.4%) (Fig. 1A and C; see also Table S1). In addition, prevalence reductions of 86% to 96% were achieved for 25 μM and 50 μM FBS0701 at 48 and 72 h of incubation, respectively (Fig. 1A and C; see also Table S1).
FIG 1.
Incubation of P. falciparum gametocytes with FBS0701 inhibits development in the mosquito. To analyze the effect of FBS0701 on malarial gametocytes, P. falciparum stage V gametocyte cultures were incubated with different concentrations of FBS0701 for 12, 48, and 72 h. Gametocytes were fed to A. gambiae mosquitoes, and the numbers of oocysts were determined in mosquito midguts at 7 days postinfection. (A) Representative experiment. Horizontal bars represent median oocyst numbers. FBS, FBS0701; N, number of mosquitoes; Prev, prevalence. (B) Percent inhibition of oocyst formation. The bars represent the pooled percent inhibition of oocyst formation from independent experiments tabulated in Table S1 in the supplemental material. (C) Percent reduction in prevalence. The bars represent the pooled percent reduction in prevalence (percent infected mosquitoes) compared to that of the blank control from the independent experiments shown in Table S1. The error bars represent the standard error of the mean.
Effect of deferoxamine on P. falciparum gametocyte infectivity.
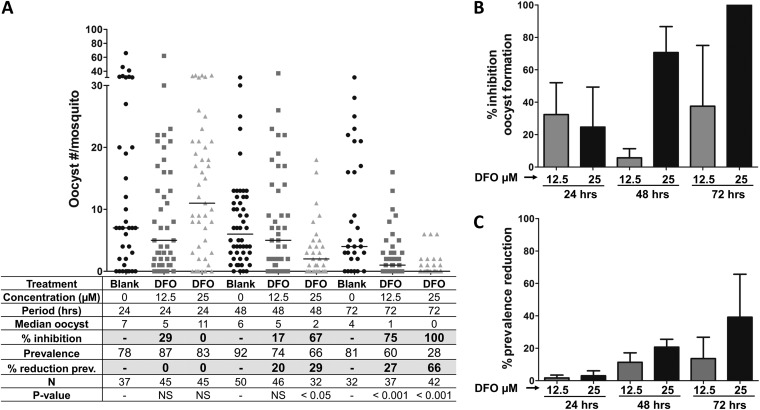
The effect of DFO, another iron chelator known to have antimalarial activity against P. falciparum (27, 28), on parasite development in the mosquito was analyzed in a similar experiment as that for FBS0701. The gametocytes were incubated with 12.5 and 25 μM DFO for 12, 24, and 72 h. Whereas DFO affected gametocyte development in the mosquito, inhibition was less effective than that for FBS0701 (Fig. 2A and B; see also Table S2 in the supplemental material). The mean percentages of inhibition of oocyst formation reached averages of 70% and 100% with a DFO concentration of 25 μM at 48 and 72 h, respectively (Fig. 2B; see also Table S2). The reduction in prevalence after DFO treatment was minimal, reaching 40% with a dose of 25 μM after 72 h of incubation (Fig. 2A and C; see also Table S2).
FIG 2.
Incubation of P. falciparum gametocytes with DFO inhibits development in the mosquito to a limited extent only. The effect of DFO on malarial gametocytes was analyzed by incubating P. falciparum stage V gametocytes with different concentrations of DFO for 12, 48, and 72 h. After treatment, gametocytes were fed to A. gambiae mosquitoes, and the numbers of oocysts were determined in mosquito midguts at 7 days postinfection. (A) Representative experiment. Horizontal bars represent median oocyst numbers. DFO, deferoxamine; N, number of mosquitoes; Prev, prevalence. (B) Percent inhibition of oocyst formation. The bars represent the pooled percent inhibition of oocyst formation from the independent experiments shown in Table S2 in the supplemental material. (C) Percent reduction in prevalence. The bars represent the pooled percent reduction in prevalence (percent infected mosquitoes) compared to that of the blank control from the independent experiments shown in Table S2. The error bars represent the standard error of the mean.
Iron quenches FBS0701 inhibitory activity on gametocyte development into oocysts.
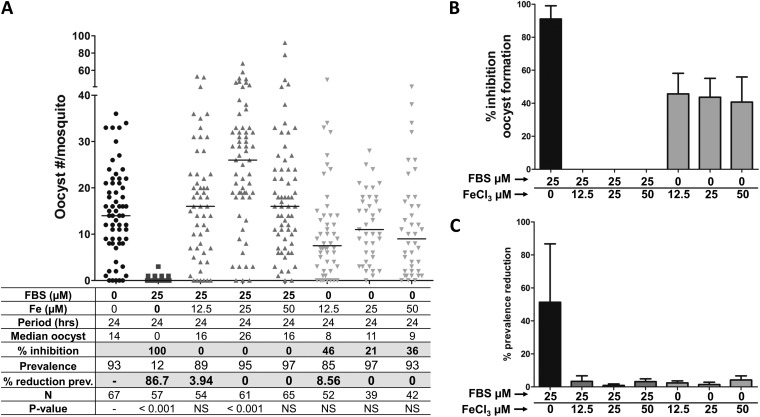
The loss of fertility of P. falciparum gametocytes incubated with FBS0701 measured by oocyst counts is presumably induced by iron chelation. To test the specificity for iron chelation, FBS0701 (25 μM) was preincubated with FeCl3 (12.5, 25, or 50 μM) and then added to the gametocyte cultures. The gametocytes were cultured with the FBS0701-FeCl3 mixture for 24 h and fed to mosquitoes, and the numbers of oocysts were determined at 7 days p.i. The controls, incubation of FBS0701 (25 μM) alone, and incubation with FeCl3 (12.5 μM, 25 μM, or 50 μM) alone were included. As shown in Fig. 3A and B and in Table S3 in the supplemental material, the preincubation of FBS0701 with FeCl3 restored gametocyte infectivity, resulting in oocyst levels comparable to those of the untreated control. Similar to our previous results (Fig. 1A and B; see also Table S1 in the supplemental material), FBS0701 at 25 μM induced a 91% reduction in oocyst formation (Fig. 3A and B; see also Table S3 in the supplemental material). Individual iron supplementations resulted in no significant change in the numbers of oocysts compared with those of the untreated samples (Fig. 3A and B; see also Table S3). The reduction in prevalence was around 50% for the cultures treated with FBS0701, whereas those treated with FBS0701-FeCl3 or FeCl3 alone reached a prevalence reduction of ≤10% (Fig. 3C; see also Table S3).
FIG 3.
Iron supplementation quenched the inhibitory activity of FBS0701. To determine if the FBS0701-induced inhibition of gametocyte infectivity was due to iron chelation, FBS0701 was preincubated with different concentrations of FeCl3 before adding it to the gametocyte culture. Gametocytes were incubated with FBS0701-FeCl3 for 24 h and then fed to A. gambiae mosquitoes. In addition, the gametocyte cultures were incubated either with 25 μM FBS0701 alone or increasing concentrations of FeCl3 alone. The numbers of oocysts were determined in mosquito midguts at 7 days postinfection. (A) Representative experiment. Horizontal bars represent median oocyst numbers. FBS, FBS0701; N, number of mosquitoes; prev, prevalence. (B) Percent inhibition of oocyst formation. The bars represent the pooled percent inhibition of oocyst formation from the independent experiments shown in Table S3 in the supplemental material. (C) Percent reduction in prevalence. The bars represent the pooled percent reduction in prevalence (percent infected mosquitoes) compared to that of the blank control from the independent experiments shown in Table S3. The error bars represent the standard error of the mean.
Reduction in mosquito infectivity results from FBS0701 inhibition of gametocyte activation.
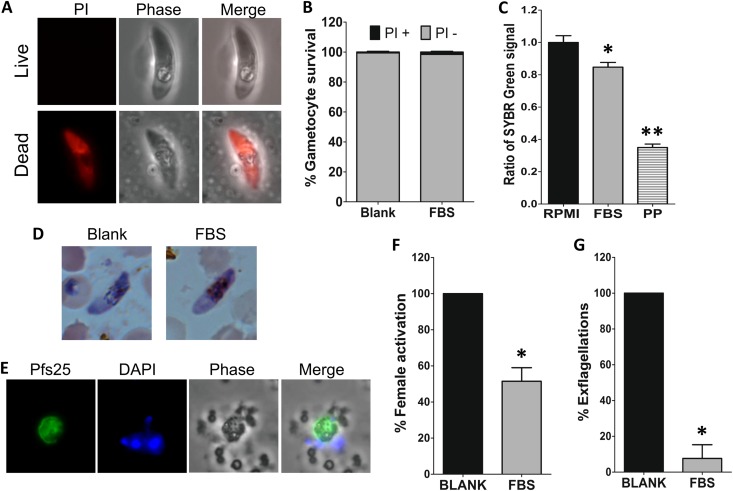
The reduction in the numbers of oocysts detected after gametocyte treatment with FBS0701 might result from dead gametocytes, the failure of gametocytes to be activated into gametes, or a failure to develop into the subsequent mosquito midgut stages (zygotes, ookinetes, and oocysts). To determine viability after FBS0701 treatment, gametocytes incubated in the presence (25 μM) or absence of FBS0701 for 48 h were stained with propidium iodide. Propidium iodide stains nucleic acids, and it only enters cells that have a permeable plasma membrane, which is a sign of cell death (Fig. 4A). No difference in propidium iodide staining was observed between the control and the FBS0701-treated gametocytes, as for both treatments, ∼99% of the gametocytes were negative for propidium iodide staining (Fig. 4B).
FIG 4.
FBS0701 inhibits P. falciparum gametocyte activation. (A) Propidium iodide staining of live (top) or dead (bottom) gametocytes. (B) Quantification of propidium iodide staining of gametocytes incubated in the absence or presence of 25 μM FBS0701 (FBS) for 48 h (P > 0.05, Fisher's exact two-tailed test). (C) Relative fluorescence of SYBR green I/CyQUANT staining of gametocytes incubated in the presence of RPMI, 25 μM FBS0701, or 10 μM pyrvinium pamoate (PP) for 48 h. The values were normalized to the RPMI control. Statistical significance was determined by one-way ANOVA with the Bonferroni multiple-comparison test (*, P < 0.05; **, P < 0.001). (D) Light microscopy of Giemsa-stained gametocytes. (E) Immunofluorescence detection of Pfs25 protein on P. falciparum female gametes after 4 h of incubation in gametocyte activation medium. DAPI, 4′,6-diamidino-2-phenylindole. (F) Percent female gametocyte activation determined by Pfs25 detection in the presence or absence of 25 μM FBS0701 for 48 h. The results were normalized to the blank control. Statistical significance was determined by Student's t test (*, P = 0.0008). (G) Percent male gametocyte exflagellation after incubation with 25 μM FBS0701 for 48 h. The results were normalized to the blank control. The error bars represent the standard error of the mean. Statistical significance was determined by Student's t test (*, P < 0.0001).
A second viability assay was performed using a combination of the SYBR green I DNA probe along with a background suppressor, CyQUANT, which has recently been used to screen drugs with transmission-blocking activity (24). This study included the induction of gametocyte exflagellation and the use of a background suppressor that quenches DNA fluorescence from dead cells to obtain a high signal-to-noise ratio. This assay does not differentiate between male and female gametocyte killings. The assay measures both killing and the inhibition of exflagellation, with the inhibition of male exflagellation accounting for about 20% of the signal decrease. This is reflective of a female-to-male predominance of about 4:1. Drugs inhibiting exflagellation but that do not kill gametocytes would report a low or intermediate inhibition of up to 20%, but an inhibition of >20% indicates killing (24). The SYBR green I fluorescence signal obtained after incubation with FBS0701 decreased significantly (1.18-fold and 2.8-fold) using pyrvinium pamoate as a positive control (Fig. 4C). An inhibition rate of 23.4% with FBS0701 was calculated based on the average of two biological replicates. In addition, the morphologies of the gametocytes incubated with FBS0701 are similar to those of the control gametocytes, based on light microscopy of Giemsa-stained smears (Fig. 4D). These results suggest that gametocytes treated with FBS0701 are alive, even while activation was inhibited.
The activation of gametocytes into gametes is the first step of parasite development in the mosquito midgut lumen. During this process, the gametocytes round up and egress from the RBC, and the male gametocytes undergo exflagellation. To determine if FBS0701 inhibits female gamete activation, gametocytes were incubated for 4 h in gametocyte activation medium and then labeled with anti-Pfs25 antibodies. Pfs25 is expressed only on the surface of female gametes and not in nonactivated gametocytes. The incubation of gametocytes with 25 μM FBS0701 for 48 h reduced female gamete activation by 50% (Fig. 4F). To determine the effect of FBS0701 on male gametocyte activation, exflagellation was measured in gametocyte cultures incubated in the presence (25 μM) or absence of FBS0701 for 48 h. Incubation with FBS0701 resulted in a 92% inhibition of male gamete exflagellation (Fig. 4G). Together, these results show that treatment with FBS0701 does not kill gametocytes but highly impairs their activation into gametes.
FBS0701 does not affect mosquito midgut stages of P. falciparum.
To test if FBS0701 affects the development of Plasmodium stages (zygotes and ookinetes) in the mosquito midgut, increasing concentrations of FBS0701 were added to gametocyte cultures (without preincubation) and immediately fed to A. gambiae mosquitoes. The numbers of oocysts were assessed in the mosquito midguts at 7 days p.i. to determine the effect of the drug on parasite development. Treatment with FBS0701 did not affect the development of the parasite in the mosquito midgut (Fig. 5; see also Table S4 in the supplemental material). None of the FBS0701 treatments induced a significant reduction in oocyst formation, and they did not reduce mosquito infection prevalence.
FIG 5.
FBS0701 does not affect Plasmodium sexual development in the mosquito midgut lumen. To analyze the effect of FBS0701 on the sexual developmental stages of Plasmodium in the mosquito midgut (gametes, zygotes, and ookinetes), P. falciparum gametocyte cultures were fed to A. gambiae mosquitoes after adding different concentrations of FBS0701 immediately before mosquito feeding. The numbers of oocysts were determined in mosquito midguts at 7 days p.i. (A) Representative experiment. Horizontal bars represent median oocyst numbers. FBS, FBS0701; N, number of mosquitoes; prev, prevalence. (B) Percent inhibition of oocyst formation. The bars represent the pooled percent inhibition of oocyst formation from the independent experiments shown in Table S4 in the supplemental material. (C) Percent reduction in prevalence. The bars represent the pooled percent reduction in prevalence (percent infected mosquitoes) compared to that of the blank control from the independent experiments shown in Table S4. The error bars represent the standard error of the mean.
DISCUSSION
Previous studies have shown that iron chelation affects Plasmodium parasite development and could be developed as an antimalarial therapy (28). The new iron chelator FBS0701 was found to have antimalarial properties against Plasmodium blood-stage infections in vitro and in vivo (22). In a previous publication, FBS0701 was shown to affect P. falciparum gametocytogenesis, having a stronger inhibitory effect on early stage gametocytes (∼90% inhibition) than on stage IV/V gametocytes (∼50% inhibition) with the same dose of 100 μM (22). However, that study did not test the effect of FBS0701 on stage IV/V gametocyte transmission to mosquitoes. Here, we demonstrate that as previously shown, the incubation of stage V gametocytes with FBS0701 does not reduce gametocyte stage numbers but induces a significant decrease in gametocyte fertility, as measured by the number of oocysts that developed in the mosquito. The reduction in mosquito infectivity was a result of a drastic reduction in gametocyte activation, especially male gametocyte activation. Importantly, some treatments induced a dramatic reduction (up to 100%) in mosquito infection. In addition, we showed that the reduction by FBS0701 on mosquito infectivity was a result of iron chelation, as preincubation of the drug with iron reduced the inhibitory effect and restored gametocyte infectivity.
Similar to FBS0701, DFO is an antimalarial iron chelator. In the present study, we report that the inhibition of stage V gametocyte fertility by DFO was lower than that by FBS0701. This is not surprising, since the 50% inhibitory concentration (IC50) of DFO against P. falciparum blood-stage organisms ranges from 4 to 35 μM (28), while that of FBS0701 is lower, at 6 to 17 μM (22). In addition, FBS0701 is more efficient at chelating iron in mice and monkeys than is DFO (29, 30). Moreover, Ferrer et al. (22) showed that FBS0701 can chelate and remove intracellular erythrocytic iron. Because DFO is a lipophobic iron chelator, it might not access and chelate intracellular iron with the same efficiency as does FBS0701. This might explain the higher inhibitory effect of FBS0701 against blood-stage Plasmodium organisms and gametocytes.
One of the mechanisms by which FBS0701 iron chelation might affect gametocyte fertility is by reducing heme availability for function in the mitochondrial electron transport system. Recent studies suggest that gametocytes switch to a nonglycolytic energy metabolism with a more active tricarboxylic acid (TCA) cycle and oxidative phosphorylation (31–34). The TCA cycle would be more active during the sexual stages in the production of succinyl CoA for heme biosynthesis (33). Heme is an essential cofactor for some of the enzymes on the electron transport chain, such as cytochrome b and cytochrome c. Indeed, there is an increased expression of cytochrome b in the sexual stages (15, 35). It is conceivable that FBS0701 reduces the heme required for proper mitochondrial function by chelating the iron contained in the heme molecule. In addition, the electron transport chain depends on iron-sulfur proteins, and these might be affected by FBS0701. Besides energy production, the electron transport system can also be used for pyrimidine biosynthesis. It has been suggested that the mitochondrial activity seen in the sexual stages is meant to fulfill this goal (34). Male gametocytes undergo fast DNA replication during activation, in which each male gamete increases its DNA content from 1 N to 8 N in a short period of time. It is conceivable that FBS0701 interferes with DNA replication during male gametocyte activation by chelating the iron needed for pyrimidine biosynthesis.
The cofeeding of P. falciparum gametocytes with FBS0701 to A. gambiae mosquitoes did not reduce the numbers of oocysts. These results suggest that iron chelation by FBS0701 has no effect on the development of parasite stages in the mosquito midgut lumen, which includes zygotes and ookinetes. These stages would be exposed to the drug present in the ingested blood meal for a period of ∼16 to 24 h when the ookinete invades and traverses the midgut epithelium to form an oocyst on the basal side of the midgut. These results are surprising, since it is suggested that the transition of malaria to the mosquito host is accompanied by an increase in mitochondrial activity, including in the TCA cycle and the electron transport chain, which requires iron for proper function (16, 31, 32, 36–39). As proposed for stage V gametocytes, FBS0701 is expected to chelate the iron required for mitochondrial function during parasite development in the mosquito midgut lumen. However, mosquitoes start the process of blood meal digestion immediately after feeding is completed. During this process, large amounts of hemoglobin from the RBCs are released and degraded, which results in an increase in free heme iron in the midgut (40). It is possible that the large amounts of free iron released during RBC hemoglobin digestion bind to FBS0701 and compromise its inhibitory effect against the mosquito midgut stages of the parasite. Further studies will be needed to test this hypothesis.
There are some theoretical concerns about the use of iron chelators to treat malaria, since populations at risk of infection suffer from iron-deficient anemia. Normal body iron stores range from around 2 to 6 g. Based on the pharmacodynamics of the drug, 32 mg/kg of body weight of FBS0701 would remove <20 mg of iron from the host over 24 h (21). This amount should not significantly affect the total iron levels in a 3- to 5-day treatment regimen for malaria. In the present study, a dose of 25 μM FBS0701 for 48 h was sufficient to block parasite development in the mosquito. A maximum concentration of drug in serum (Cmax) of 154.7 μM FBS0701 is achieved with a human oral dose of 32 mg/kg (21). These levels would be sufficient to inhibit the development of gametocytes. While the classic cutoff for malaria drugs is <1 μM, there are examples with the antibacterials, like clindamycin or tetracycline, in which a drug with a micromolar IC50 can still be clinically relevant against Plasmodium (41, 42). For Staphylococcus aureus and many antifungal drugs for bloodstream infections, the clinical breakpoints are in the low micromolar range (41–44). A phase 2 efficacy study confirmed that doses of 16 mg/kg and 32 mg/kg were well tolerated in humans for 7 days (20). Therefore, we propose that a short course of iron chelation will inactivate gametocytes while removing inconsequential amounts of iron from humans. The drug should not be used with the artemisinins. Newer drugs that may be useful in combination with FBS0701 as well as a postartemisinin dose are reaching the clinical pipeline. At present, we do not suggest that FBS0701 be used by itself as a prophylactic or transmission blocker. A possible validation in either a human challenge model or in uncomplicated malaria would be to treat asexual stages with a quinoline followed by randomization to an iron chelator or placebo to look at mosquito infectivity.
In summary, we have shown that the iron chelator FBS0701 has a negative effect on the fertility of Plasmodium gametocytes to its mosquito vector. We hypothesize that FBS0701 interferes with energy production and the proper function of the electron transport system of gametocytes and/or gametocyte activation. These results encourage further studies on the role of iron metabolism in Plasmodium gametocyte infectivity to the mosquito vector. Moreover, this study encourages the further development of FBS0701 and iron chelation as a potential malaria transmission-blocking alternative.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Johns Hopkins Malaria Research Institute mosquito and P. falciparum core facilities for help with mosquito rearing and parasite cultures.
This work received financial support from the National Institutes of Health (NIH) (grant R01AI084940). Additional support was provided by the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation. The supply of human blood was supported by NIH grant RR00052.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04642-14.
REFERENCES
- 1.Smith C, Vega-Rodriguez J, Jacobs-Lorena M. 2014. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Mem Inst Oswaldo Cruz 109:644–661. doi: 10.1590/0074-0276130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smalley ME, Sinden RE. 1977. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology 74:1–8. doi: 10.1017/S0031182000047478. [DOI] [PubMed] [Google Scholar]
- 4.Shute P, Maryon M. 1948. The gametocytocidal action of paludrine upon infections of Plasmodium falciparum. Parasitology 38:264–270. doi: 10.1017/S0031182000023210. [DOI] [PubMed] [Google Scholar]
- 5.Fleck SL, Pudney M, Sinden RE. 1996. The effect of atovaquone (566C80) on the maturation and viability of Plasmodium falciparum gametocytes in vitro. Trans R Soc Trop Med Hyg 90:309–312. doi: 10.1016/S0035-9203(96)90266-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, Song YZ. 1994. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin Med J (Engl) 107:709–711. [PubMed] [Google Scholar]
- 7.Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GAT. 2005. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med 2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. 2006. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg 75:402–415. [PubMed] [Google Scholar]
- 9.Vale N, Moreira R, Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur J Med Chem 44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Peatey CL, Leroy D, Gardiner DL, Trenholme KR. 2012. Anti-malarial drugs: how effective are they against Plasmodium falciparum gametocytes? Malar J 11:34. doi: 10.1186/1475-2875-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershko C, Gordeuk VR, Thuma PE, Theanacho EN, Spira DT, Hider RC, Peto TEA, Brittenham GM. 1992. The antimalaria effect of iron chelators: studies in animal-models and in humans with mild falciparum malaria. J Inorg Biochem 47:267–277. doi: 10.1016/0162-0134(92)84072-U. [DOI] [PubMed] [Google Scholar]
- 12.Loyevsky M, Sacci JB Jr, Boehme P, Weglicki W, John C, Gordeuk VR. 1999. Plasmodium falciparum and Plasmodium yoelii: effect of the iron chelation prodrug dexrazoxane on in vitro cultures. Exp Parasitol 91:105–114. doi: 10.1006/expr.1998.4371. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson CT, Bayne MT, Gordeuk VR, Brittenham GM, Aikawa M. 1991. Stage-specific ultrastructural effects of desferrioxamine on Plasmodium falciparum in vitro. Am J Trop Med Hyg 45:593–601. [DOI] [PubMed] [Google Scholar]
- 14.Raventos-Suarez C, Pollack S, Nagel RL. 1982. Plasmodium falciparum: inhibition of in vitro growth by desferrioxamine. Am J Trop Med Hyg 31:919–922. [DOI] [PubMed] [Google Scholar]
- 15.Krungkrai J, Burat D, Kudan S, Krungkrai S, Prapunwattana P. 1999. Mitochondrial oxygen consumption in asexual and sexual blood stages of the human malarial parasite, Plasmodium falciparum. Southeast Asian J Trop Med Public Health 30:636–642. [PubMed] [Google Scholar]
- 16.Krungkrai J, Prapunwattana P, Krungkrai SR. 2000. Ultrastructure and function of mitochondria in gametocytic stage of Plasmodium falciparum. Parasite 7:19–26. doi: 10.1051/parasite/2000071019. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya AB, Mather MW. 2009. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol 63:249–267. doi: 10.1146/annurev.micro.091208.073424. [DOI] [PubMed] [Google Scholar]
- 18.Learngaramkul P, Petmitr S, Krungkrai SR, Prapunwattana P, Krungkrai J. 1999. Molecular characterization of mitochondria in asexual and sexual blood stages of Plasmodium falciparum. Mol Cell Biol Res Commun 2:15–20. doi: 10.1006/mcbr.1999.0145. [DOI] [PubMed] [Google Scholar]
- 19.Young JA, Fivelman QL, Blair PL, de la Vega P, Le Roch KG, Zhou Y, Carucci DJ, Baker DA, Winzeler EA. 2005. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol 143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Neufeld EJ, Galanello R, Viprakasit V, Aydinok Y, Piga A, Harmatz P, Forni GL, Shah FT, Grace RF, Porter JB, Wood JC, Peppe J, Jones A, Rienhoff HY Jr. 2012. A phase 2 study of the safety, tolerability, and pharmacodynamics of FBS0701, a novel oral iron chelator, in transfusional iron overload. Blood 119:3263–3268. doi: 10.1182/blood-2011-10-386268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rienhoff HY Jr, Viprakasit V, Tay L, Harmatz P, Vichinsky E, Chirnomas D, Kwiatkowski JL, Tapper A, Kramer W, Porter JB, Neufeld EJ. 2011. A phase 1 dose-escalation study: safety, tolerability, and pharmacokinetics of FBS0701, a novel oral iron chelator for the treatment of transfusional iron overload. Haematologica 96:521–525. doi: 10.3324/haematol.2010.034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrer P, Tripathi AK, Clark MA, Hand CC, Rienhoff HY Jr, Sullivan DJ Jr. 2012. Antimalarial iron chelator, FBS0701, shows asexual and gametocyte Plasmodium falciparum activity and single oral dose cure in a murine malaria model. PLoS One 7:e37171. doi: 10.1371/journal.pone.0037171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurd H, Taylor PJ, Adams D, Underhill A, Eggleston P. 2005. Evaluating the costs of mosquito resistance to malaria parasites. Evolution 59:2560–2572. doi: 10.1554/05-211.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. 2014. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS One 9:e105817. doi: 10.1371/journal.pone.0105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delves MJ, Ruecker A, Straschil U, Lelièvre J, Marques S, López-Barragán MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordeuk VR, Thuma PE, Brittenham GM, Zulu S, Simwanza G, Mhangu A, Flesch G, Parry D. 1992. Iron chelation with desferrioxamine B in adults with asymptomatic Plasmodium falciparum parasitemia. Blood 79:308–312. [PubMed] [Google Scholar]
- 28.Mabeza GF, Loyevsky M, Gordeuk VR, Weiss G. 1999. Iron chelation therapy for malaria: a review. Pharmacol Ther 81:53–75. doi: 10.1016/S0163-7258(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 29.Bergeron RJ, Wiegand J, McManis JS, Bharti N, Singh S. 2008. Design, synthesis, and testing of non-nephrotoxic desazadesferrithiocin polyether analogues. J Med Chem 51:3913–3923. doi: 10.1021/jm800154m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergeron RJ, Wiegand J, Bharti N, McManis JS, Singh S. 2011. Desferrithiocin analogue iron chelators: iron clearing efficiency, tissue distribution, and renal toxicity. Biometals 24:239–258. doi: 10.1007/s10534-010-9389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daily JP, Scanfeld D, Pochet N, Le Roch K, Plouffe D, Kamal M, Sarr O, Mboup S, Ndir O, Wypij D, Levasseur K, Thomas E, Tamayo P, Dong C, Zhou Y, Lander ES, Ndiaye D, Wirth D, Winzeler EA, Mesirov JP, Regev A. 2007. Distinct physiological states of Plasmodium falciparum in malaria-infected patients. Nature 450:1091–1095. doi: 10.1038/nature06311. [DOI] [PubMed] [Google Scholar]
- 32.MacRae JI, Dixon MW, Dearnley MK, Chua HH, Chambers JM, Kenny S, Bottova I, Tilley L, McConville MJ. 2013. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol 11:67. doi: 10.1186/1741-7007-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogi T, Kita K. 2010. Diversity in mitochondrial metabolic pathways in parasitic protists Plasmodium and Cryptosporidium. Parasitol Int 59:305–312. doi: 10.1016/j.parint.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Petmitr S, Krungkrai J. 1995. Mitochondrial cytochrome b gene in two developmental stages of human malarial parasite Plasmodium falciparum. Southeast Asian J Trop Med Public Health 26:600–605. [PubMed] [Google Scholar]
- 35.Lang-Unnasch N, Murphy AD. 1998. Metabolic changes of the malaria parasite during the transition from the human to the mosquito host. Annu Rev Microbiol 52:561–590. doi: 10.1146/annurev.micro.52.1.561. [DOI] [PubMed] [Google Scholar]
- 36.Sinden RE. 1978. Cell biology, p 85–168. In Killick-Kendrick R, Peters W (ed), Rodent malaria. Academic Press, London, United Kingdom. [Google Scholar]
- 37.Hall N, Karras M, Raine JD, Carlton JM, Kooij TWA, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, James K, Rutherford K, Harris B, Harris D, Churcher C, Quail MA, Ormond D, Doggett J, Trueman HE, Mendoza J, Bidwell SL, Rajandream M-A, Carucci DJ, Yates JR III, Kafatos FC, Janse CJ, Barrell B, Turner CM, Waters AP, Sinden RE. 2005. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 38.Lovegrove FE, Peña-Castillo L, Liles WC, Hughes TR, Kain KC. 2008. Plasmodium falciparum shows transcriptional versatility within the human host. Trends Parasitol 24:288–291. doi: 10.1016/j.pt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 39.LeRoux M, Lakshmanan V, Daily JP. 2009. Plasmodium falciparum biology: analysis of in vitro versus in vivo growth conditions. Trends Parasitol 25:474–481. doi: 10.1016/j.pt.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Zhou G, Kohlhepp P, Geiser D, del Carmen Frasquillo M, Vazquez-Moreno L, Winzerling JJ. 2007. Fate of blood meal iron in mosquitoes. J Insect Physiol 53:1169–1178. doi: 10.1016/j.jinsphys.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pradines B, Spiegel A, Rogier C, Tall A, Mosnier J, Fusai T, Trape JF, Parzy D. 2000. Antibiotics for prophylaxis of Plasmodium falciparum infections: in vitro activity of doxycycline against Senegalese isolates. Am J Trop Med Hyg 62:82–85. [DOI] [PubMed] [Google Scholar]
- 42.Burkhardt D, Wiesner J, Stoesser N, Ramharter M, Uhlemann AC, Issifou S, Jomaa H, Krishna S, Kremsner PG, Borrmann S. 2007. Delayed parasite elimination in human infections treated with clindamycin parallels ‘delayed death' of Plasmodium falciparum in vitro. Int J Parasitol 37:777–785. doi: 10.1016/j.ijpara.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Tenover FC, Moellering RC Jr. 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 44:1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 44.Kuper KM, Coyle EA, Wanger A. 2012. Antifungal susceptibility testing: a primer for clinicians. Pharmacotherapy 32:1112–1122. doi: 10.1002/phar.1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.