Abstract
Intragastric Klebsiella pneumoniae infections of mice can cause liver abscesses, necrosis of liver tissues, and bacteremia. Lithium chloride, a widely prescribed drug for bipolar mood disorder, has been reported to possess anti-inflammatory properties. Using an intragastric infection model, the effects of LiCl on K. pneumoniae infections were examined. Providing mice with drinking water containing LiCl immediately after infection protected them from K. pneumoniae-induced death and liver injuries, such as necrosis of liver tissues, as well as increasing blood levels of aspartate aminotransferase and alanine aminotransferase, in a dose-dependent manner. LiCl administered as late as 24 h postinfection still provided protection. Monitoring of the LiCl concentrations in the sera of K. pneumoniae-infected mice showed that approximately 0.33 mM LiCl was the most effective dose for protecting mice against infections, which is lower than the clinically toxic dose of LiCl. Surveys of bacterial counts and cytokine expression levels in LiCl-treated mice revealed that both were effectively inhibited in blood and liver tissues. Using in vitro assays, we found that LiCl (5 μM to 1 mM) did not directly interfere with the growth of K. pneumoniae but made K. pneumoniae cells lose the mucoid phenotype and become more susceptible to macrophage killing. Furthermore, low doses of LiCl also partially enhanced the bactericidal activity of macrophages. Taken together, these data suggest that LiCl is an alternative therapeutic agent for K. pneumoniae-induced liver infections.
INTRODUCTION
Klebsiella pneumoniae is a well-known human nosocomial pathogen that causes urinary tract infections, pneumonia, and septicemia in immunocompromised individuals (1). In the past 20 years, a predominant, community-acquired, invasive K. pneumoniae infection type has emerged in Asia (2–8) and has also been reported worldwide (9, 10). The resulting infections are characterized by primary liver abscesses (2–10), and some patients develop extrahepatic complications such as endophthalmitis, meningitis, and necrotizing fasciitis (3, 6, 8). The mechanism by which K. pneumoniae induces primary liver abscesses involves both microbial and host factors. Several genetic loci, such as the cps cluster (11), the wb cluster (12), rmpA (13), htrA (14), and magA (8, 15), have been identified as K. pneumoniae virulence genes. The major virulence factors in the invasive K. pneumoniae isolates from patients with liver abscesses in Taiwan are the magA and rmpA genes and capsular serotype K1 or K2 (16, 17). The most important risk factor for patients with K. pneumoniae-induced liver abscesses is diabetes mellitus (2, 4–6, 8); however, a few infected individuals exhibit no apparent underlying diseases (8). Despite intensive care with catheter drainage and antibiotic therapy, the mortality rate has remained higher than 10%, especially among patients with metastatic complications (17–19). Moreover, the occurrence of K. pneumoniae isolates displaying resistance to carbapenems and third-generation cephalosporins has greatly increased recently (20, 21). Consequently, the development of alternative therapeutic and prophylactic agents for control of K. pneumoniae infections is necessary.
Innate immune cells use pathogen recognition receptors (PRRs) such as Toll-like receptors (TLRs) to recognize the pathogen-associated molecular patterns (PAMPs) of microbes or virulence factors. This recognition can induce cells to produce inflammatory cytokines and other molecules to help eliminate the pathogens and direct pathogen-specific adaptive immune responses. The release of inflammatory cytokines can promote cell infiltration and tissue damage, which are characteristic of inflammation, although excessive or prolonged inflammation can cause severe injury to the host, such as septic shock (22). For more than 50 years, LiCl has been widely used to treat bipolar mood disorder. In spite of its important clinical applications, the molecular mechanisms by which LiCl exerts its therapeutic effects on mental disorders are still not well understood (23). Using different study models, LiCl has been shown to directly inhibit various enzymes and targets in vitro, including inositol monophosphatases (IMPAs) (24), bisphosphate 3′-nucleotidase (25), cyclooxygenase (26), isoforms of glycogen synthase kinase 3 (GSK3) (i.e., GSK3α and -β) (27–29), and β-arrestin 2 (23). Among these targets, GSK3β, a serine/threonine kinase that phosphorylates more than 50 substrates including p65-NF-κB, is a critical regulator of the innate immune response against bacterial infections (30). A number of studies have documented that GSK3β activity is crucial in regulating inflammatory responses during bacterial infections, due to its role in the expression of either proinflammatory or anti-inflammatory cytokines to promote or to inhibit the process (31).
The effect of LiCl on K. pneumoniae infections has not been demonstrated. In the present study, the therapeutic effects of LiCl, a clinically used GSK3β inhibitor, on K. pneumoniae infections were evaluated. Using an intragastric infection model, which mimics the clinical infection route of K. pneumoniae liver abscesses (32, 33), we demonstrated that providing LiCl-treated drinking water inhibited K. pneumoniae-induced death and liver injury in mice by decreasing the bacterial burden and cytokine production in blood and liver tissues.
MATERIALS AND METHODS
Bacteria.
K. pneumoniae NK-9 (capsular serotype K1) with hypermucoviscosity was isolated from a patient with primary liver abscesses at the National Cheng Kung University Hospital. K. pneumoniae NK-9 was cultured in tryptic soy broth (TSB) (Difco Laboratories, Detroit, MI) for 18 h at 37°C and then was subcultured in fresh broth (1:50 [vol/vol]) for another 3 h. The concentration of bacteria was determined with a spectrophotometer (Beckman Instruments, Somerset, NJ), with an optical density at 600 nm of 1 being equal to 1 × 109 CFU/ml. The exact concentration was confirmed by serial dilutions and plate counting.
Mice.
C57BL/6 (B6) mice were purchased from the National Laboratory Animal Center in Taiwan. The animals were maintained on standard laboratory chow and water, available ad libitum, in the animal center at I-Shou University. The animals were raised and cared for in accordance with guidelines established by the Ministry of Science and Technology in Taiwan. All procedures for the treatment, care, and handling of the animals were reviewed and approved by the Institutional Animal Care and Use Committee at I-Shou University (protocol ISU101036). Five- to 6-week-old male mice (body weight, 22 ± 1.5 g per mouse) were used in all experiments.
Intragastric model of infection and LiCl treatment.
To induce liver abscesses, groups of 8 to 16 mice were given 0.2 ml of 0.2 M NaHCO3 through a sterile gastric gavage tube, to neutralize acidity. After the bicarbonate treatment, 1 × 109 K. pneumoniae NK-9 cells in 0.2 ml of sterile phosphate-buffered saline (PBS) were immediately administered through the same route (32, 33). The 70% lethal dose (LD70) of K. pneumoniae NK-9 cells administered intragastrically in B6 mice was 1 × 109 K. pneumoniae cells. The animals were observed every day for a total of 9 days. To determine the effects of LiCl, various concentrations of the drug (Sigma catalog no. L9650) were added to the drinking water, which was administered immediately postinfection and provided to the mice ad libitum. In another LiCl therapeutic experiment, LiCl-treated drinking water was provided at 24 or 48 h postinfection, following which the survival rates of the K. pneumoniae-infected mice were examined for a total of 9 days.
In some LiCl experiments, groups of six B6 mice were inoculated intragastrically with 1 × 109 K. pneumoniae NK-9 cells per mouse. LiCl (10 or 400 μg/ml) was administered with the drinking water immediately postinfection. At various times after infection, serum samples were collected from the mice to examine LiCl concentrations in the serum, and the livers were removed, fixed in 3.7% formaldehyde, and embedded in paraffin. Tissue slices (5 μm thick) were prepared and stained with hematoxylin and eosin, and the degree of liver inflammation was determined as a histopathology score, in a blinded manner. Four different sections of the largest liver lobule of each mouse were examined and scored as follows: score of 1, less than 5 microabscesses in each liver section and no necrotic region present; score of 2, between 5 and 10 microabscesses in each liver section and no necrotic region present; score of 3, between 5 and 10 microabscesses in each liver section and necrotic regions present; score of 4, between 10 and 15 microabscesses in each liver section and necrotic regions present. The average score for each group was generated by examination of liver sections from six mice (33). In another LiCl experimental group, groups of four B6 mice were inoculated intragastrically with 5 × 108 K. pneumoniae NK-9 cells per mouse. LiCl (10, 100, or 400 μg/ml) was administered with the drinking water immediately postinfection. At 18 or 72 h after infection, mouse sera were collected, and some heparin-containing blood or liver tissue homogenized in PBS was aseptically collected, serially diluted, poured on LB agar plates, and incubated overnight at 37°C. The number of CFU of K. pneumoniae was then quantitated and expressed as the mean ± standard deviation. The limit of detection was 10 CFU per g or ml.
Determination of LiCl concentrations in sera.
Mouse sera were collected, and the concentrations of LiCl were determined using a colorimetric assay (34) and then detected using a Dimension RxL Max Integrated Chemistry System 50/60 Hz (Siemens Healthcare Diagnostics, Germany). The average concentration in each group was generated by examination of sera from six mice and was expressed as the mean ± standard deviation.
Determination of aspartate aminotransferase and alanine aminotransferase levels in sera.
Mouse sera were collected at 18 or 72 h postinfection, and the concentrations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined using GOT-JS and GPT-JS kits (Denka Seiken Co., Ltd., Niigata, Japan), respectively, and an automated biochemical analyzer (model TBA-200FR; Toshiba Co., Tokyo, Japan) (33).
Determination of cytokine levels in sera and liver tissues.
Groups of four B6 mice were inoculated intragastrically with 5 × 108 K. pneumoniae NK-9 cells per mouse. Various concentrations of LiCl (10, 100, or 400 μg/ml) were administered orally with the drinking water immediately postinfection. At 18 or 72 h after infection, mouse serum or liver tissue homogenized in PBS was collected for the detection of cytokines. Inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), interleukin-1β (IL-1β), gamma interferon (IFN-γ), interleukin-12 p70 (IL-12 p70), and keratinocyte-derived chemokine (KC), and anti-inflammatory cytokines, including interleukin-10 (IL-10) and interleukin-13 (IL-13), in the sera of mice were assessed using BD Cytometric Bead Array (CBA) flex sets (BD Biosciences, San Jose, CA). With this detection system, the limit of cytokine detection was 5 pg/ml. In this intragastric infection model, IFN-γ, IL-12, IL-4, and IL-13 were not detected in either the sera or liver tissue homogenates after K. pneumoniae NK-9 infection.
Bacterial growth curves.
K. pneumoniae NK-9 was cultured in TSB for 18 h at 37°C and then subcultured in fresh broth (1:50, 1:100, 1:200, or 1:400 [vol/vol]) for 3 h. At the time of subculture, various concentrations of LiCl were added to the bacterial suspension, and bacterial growth at different time points was determined by measuring the absorbance at 600 nm with a spectrophotometer. For exact quantification of bacterial counts, the bacterial suspensions were serially diluted, plated on LB agar, and cultured overnight at 37°C (32). The results of one of three experiments are reported.
String test.
K. pneumoniae NK-9 was cultured overnight at 37°C on TSB agar plates containing various concentrations of LiCl (5 μM to 1 mM), and then the length of the sticky string from each single colony was determined by sticking using a standard inoculation loop (17). The average length of strings in each group was determined by examination of six single colonies and was expressed as the mean ± standard deviation.
Clearance of LiCl-pretreated K. pneumoniae by RAW264.7 cells.
K. pneumoniae NK-9 cells were cultured in TSB for 18 h at 37°C and then subcultured in fresh broth (1:50 [vol/vol]). Various concentrations of LiCl were added to the bacterial suspensions at the time of subculture, and cells were cultured for another 3 h. After the 3 h of incubation, LiCl-treated K. pneumoniae NK-9 cells were washed twice with PBS and then were used to infect RAW264.7 cells. RAW264.7 macrophage cells were grown at 37°C in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY) containing 5% fetal bovine serum (FBS) (HyClone; GE Healthcare Life Sciences, Piscataway, NJ). The RAW264.7 cells (4 × 105 cells/well) in 24-well culture plates were cocultured with K. pneumoniae NK-9 cells at a ratio of bacteria to RAW264.7 cells of 250:1. The plates were centrifuged for 5 min at 500 × g to enhance the contact of bacteria and cells. The cells were incubated for 2 h at 37°C to permit phagocytosis and infection. Following this, the free bacteria outside the cells were washed away with PBS, DMEM containing 5% FBS and 100 μg/ml gentamicin was added, and the cells were cultured for another 4 h or 14 h. At the indicated times, the cells were washed and hydrolyzed with 1 ml of sterile distilled water (pH 11) (35), and intracellular remnant bacteria were determined by plate counting. The results of three experiments are expressed as the mean ± standard deviation.
Detection of bactericidal activity of RAW264.7 cells following LiCl treatment.
RAW264.7 macrophage cells were grown in DMEM containing 5% FBS. RAW264.7 cells (4 × 105 cells/well) were cocultured with K. pneumoniae NK-9 cells at a ratio of bacteria to RAW264.7 cells of 200:1, in 24-well culture plates. The plates were centrifuged for 5 min at 500 × g to enhance the contact of bacteria and cells. The cells were incubated for 2 h at 37°C to permit phagocytosis and infection. Subsequently, the free bacteria outside the cells were washed away with PBS, DMEM containing 5% FBS, 100 μg/ml gentamicin, and various concentrations of LiCl was added, and the cells were cultured for another 4 h or 14 h. At the indicated times, the cells were washed and hydrolyzed with 1 ml of sterile distilled water (pH 11) (35), and intracellular remnant bacteria were determined by plate counting. The results of three experiments are expressed as the mean ± standard deviation.
Statistics.
The statistical analysis was carried out using Prism 3.0 software (GraphPad Software, San Diego, CA). For the mouse model in Fig. 1, survival curves were compared for significance using the log rank test. For the histological data in Fig. 2I, the significance of differences between the treatment groups was determined using the Wilcoxon signed-rank test. The data shown in the tables were compared for significance using analysis of variance (ANOVA). In Fig. 3 and 4, treatment groups were compared for significance using the t test. Statistical significance was set at a P value of <0.05.
FIG 1.
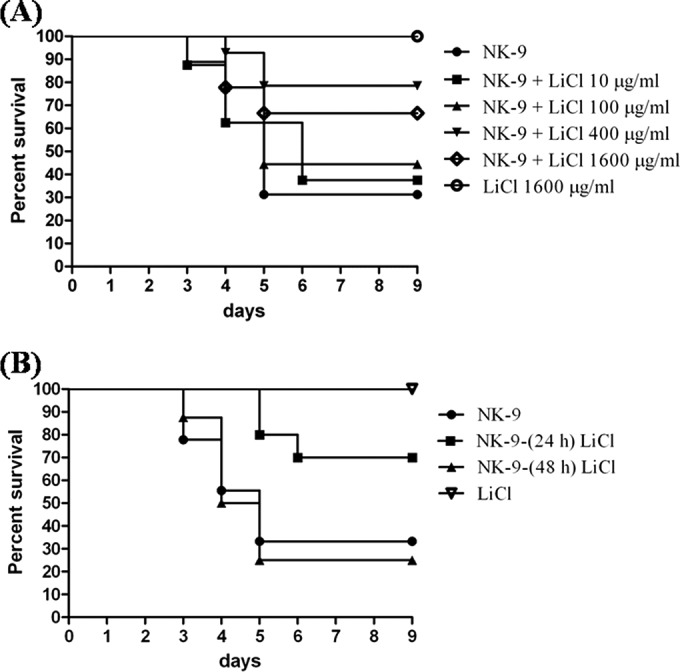
Inhibition of K. pneumoniae-induced death by LiCl. (A) Oral administration of LiCl inhibiting K. pneumoniae NK-9-induced death in B6 mice in a dose-dependent manner. Groups of eight to 16 B6 mice were inoculated, via the intragastric route, with 1 × 109 K. pneumoniae NK-9 cells per mouse. Various doses of LiCl were administered with the drinking water immediately postinfection. LiCl alone (1,600 μg/ml) had no effect on mice. Survival curves were compared for significance using the log rank test for the NK-9 plus LiCl (400 μg/ml) group versus the NK-9 group (P = 0.0087). (B) Therapeutic effects of LiCl against K. pneumoniae NK-9 infections in B6 mice. Groups of eight to 10 B6 mice were inoculated intragastrically with 1 × 109 K. pneumoniae NK-9 cells per mouse. LiCl (400 μg/ml) was administered with the drinking water at 24 h or 48 h postinfection. Survival curves were compared for significance using the log rank test for the NK-9 plus LiCl (24 h postinfection) group versus the NK-9 group (P = 0.048).
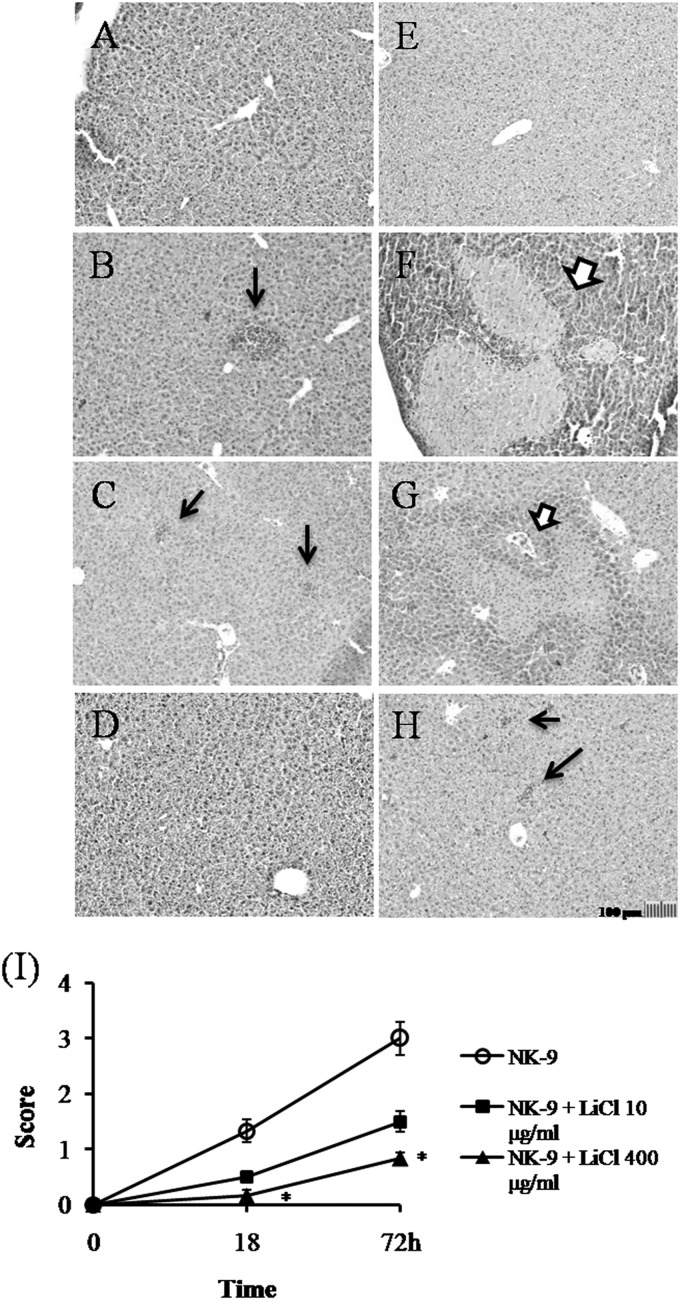
FIG 2.
Inhibition of K. pneumoniae-induced liver damage by LiCl. Groups of six B6 mice were inoculated intragastrically with 1 × 109 K. pneumoniae NK-9 cells per mouse. Various concentrations of LiCl were administered as described for Fig. 1A. The mice were sacrificed at 18 or 72 h postinfection, and liver sections were prepared as described in Materials and Methods. (A to H) Representative tissue sections. (A) Drinking water only, without K. pneumoniae, with sacrifice at 72 h. (B) Drinking water plus K. pneumoniae, with sacrifice at 18 h. (C) LiCl (10 μg/ml) plus K. pneumoniae, with sacrifice at 18 h. (D) LiCl (400 μg/ml) plus K. pneumoniae, with sacrifice at 18 h. (E) LiCl (400 μg/ml) without K. pneumoniae, with sacrifice at 72 h. (F) Drinking water plus K. pneumoniae, with sacrifice at 72 h. (G) LiCl (10 μg/ml) plus K. pneumoniae, with sacrifice at 72 h. (H) LiCl (400 μg/ml) plus K. pneumoniae, with sacrifice at 72 h. Thick arrows, necrotic regions; thin arrows, liver abscesses. Magnification, ×100. (I) Degree of liver inflammation determined by histological examination, as described in Materials and Methods. *, P < 0.05, compared with K. pneumoniae-treated mice.
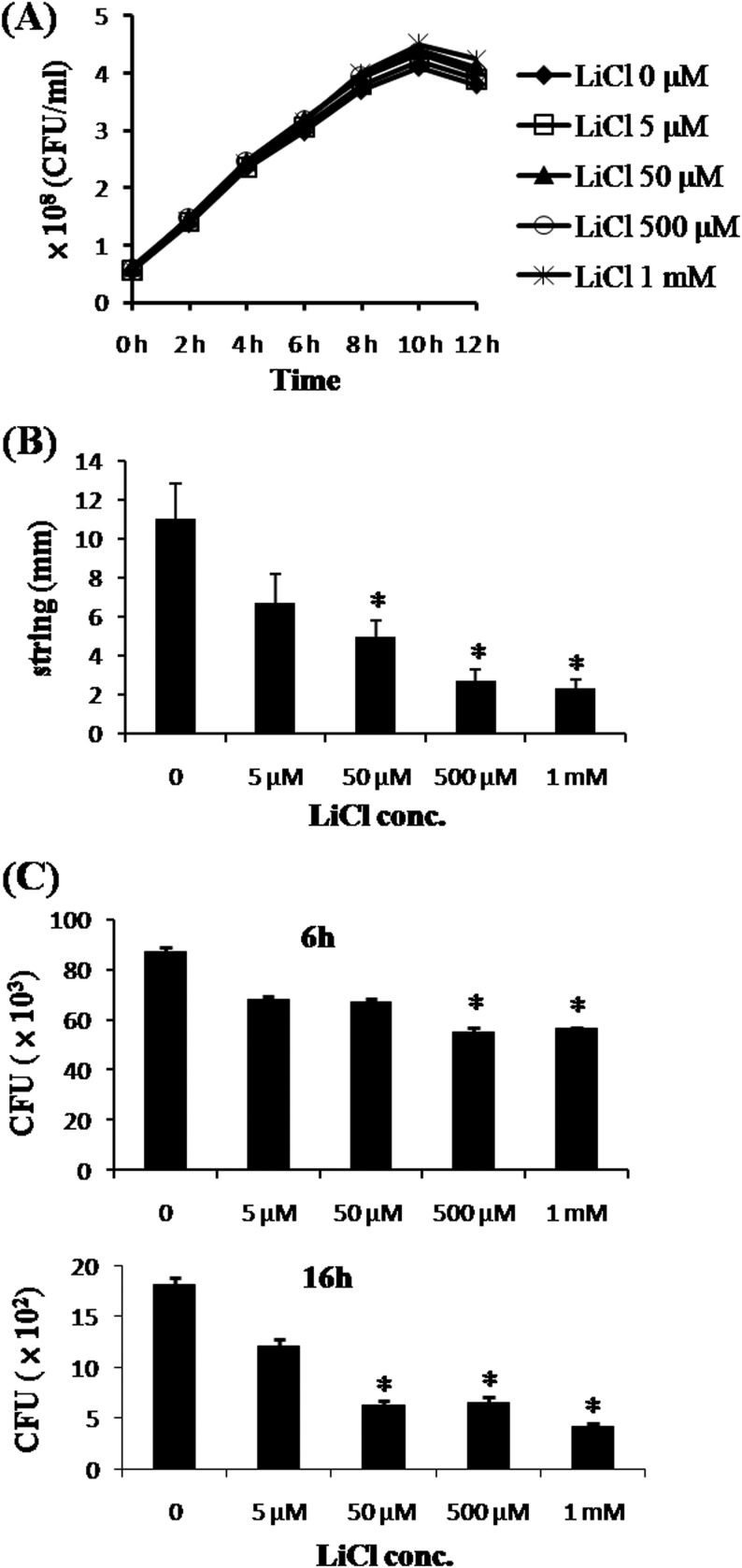
FIG 3.
In vitro effects of LiCl on K. pneumoniae NK-9 cells. (A) Effects of LiCl on the in vitro growth of K. pneumoniae. K. pneumoniae NK-9 cells (5.8 × 107 CFU/ml) were incubated with various concentrations of LiCl for different times, and the amounts of bacteria at each time point were determined by counting colonies on LB agar plates, as described in Materials and Methods. (B) Effects of LiCl on the mucoid phenotype of K. pneumoniae. K. pneumoniae NK-9 cells were cultured on agar plates containing various concentrations of LiCl, and the mucoid strings of the colonies were determined as described in Materials and Methods. (C) Clearance of LiCl-pretreated K. pneumoniae by macrophages. The same amounts of K. pneumoniae NK-9 cells were pretreated with various concentrations of LiCl for 3 h. Subsequently, LiCl-pretreated K. pneumoniae cells were infected for 2 h with RAW264.7 cells (4 × 105 cells/well) at a ratio of bacteria to RAW264.7 cells of 250:1. After removal of the extracellular bacteria with gentamicin, the live intracellular bacteria were quantitated at 6 or 16 h postinfection, as described in Materials and Methods. *, P < 0.05, compared with the K. pneumoniae-infected group not treated with LiCl.
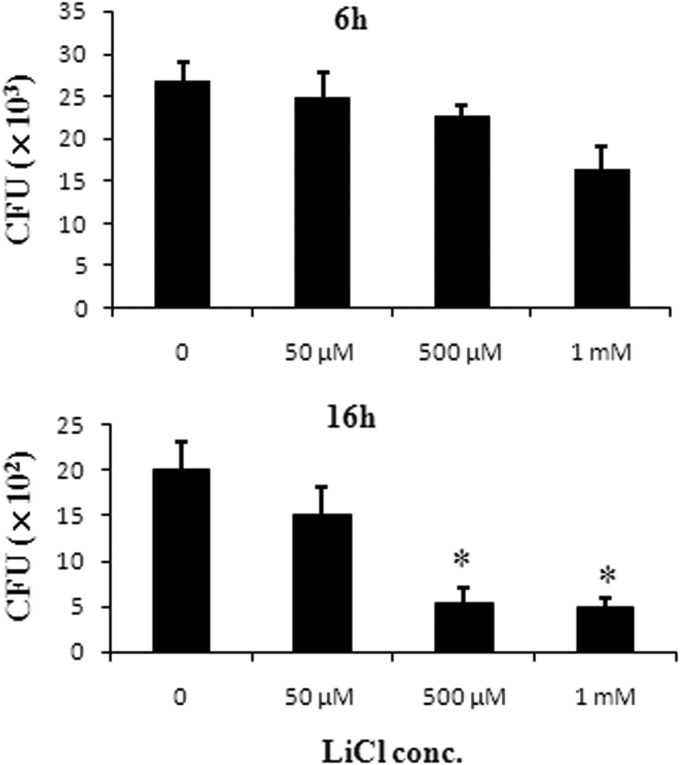
FIG 4.
Effects of LiCl on the bactericidal activity of macrophages. RAW264.7 cells (4 × 105 cells/well) were cocultured for 2 h with K. pneumoniae NK-9 cells at a ratio of bacteria to RAW264.7 cells of 200:1. Subsequently, the free bacteria outside the cells were washed, DMEM containing gentamicin and various concentrations of LiCl was added to the cells, and the cells were cultured for another 4 h or 14 h. At the indicated times, the cells were lysed and the live intracellular bacteria were counted as described in Materials and Methods. *, P < 0.05, compared with the group not treated with LiCl.
RESULTS
LiCl inhibits K. pneumoniae-induced death.
To examine the effects of LiCl on K. pneumoniae infections in mice, we chose to add LiCl to the drinking water (which is provided ad libitum for the mice) for 7 days instead of injecting it intragastrically, to reduce the emotional stress on the mice. Without treatment, approximately 70% of the mice (11 of 16 mice) died within 5 days after intragastric inoculation of 1 × 109 K. pneumoniae NK-9 cells (the LD70 for mice). In contrast, treatment with LiCl immediately following inoculation inhibited K. pneumoniae-induced death in a dose-dependent manner; 10 μg/ml LiCl resulted in 38% survival (3 of 8 mice), 100 μg/ml LiCl resulted in 44% survival (4 of 9 mice), and 400 μg/ml LiCl yielded 79% survival (11 of 14 mice). As the dose of LiCl was increased to 1,600 μg/ml, the survival rate fell to 67% (6 of 9 mice); the survival rate was higher than that of the K. pneumoniae-infected group (30%), and no statistically significant differences were observed, compared to the results for the K. pneumoniae-infected group. No mice died in the LiCl-only (1,600 μg/ml) control group (Fig. 1A). In addition, providing mice with LiCl-treated drinking water (400 μg/ml) 3 h before the infection resulted in 63% survival (5 of 8 mice) (data not shown). This result indicates that pretreatment with LiCl did not seem able to increase the survival rate of infected mice, compared to the results with LiCl given immediately following inoculation.
The consumption of drinking water by each LiCl-treated mouse was approximately 4 ± 0.4 ml per day and was dependent on mouse lethargy and not the dose of LiCl administered (data not shown). We monitored the concentrations of LiCl in the sera of infected mice that were treated with LiCl-containing water ad libitum at 72 h postinfection, and we found that the average concentrations of LiCl in the sera of the 400-μg/ml and 1,600-μg/ml LiCl-treated mice were 0.33 ± 0.04 mM and 0.61 ± 0.03 mM, respectively. Both of these concentrations were lower than the clinically acute toxic doses of LiCl (>1.2 mM) (36). To extrapolate the oral doses of LiCl, we treated mice with different concentrations of LiCl administered intragastrically, and the average concentrations of LiCl in the sera were determined 2 h after treatment. Intragastric administration of 20 mg/kg and 50 mg/kg of LiCl to mice resulted in concentrations of LiCl in the sera of 0.35 ± 0.02 mM and 0.57 ± 0.04 mM, respectively. These two oral doses of LiCl caused approximately equivalent serum concentrations of LiCl, compared with treatment with drinking water containing LiCl at 400 μg/ml and 1,600 μg/ml, respectively.
Histological examinations showed that the liver tissue had abscess formation at 18 h postinfection (Fig. 2B), and massive necrosis of hepatocytes appeared 72 h after K. pneumoniae NK-9 infection (Fig. 2F). However, treatment with 400 μg/ml of LiCl significantly inhibited abscess formation and necrosis of the liver tissue (Fig. 2D and H). Compared with the water-treated mice, no significant histological differences were found in the liver tissue of the LiCl-only control mice (Fig. 2A and E). The degree of inflammation in the liver tissues was quantitated by histological examination and is shown in Fig. 2I. Furthermore, there was a therapeutic effect of LiCl on K. pneumoniae infection. The addition of LiCl (400 μg/ml) to the drinking water for 7 days starting at 24 h postinfection protected 70% of the mice (7 of 10 mice) from death, while the addition of LiCl at 48 h postinfection had no protective effect (Fig. 1B). These results indicate that LiCl could inhibit K. pneumoniae-induced death in this intragastric infection model.
LiCl decreases bacterial burdens, liver damage, and cytokine production.
The bacterial counts in the liver and blood were quantitated further. After intragastric inoculation of 5 × 108 K. pneumoniae NK-9 cells, bacterial counts in both the liver (per gram of tissue) and blood (per ml) were approximately 105 CFU at 18 h, with the numbers increasing to 107 to 108 CFU at 72 h postinfection. In contrast, LiCl treatment significantly decreased the bacterial loads in both the liver and the blood, in a dose-dependent manner (Table 1). Liver functionality was also impaired, as shown by the increased levels of AST and ALT in the serum after K. pneumoniae NK-9 infection. Comparatively, the AST and ALT levels in the LiCl-treated mice were significantly inhibited (Table 2). Cytokine expression, including expression of the inflammatory cytokines TNF-α, IL-6, MCP-1, and KC and the anti-inflammatory cytokine IL-10, in the blood after K. pneumoniae NK-9 infection was elevated at 18 h, with the levels peaking at 72 h postinfection. However, LiCl treatment significantly inhibited cytokine production in the blood (Table 3). Interestingly, although there was no IL-1β expression in the blood, expression was induced in the liver tissue at 18 h postinfection; following LiCl treatment, the expression levels decreased (Table 4). Although the expression profiles of the cytokines were different in blood and liver tissue, they were all significantly inhibited by LiCl treatment (Tables 3 and 4). These results suggested that LiCl could decrease the bacterial burdens, liver impairment, and inflammation in K. pneumoniae NK-9-infected mice.
TABLE 1.
Bacterial counts in blood and liver tissues after LiCl treatment of K. pneumoniae infectiona
| Bacterial count (log10 CFU/ml) at: |
||||
|---|---|---|---|---|
| Treatment | 18 h |
72 h |
||
| Blood | Liver | Blood | Liver | |
| NK-9 | 5.0 ± 0.8 | 5.5 ± 0.5 | 7.7 ± 1.5 | 7.0 ± 1.0 |
| NK-9 + 10 μg/ml LiCl | 2.4 ± 0.3 | 4.5 ± 2.0 | 5.9 ± 0.9 | 5.3 ± 0.5 |
| NK-9 + 100 μg/ml LiCl | 2.4 ± 0.6 | 3.9 ± 0.4 | 3.9 ± 0.8b | 3.9 ± 0.2b |
| NK-9 + 400 μg/ml LiCl | <1 | 1.5 ± 0.5b | <1 | 1.5 ± 0.2b |
Groups of four mice were inoculated, via the intragastric route, with 5 × 108 K. pneumoniae NK-9 cells per mouse. Various concentrations of LiCl were administered immediately postinfection by addition to the drinking water. The mice were sacrificed at 18 or 72 h postinfection, and the bacterial counts were detected by plate counting. Data are presented as mean ± standard deviation. The statistical analysis was performed using repeated-measures ANOVA.
P < 0.05, compared with results for NK-9-treated mice.
TABLE 2.
Serum AST and ALT levels after LiCl treatment of K. pneumoniae infectiona
| Time (h) | AST level (U/liter) |
ALT level (U/liter) |
||||
|---|---|---|---|---|---|---|
| NK-9 | NK-9 + LiCl | LiCl | NK-9 | NK-9 + LiCl | LiCl | |
| 0 | 102 ± 18 | 114 ± 7 | 115 ± 5 | 43 ± 3 | 38 ± 8 | 41 ± 3 |
| 18 | 698 ± 36 | 188 ± 27b | 103 ± 8 | 375 ± 50 | 45 ± 10b | 38 ± 5 |
| 72 | 792 ± 85 | 220 ± 25b | 101 ± 7 | 412 ± 54 | 85 ± 13b | 42 ± 3 |
Groups of four mice were inoculated, via the intragastric route, with 5 × 108 K. pneumoniae NK-9 cells per mouse. LiCl (400 μg/ml) was administered immediately postinfection by addition to the drinking water. The mice were sacrificed at 0, 18, or 72 h postinfection, sera were collected, and AST and ALT levels were measured. Data are presented as mean ± standard deviation. The statistical analysis was performed using repeated-measures ANOVA.
P < 0.05, compared with results for NK-9-treated mice.
TABLE 3.
Cytokine expression in blood after LiCl treatment of K. pneumoniae infectiona
| Treatment | Cytokine level (pg/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 h |
72 h |
|||||||||||
| TNF-α | IL-6 | MCP-1 | KC | IL-1β | IL-10 | TNF-α | IL-6 | MCP-1 | KC | IL-1β | IL-10 | |
| None | <5b | <5b | <10b | 22 ± 10 | <5b | <5b | <5b | <5b | <10b | 22 ± 10 | <5b | <5b |
| 400 μg/ml LiCl | <5b | <5b | <10b | 23 ± 8 | <5b | <5b | <5b | <5b | <10b | 25 ± 8 | <5b | <5b |
| NK-9 | 164 ± 10 | 888 ± 68 | 954 ± 216 | 878 ± 49 | <5b | 46 ± 11 | 251 ± 15 | 15,898 ± 99 | 988 ± 216 | 2,461 ± 98 | <5b | 630 ± 55 |
| NK-9 + 10 μg/ml LiCl | 29 ± 10 | 364 ± 89 | 1,281 ± 87 | 350 ± 98 | <5b | 12 ± 5 | 80 ± 11 | 860 ± 279 | 398 ± 78 | 521 ± 98 | <5b | 23 ± 15 |
| NK-9 + 100 μg/ml LiCl | 33 ± 2 | 273 ± 95 | 868 ± 49 | 430 ± 49 | <5b | 16 ± 3 | 59 ± 8 | 1,019 ± 106 | 152 ± 58 | 596 ± 23 | <5b | 17 ± 5 |
| NK-9 + 400 μg/ml LiCl | 13 ± 3c | 90 ± 11c | 299 ± 30c | 187 ± 21c | <5b | <5b | 5 ± 1c | 21 ± 5c | 18 ± 4c | 53 ± 10c | <5b | <5b |
Groups of four mice were inoculated, via the intragastric route, with 5 × 108 K. pneumoniae NK-9 cells per mouse. Various concentrations of LiCl were administered immediately postinfection by addition to the drinking water. The mice were sacrificed at 18 or 72 h postinfection, and cytokine expression in sera was detected with CBA flex sets. Data are presented as mean ± standard deviation. The statistical analysis was performed using repeated-measures ANOVA.
Minimal cytokine concentration detected using a CBA flex kit.
P < 0.05, compared with results for NK-9-treated mice.
TABLE 4.
Cytokine expression in liver tissues after LiCl treatment of K. pneumoniae infectiona
| Treatment | Cytokine level (pg/ml) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 h |
72 h |
|||||||||||
| TNF-α | IL-6 | MCP-1 | KC | IL-1β | IL-10 | TNF-α | IL-6 | MCP-1 | KC | IL-1β | IL-10 | |
| None | 6 ± 1 | <5b | <10b | <10b | <5b | 13 ± 8 | 6 ± 1 | <5b | <10b | <10b | <5b | 13 ± 8 |
| 400 μg/ml LiCl | 6 ± 1 | <5b | 11 ± 1 | <10b | <5b | 12 ± 6 | 7 ± 2 | <5b | <10b | <10b | <5b | 15 ± 3 |
| NK-9 | 113 ± 15 | 18 ± 5 | 1,417 ± 89 | 333 ± 78 | 197 ± 30 | 53 ± 6 | 41 ±10 | 114 ± 10 | 649 ± 78 | 428 ± 50 | 42 ± 5 | 60 ± 10 |
| NK-9 + 10 μg/ml LiCl | 45 ± 10 | 7 ± 2 | 971 ± 78 | 163 ± 23 | 54 ± 18 | 27 ± 6 | 38 ± 11 | 20 ± 7 | 327 ± 20 | 242 ± 32 | 39 ± 5 | 43 ± 6 |
| NK-9 + 100 μg/ml LiCl | 43 ± 5 | 7 ± 2 | 918 ± 58 | 89 ± 70 | 42 ± 28 | 23 ± 4 | 29 ± 2 | 18 ± 9 | 286 ± 33 | 166 ± 34 | 37 ± 5 | 32 ± 5 |
| NK-9 + 400 μg/ml LiCl | 19 ± 3c | <5b | 362 ± 60c | 73 ± 18c | 46 ± 6c | 14 ± 2c | 16 ± 2c | <5b | 49 ± 10c | 13 ± 3c | 12 ± 6c | 18 ± 5c |
Groups of four mice were inoculated, via the intragastric route, with 5 × 108 K. pneumoniae NK-9 cells per mouse. Various concentrations of LiCl were administered immediately postinfection by addition to the drinking water. The mice were sacrificed at 18 or 72 h postinfection, and cytokine expression in liver homogenates was detected with CBA flex sets. Data are presented as mean ± standard deviation. The statistical analysis was performed using repeated-measures ANOVA.
Minimal cytokine concentration detected using a CBA flex kit.
P < 0.05, compared with results for NK-9-treated mice.
LiCl makes K. pneumoniae NK-9 cells more susceptible to macrophage killing.
To examine the in vitro growth of K. pneumoniae NK-9 cells with LiCl, we subcultured bacterial suspensions with fresh TSB at a 1:50 dilution, and different concentrations of LiCl were added and incubated for various times. LiCl had no direct antimicrobial activity with respect to in vitro growth of K. pneumoniae NK-9 cells in TSB cultures (Fig. 3A). Even when the initial bacterial concentration was decreased by increasing the dilution with fresh TSB to 1:400, LiCl (5 μM to 1 mM) had no direct effects on the growth curves of K. pneumoniae NK-9 cells (data not shown). The K. pneumoniae NK-9 strain has a hypermucoviscous phenotype, which might be indicative of the extent of capsular polysaccharide expression and related to resistance to phagocytosis (17). Using the string test, we found that LiCl dose-dependently caused K. pneumoniae NK-9 cells to lose the mucoid phenotype (Fig. 3B). Moreover, pretreatment of K. pneumoniae NK-9 cells with LiCl caused K. pneumoniae to be more susceptible to macrophage killing, in a dose-dependent manner, at both 6 h and 16 h postinfection (Fig. 3C). These results indicate that LiCl could modify capsular polysaccharides of K. pneumoniae that are involved in resistance to macrophage killing while not affecting the growth in vitro.
LiCl partially enhances macrophage bactericidal activity.
The effect of LiCl on macrophage bactericidal activity was examined next. A previous study indicated that LiCl (>3 mM) induced macrophage apoptosis (37). We first examined the effects of LiCl (5 μM to 2 mM) on the viability of RAW264.7 cells in 24-h cultures, and we found that concentrations of LiCl lower than 2 mM could not induce macrophage death (data not shown). To test the effects of LiCl on the bactericidal activity of macrophages, we infected RAW264.7 cells with K. pneumoniae NK-9 cells for 2 h, washed the extracellular bacteria, and killed them using gentamicin. We added various concentrations of LiCl, and the bactericidal activity of the macrophages was examined by counting the number of surviving intracellular bacteria. As shown in Fig. 4, LiCl treatment was able to partially decrease the live bacterial counts within the macrophages, indicating that it could enhance the bactericidal activity of macrophages.
DISCUSSION
A predominant, community-acquired, primary K. pneumoniae type causing liver abscesses has emerged in Asia over the past 2 decades (2–8). Its complications are associated with high mortality rates, especially in metastatic meningitis (17–19), and severe visual deficits, especially in endophthalmitis (17). Early diagnosis and antibiotic treatment can decrease K. pneumoniae-induced mortality rates and visual or brain damage (17). In Taiwan, most of the K. pneumoniae strains isolated from primary liver abscesses are susceptible to cephalosporins and aminoglycosides (2, 17) but constitutively resistant to amoxicillin and ampicillin (38). However, nosocomial liver abscesses caused by extended-spectrum β-lactamase-producing K. pneumoniae have been reported (20). Moreover, the frequency of K. pneumoniae isolates that are resistant to carbapenems and third-generation cephalosporins is increasing in all regions of the United States (21). Hence, it is of utmost importance to search for an alternative anti-K. pneumoniae agent. In this study, we demonstrated that a subtoxic dose of LiCl, a widely prescribed drug, could effectively inhibit K. pneumoniae-induced liver abscesses and death in mice by modulating K. pneumoniae itself, decreasing systemic inflammation, and partially enhancing the bactericidal activity of macrophages.
Use of LiCl to treat infections has been reported previously for both in vitro and in vivo models (39–47). With regard to virus infections, LiCl has been found not only to inhibit replication of the herpes simplex viruses (39) and pseudorabies herpesviruses (40) directly in in vitro assays but also to decrease the infective capacity of porcine and avian coronaviruses in host cells (41). In a rat infection model, a combination of diminazene aceturate, an anti-Trypanosoma drug, and LiCl could provide a greater therapeutic effect than diminazene aceturate alone in preventing relapse infections with Trypanosoma brucei (42). Moreover, LiCl has been reported to suppress Plasmodium berghei parasitemia (43) and to restore Plasmodium falciparum-induced neurocognitive damage in a murine model (44). With regard to bacterial infections, LiCl has been shown to decrease mortality rates, to various degrees, in Francisella tularensis-infected (45), Streptococcus pyogenes-infected (46), and Burkholderia pseudomallei-infected (47) mice. Although the inhibitory effects of LiCl against infections have been demonstrated in a variety of models, the detailed mechanisms in these different infective models are not clearly understood.
LiCl has been used in the treatment of bipolar disorders for a long time, but it has limitations due to its adverse effects, such as damage to renal, thyroid, and parathyroid functions, teratogenicity, and weight gain (36) with long-term treatment. Safe therapeutic serum concentrations of lithium, with minimal side effects with long-term administration, for the treatment of human bipolar disorders are 0.4 to 0.8 mM, while serum concentrations over 1.2 mM LiCl can cause acute toxicity (36). In our model, by adding LiCl to the drinking water, we demonstrated that the most effective dose of LiCl in K. pneumoniae-infected mice was 400 μg per ml, which corresponded to a serum concentration of 0.33 mM. Even when the LiCl dose in the water was up to 1,600 μg per ml, corresponding to 0.61 mM LiCl in the serum, the survival rate of K. pneumoniae-infected mice was not increased (Fig. 1A). These results indicated that the most appropriate serum concentration of LiCl, which provided protection against K. pneumoniae infection, was limited and lower than that used for treatment of bipolar disorders. Similar results have been reported for the B. pseudomallei mouse model (47). Tay et al. indicated that intraperitoneal administration of LiCl (100 mg/kg) could provide the best protection against B. pseudomallei infection, while the survival rate fell with doses of up to 200 mg/kg LiCl (47).
Based on our results, we demonstrated that a subtherapeutic dose of LiCl was able to control bacterial burdens and, as a result, to inhibit local liver damage, systemic inflammation, and death in mice. The effects of LiCl on bacterial burdens can be divided into two parts. The first is the direct effect of LiCl on K. pneumoniae NK-9 cells. Previous studies indicated that high doses of LiCl (>100 mM) could alter bacterial cell walls and thus viability (48, 49). As shown in Fig. 3A, low doses of LiCl did not affect the in vitro growth curve of K. pneumoniae. However, they could modify the expression of capsular polysaccharides of K. pneumoniae and made the bacteria more susceptible to killing by macrophages (Fig. 3B and C). Capsular polysaccharides and lipopolysaccharides are two important virulence factors of K. pneumoniae (1) that interact with the PRRs on innate cells, and these interactions stimulate innate cells to release cytokines and other mediators (50). In addition to capsular polysaccharides, we cannot rule out subtle modifications of cell walls by low doses of LiCl, and the detailed mechanisms involved in the modification of K. pneumoniae capsules or cell walls by low doses of LiCl need further investigation.
The second effect of LiCl on bacterial burdens is its partial enhancement of the bactericidal activity of macrophages. A previous study indicated that LiCl (>3 mM) induced selective macrophage apoptosis in atherosclerotic plaques through inhibition of inositol monophosphatase (37). In our study, we found that LiCl (5 μM to 1 mM) alone could not induce macrophage death (data not shown). After K. pneumoniae infection, LiCl treatment enhanced the bactericidal activity of macrophages (Fig. 4). The detailed mechanisms are not well understood, but our unpublished data indicated that low doses of LiCl could inhibit K. pneumoniae-infected cell death. By inhibiting the death of macrophages, their killing activity would be enhanced. Controversial results regarding the effects of lithium on phagocytes and their bactericidal activity have been noted in humans and in animal models (51–53). The results from in vitro assays showed that lithium increases the numbers of leukocytes but decreases the bactericidal capacity of granulocytes in humans (51). However, another study indicated that lithium can enhance the phagocytic activity of leukocytes through inhibition of adenylate cyclase and cyclic AMP production (52). An in vivo study demonstrated that lithium stimulated the phagocytic functions of neutrophils in normal control animals but inhibited phagocytic activity in infected animals (53). Because most studies used relevant LiCl serum concentrations higher than 1 mM, we hypothesize that the lithium concentrations and assay methods used in the different models could be the cause of the controversial results.
In the F. tularensis infection model, Zhang et al. indicated that LiCl-mediated inhibition occurred through inhibition of GSK3β, with decreasing production of proinflammatory cytokines and increasing production of anti-inflammatory cytokines such as IL-10 in sera to prevent mice from undergoing septic shock (45). Based on our results shown in Tables 3 and 4, we found that intragastric infection by K. pneumoniae induced production of both inflammatory and anti-inflammatory cytokines in both sera and liver tissues. After LiCl treatment, both were inhibited in a dose-dependent manner. Enhanced IL-10 production was not found in either the sera or liver tissues after LiCl treatment. These data indicate that the inhibitory effects of LiCl on K. pneumoniae infections could be independent of anti-inflammatory cytokine production. However, we did not exclude the possibility that the levels of IL-10 or other anti-inflammatory mediators could be increased in other tissues, such as the spleen and lungs, as that has been reported for B. pseudomallei-infected mice following LiCl treatment (47). Using an in vitro infection assay, we found that the GSK3β activity in macrophages was inhibited by low doses of LiCl (unpublished data). Because LiCl has several targets (23–29), the detailed molecular mechanisms of the effects of LiCl on macrophage functions against K. pneumoniae infections need to be further examined. In conclusion, in addition to its previously reported anti-inflammatory effects, we suggest that nontoxic doses of LiCl were able to prevent K. pneumoniae infections in mice through modulation of K. pneumoniae itself and enhancement of the bactericidal activity of macrophages.
ACKNOWLEDGMENTS
This work was supported by grants NSC101-2320-B214-004, MOST102-2320-B214-006, and MOST103-2320-B214-006 from the Ministry of Science and Technology, Taiwan.
REFERENCES
- 1.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, Wann SR, Lin HH. 1998. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 26:1434–1438. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 3.Hu BS, Lau YJ, Shi ZY, Lin YH. 1999. Necrotizing fasciitis associated with Klebsiella pneumoniae liver abscess. Clin Infect Dis 29:1360–1361. doi: 10.1086/313471. [DOI] [PubMed] [Google Scholar]
- 4.Lai YC, Yang SL, Peng HL, Chang HY. 2000. Identification of genes present specifically in a virulent strain of Klebsiella pneumoniae. Infect Immun 68:7149–7151. doi: 10.1128/IAI.68.12.7149-7151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau YJ, Hu BS, Wu WL, Lin YH, Chang HY, Shi ZY. 2000. Identification of a major cluster of Klebsiella pneumoniae isolates from patients with liver abscess in Taiwan. J Clin Microbiol 38:412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung CP, Chang FY, Lee SC, Hu BS, Kuo BT, Liu CY, Siu LK. 2002. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50:420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmori S, Shiraki K, Ito K, Inoue H, Ito T, Sakai T, Takase K, Nakano T. 2002. Septic endophthalmitis and meningitis associated with Klebsiella pneumoniae liver abscess. Hepatol Res 22:307–312. doi: 10.1016/S1386-6346(01)00153-X. [DOI] [PubMed] [Google Scholar]
- 8.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. 2004. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahill M, Chang B, Murray A. 2000. Bilateral endogenous bacterial endophthalmitis associated with pyogenic hepatic abscess. Br J Ophthalmol 84:1436. doi: 10.1136/bjo.84.12.1432e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lederman ER, Crum NF. 2005. Pyrogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol 100:322–331. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 11.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. 1995. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol 177:1788–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merino S, Altarriba M, Izquierdo L, Nogueras MM, Regue M, Tomas JM. 2000. Cloning and sequencing of the Klebsiella pneumoniae O5 wb gene cluster and its role in pathogenesis. Infect Immun 68:2435–2440. doi: 10.1128/IAI.68.5.2435-2440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arakawa Y, Ohta M, Wacharotayankun R, Mori M, Kido N, Ito H, Komatsu T, Sugiyama T, Kato N. 1991. Biosynthesis of Klebsiella K2 capsular polysaccharide in Escherichia coli HB101 requires the functions of rmpA and the chromosomal cps gene cluster of the virulent strain Klebsiella pneumoniae Chedid (O1:K2). Infect Immun 59:2043–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortés G, de Astorza B, Benedi VJ, Alberti S. 2002. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect Immun 70:4772–4776. doi: 10.1128/IAI.70.9.4772-4776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT. 2006. Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 193:645–654. doi: 10.1086/499968. [DOI] [PubMed] [Google Scholar]
- 16.Wu KM, Li LH, Yan JJ, Tsao N, Liao TL, Tsai HC, Fung CP, Chen HJ, Liu YM, Wang JT, Fang CT, Chang SC, Shu HY, Liu TT, Chen YT, Shiau YR, Lauderdale TL, Su IJ, Kirby R, Tsai SF. 2009. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol 191:4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. 2012. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 18.Fang CT, Chen YC, Chang SC, Shau WY, Luh KT. 2000. Klebsiella pneumoniae meningitis: timing of antimicrobial therapy and prognosis. QJM 93:45–53. doi: 10.1093/qjmed/93.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, McCormack JG, Yu VL. 2002. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 8:160–166. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JC, Siu LK, Fung CP, Yeh KM, Chang FY. 2007. Nosocomial liver abscess caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. J Clin Microbiol 45:266–269. doi: 10.1128/JCM.01413-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braykov NP, Eber MR, Klein EY, Morgan DJ, Laxminarayan R. 2013. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999-2010. Infect Control Hosp Epidemiol 34:259–268. doi: 10.1086/669523. [DOI] [PubMed] [Google Scholar]
- 22.Kumar H, Kawai T, Akira S. 2009. Pathogen recognition in the innate immune response. Biochem J 420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 23.Freland L, Beaulieu JM. 2012. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front Mol Neurosci 5:14. doi: 10.3389/fnmol.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berridge MJ, Downes CP, Hanley MR. 1989. Neural and development actions of lithium: a unifying hypothesis. Cell 59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 25.Spiegelberg BD, Dela Cruz J, Law TH, York JD. 2005. Alteration of lithium pharmacology through manipulation of phosphoadenosine phosphate metabolism. J Biol Chem 280:5400–5405. doi: 10.1074/jbc.M407890200. [DOI] [PubMed] [Google Scholar]
- 26.Rapoport SI, Bosetti F. 2002. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry 59:592–596. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- 27.Klein PS, Melton DA. 1996. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stambolic V, Ruel L, Woodgett JR. 1996. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signaling in intact cells. Curr Biol 6:1664–1668. doi: 10.1016/S0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 29.Phiel CJ, Klein PS. 2001. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol 41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- 30.Beurel E, Michalek SM, Jope RS. 2010. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol 31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortés-Vieyra R, Bravo-Patiño A, Valdez-Alarcón JJ, Juárez MC, Finlay BB, Baizabal-Aguirre VM. 2012. Role of glycogen synthase kinase-3 beta in the inflammatory response caused by bacterial pathogens. J Inflamm (Lond) 9:23. doi: 10.1186/1476-9255-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo CF, Wang YH, Lei HY, Wang CH, Tsao N. 2007. Concanavalin A protects mice from a lethal inoculation of intragastric Klebsiella pneumoniae and reduces the induced liver damage. Antimicrob Agents Chemother 51:3122–3130. doi: 10.1128/AAC.01379-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hung CH, Kuo CF, Wang CH, Wu CM, Tsao N. 2011. Experimental phage therapy in treating Klebsiella pneumoniae-mediated liver abscesses and bacteremia in mice. Antimicrob Agents Chemother 55:1358–1365. doi: 10.1128/AAC.01123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapoteau E, Czech BP, Zazulak W, Kumar A. 1992. First practical colorimetric assay of lithium in serum. Clin Chem 38:1654–1657. [PubMed] [Google Scholar]
- 35.Decleva E, Menegazzi R, Busetto S, Patriarca P, Dri P. 2006. Common methodology is inadequate for studies on the microbicidal activity of neutrophils. J Leukoc Biol 79:87–94. doi: 10.1189/jlb.0605338. [DOI] [PubMed] [Google Scholar]
- 36.Malhi GS, Berk M. 2012. Is the safety of lithium no longer in the balance? Lancet 379:690–692. doi: 10.1016/S0140-6736(11)61703-0. [DOI] [PubMed] [Google Scholar]
- 37.De Meyer I, Martinet W, Van Hove CE, Schrijvers DM, Hoymans VY, Van Vaeck L, Fransen P, Bult H, De Meyer GR. 2011. Inhibition of inositol monophosphatase by lithium chloride induces selective macrophage apoptosis in atherosclerotic plaques. Br J Pharmacol 162:1410–1423. doi: 10.1111/j.1476-5381.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang SC, Fang CT, Hsueh PR, Chen YC, Luh KT. 2000. Klebsiella pneumoniae isolates causing liver abscess in Taiwan. Diagn Microbiol Infect Dis 37:279–284. doi: 10.1016/S0732-8893(00)00157-7. [DOI] [PubMed] [Google Scholar]
- 39.Ziaie Z, Kefalides NA. 1989. Lithium chloride restores host protein synthesis in herpes simplex virus-infected endothelial cells. Biochem Biophys Res Commun 160:1073–1078. doi: 10.1016/S0006-291X(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 40.Sui X, Yin J, Ren X. 2010. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res 85:346–353. doi: 10.1016/j.antiviral.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren X, Meng F, Yin J, Li G, Li X, Wang C, Herrler G. 2011. Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis coronavirus. PLoS One 6:e18669. doi: 10.1371/journal.pone.0018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odika IE, Asuzu IU, Anika SM. 1995. The chemotherapeutic efficacy of diminazene aceturate and lithium chloride against relapse infection of Trypanosoma brucei in rats. Trop Med Parasitol 46:99–102. [PubMed] [Google Scholar]
- 43.Zakaria NA, Embi N, Sidek HM. 2010. Suppression of Plasmodium berghei parasitemia by LiCl in an animal infection model. Trop Biomed 27:624–631. [PubMed] [Google Scholar]
- 44.Dai M, Freeman B, Shikani HJ, Bruno FP, Collado JE, Macias R, Reznik SE, Davies P, Spray DC, Tanowitz HB, Weiss LM, Desruisseaux MS. 2012. Altered regulation of Akt signaling with murine cerebral malaria, effects on long-term neuro-cognitive function, restoration with lithium treatment. PLoS One 7:e44117. doi: 10.1371/journal.pone.0044117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P, Katz J, Michalek SM. 2009. Glycogen synthase kinase-3β (GSK3β) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Mol Immunol 46:677–678. doi: 10.1016/j.molimm.2008.08.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang YT, Chen CL, Lin CF, Lu SL, Cheng MH, Kuo CF, Lin YS. 2013. Regulatory role of GSK-3β on NF-κB, nitric oxide, and TNF-α in group A streptococcal infection. Mediators Inflamm 2013:720689. doi: 10.1155/2013/720689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tay TF, Maheran M, Too SL, Hasidah MS, Ismail G, Embi N. 2012. Glycogen synthase kinase-3β inhibition improved survivability of mice infected with Burkholderia pseudomallei. Trop Biomed 29:551–567. [PubMed] [Google Scholar]
- 48.Pavlovich NV, Tkacheva TI. 1990. Natural penicillin resistance of Francisella tularensis. Antibiot Khimioter 35:25–28. (In Russian.) [PubMed] [Google Scholar]
- 49.Scolari G, Vescovo M, Zacconi C, Vescovi F. 2006. Extraction and partial characterization of proteolytic activities from the cell surface of Lactobacillus helveticus Zuc2. J Dairy Sci 89:3800–3809. doi: 10.3168/jds.S0022-0302(06)72421-3. [DOI] [PubMed] [Google Scholar]
- 50.Yang FL, Yang YL, Liao PC, Chou JC, Tsai KC, Yang AS, Sheu F, Lin TL, Hsieh PF, Wang JT, Hua KF, Wu SH. 2011. Structure and immunological characterization of the capsular polysaccharide of a pyrogenic liver abscess caused by Klebsiella pneumoniae: activation of macrophages through Toll-like receptor 4. J Biol Chem 286:21041–21051. doi: 10.1074/jbc.M111.222091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedenberg WA, Marx JJ. 1980. The effect of lithium carbonate on granulocyte and platelet function. Cancer 45:91–97. doi:. [DOI] [PubMed] [Google Scholar]
- 52.Perez HD, Kaplan HB, Goldstein IM, Shenkman L, Borkowsky W. 1980. Reversal of an abnormality of polymorphonuclear leukocyte chemotaxis with lithium. Clin Immunol Immunopathol 16:308–315. doi: 10.1016/0090-1229(80)90136-1. [DOI] [PubMed] [Google Scholar]
- 53.Türközkan N, Durmus O, Boran N. 1993. Biochemical investigation of leukocyte functions during lithium therapy. Int J Biochem 25:1501–1504. doi: 10.1016/0020-711X(93)90697-D. [DOI] [PubMed] [Google Scholar]