Abstract
Rapid identification of microorganisms and antimicrobial resistance is paramount for targeted treatment in serious bloodstream infections (BSI). The Verigene Gram-negative blood culture nucleic acid test (BC-GN) is a multiplex, automated molecular diagnostic test for identification of eight Gram-negative (GN) organisms and resistance markers from blood culture with a turnaround time of approximately 2 h. Clinical isolates from adult patients at the University Maryland Medical Center with GN bacteremia from 1 January 2012 to 30 June 2012 were included in this study. Blood culture bottles were spiked with clinical isolates, allowed to incubate, and processed by BC-GN. A diagnostic evaluation was performed. In addition, a theoretical evaluation of time to effective and optimal antibiotic was performed, comparing actual antibiotic administration times from chart review (“control”) to theoretical administration times based on BC-GN reporting and antimicrobial stewardship team (AST) review (“intervention”). For organisms detected by the assay, BC-GN correctly identified 95.6% (131/137), with a sensitivity of 97.1% (95% confidence interval [CI], 90.7 to 98.4%) and a specificity of 99.5% (95% CI, 98.8 to 99.8%). CTX-M and OXA resistance determinants were both detected. Allowing 12 h from Gram stain for antibiotic implementation, the intervention group had a significantly shorter duration to both effective (3.3 versus 7.0 h; P < 0.01) and optimal (23.5 versus 41.8 h; P < 0.01) antibiotic therapy. BC-GN with AST intervention can potentially decrease time to both effective and optimal antibiotic therapy in GN BSI.
INTRODUCTION
Rapid identification of microorganism and antimicrobial resistance is paramount for targeted treatment in serious bloodstream infections (BSI). Clinical microbiology laboratories typically require 24 to 72 h to determine the identity and antimicrobial susceptibility of an organism in BSI. In particular, Gram-negative (GN) bacteremia with inappropriate antibiotic therapy is associated with a high mortality rate (1). Furthermore, due to emergence of multidrug-resistant (MDR) GN organisms, such as carbapenem-resistant Enterobacteriaceae (CRE), early targeted treatment is favored for both preservation of broad-spectrum antibiotics and ensuring correct treatment of the pathogen.
The Verigene Gram-negative blood culture nucleic acid test (BC-GN; Nanosphere, Northbrook, IL) is a multiplex, automated molecular diagnostic test for the identification of genus, species, and genetic resistance determinants for a broad panel of the most common GN organisms isolated from blood culture, with an approximate turnaround time of 2 h from blood culture positivity (2). The assay is capable of detecting Escherichia coli, Shigella spp., Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Acinetobacter spp., Proteus spp., Citrobacter spp., and Enterobacter spp., as well as the presence of multiple resistance markers (KPC, NDM, CTX-M, VIM, IMP, and OXA-23, -40, -48, and -58).
BC-GN performance has recently been demonstrated, with a high sensitivity and specificity for organism and resistance identification (3–7). The wide variety of resistance markers included within BC-GN may be a distinct advantage over some of the currently available technologies (8). However, there are no data evaluating the impact of BC-GN implementation on patient outcomes. It has been demonstrated that antimicrobial stewardship team (AST) intervention alone and in combination with other rapid testing diagnostics improves patient outcomes compared to standard microbiology reporting (9, 10). The aim of our study was to assess the potential impact of BC-GN in combination with AST intervention on antimicrobial therapy-related outcomes.
MATERIALS AND METHODS
Study location and design.
This single-center study was conducted at the University of Maryland Medical Center (UMMC), an 850-bed tertiary care center located in downtown Baltimore, MD. In addition to routine medical and surgical services, the UMMC offers specialized care, including a trauma center, solid-organ and bone marrow transplantation, and 6 subspecialty medical and surgical intensive care units (ICUs).
Adult patients (≥18 years-old) with a first episode of GN bacteremia (excluding polymicrobial infections) during the period from 1 January 2012 to 30 June 2012 were identified from the UMMC central data repository, a relational database containing demographic, administrative, and microbiology data on all patients. Of these, patients who had Gram stain results reported prior to discharge or death, and whose archived isolates could be accessed, were included in the study cohort.
Cohort and chart abstraction.
Chart review was performed for type of infection, antibiotic type and time of administration, ICU admission, infectious disease consult, comorbidities, and disposition. In addition, detailed microbiological data were collected, such as organism identification and susceptibility profile and date and time of the following: blood culture draw, Gram stain, identification, and susceptibility report. Standard laboratory techniques during the study period included Vitek 2 (bioMérieux, Durham, NC) for organism identification and Kirby-Bauer disk diffusion for susceptibility reporting. Antibiotic susceptibilities were determined by following Clinical and Laboratory Standards Institute guidelines (11).
Laboratory procedures. (i) Spiked blood cultures.
Based on the included cases, frozen, archived clinical isolates (−80°C) were pulled and subcultured on blood agar plates. Starting with a 0.5 McFarland standard suspension of organism, 3 successive 100-fold dilutions were made for a final concentration of 1.5 × 102 CFU/ml. Bactec Standard/10 Aerobic/F blood culture bottles (Becton Dickinson, Sparks, MD) were inoculated with clinical isolates, along with 8 ml of banked packed red blood cells, and placed in a Bactec 9050 system until positivity was established.
(ii) Verigene BC-GN.
After positivity, blood culture samples were prepared and processed with BC-GN according to the manufacturer's instructions (2). External quality controls were run daily and included a known positive and negative blood sample. Testing was repeated for “No call” results once that same day. Any samples with discrepant results were retested on the Vitek 2 (original clinical isolate and spiked blood culture isolate) and sent out for additional testing, such as PCR, sequencing, and/or matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF).
(iii) Molecular determination of resistance mechanism.
All isolates with resistance determinants were confirmed with PCR. In addition, PCR was performed on isolates with susceptibility profiles consistent with resistance to ceftriaxone and/or ertapenem/meropenem in which no resistance marker was detected by BC-GN (12–14).
Theoretical antibiotic therapy comparison.
A theoretical evaluation of two antimicrobial therapy-related outcomes (time to effective antibiotic therapy and time to optimal antibiotic therapy) was performed (15), comparing actual antibiotic administration times from chart review (control) to theoretical administration times based on BC-GN reporting and AST review (intervention).
AST review.
The theoretical AST consisted of an infectious disease physician and an infectious disease pharmacist (S.L. and E.H.). Individual case report sheets were filled out by two reviewers (J.T.B. and R.B.) for each case up until the time of Gram stain report. Information included clinical, microbiological, and antimicrobial history, in addition to the BC-GN result. Treatment algorithms were derived based on literature and hospital antibiograms (see Fig. S1 and S2 in the supplemental material) and used as guidelines for antibiotic recommendations.
Outcomes and definitions.
Time to effective antibiotic therapy was defined as time from Gram stain report to the time of administration of the first antimicrobial with known susceptibility per the microbiology report. Time to optimal antibiotic therapy was defined as time from Gram stain report to the time the patient received optimal antibiotic therapy, which includes de-escalation based on known susceptibility results, need to cover other polymicrobial infections, concomitant infections, and antibiotic allergies or intolerances (15).
We considered the evaluable time to be the time from Gram stain report until 24 h after susceptibility results were reported, as this would constitute the potential time of impact of rapid reporting using BC-GN. For the implementation of the theoretical AST intervention, we considered progressively increasing intervals (3 h, 6 h, 12 h, 18 h, and 24 h) from the time of the Gram stain report and compared each theoretical administration time with the control's actual antibiotic administration time obtained from the chart. If effective or optimal antibiotic was not achieved, the time until the end of the follow-up period (24 h after the susceptibility result) was used.
Antibiotic interventions were classified as either appropriate (or inappropriate) escalation, defined as a change from a narrow-spectrum to a broad-spectrum antibiotic for a susceptible organism, or appropriate (or inappropriate) de-escalation, defined as a change from a broad-spectrum to narrow-spectrum antibiotic. Inappropriateness in de-escalation was defined as a change from a sensitive antibiotic to one that is either resistant or intermediate on a susceptibility report.
Finally, aggregate antibiotic utilization from the time of the Gram stain report until 24 h after the susceptibility report was quantified using days of therapy (DOT) per 1,000 patient days and compared between the control and intervention groups.
Statistical analysis.
Comparison of time to appropriate and effective antibiotic therapy was performed using a paired sample t test for the differences between mean antibiotic times in the control and intervention groups. In addition, time to event analysis for both outcomes was performed using proportional hazards regression, with a sandwich variance estimate approach for clustered data. Differences between antibiotic days of therapy in the control and intervention groups were evaluated using Poisson 95% confidence intervals (CI). All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Cohort description.
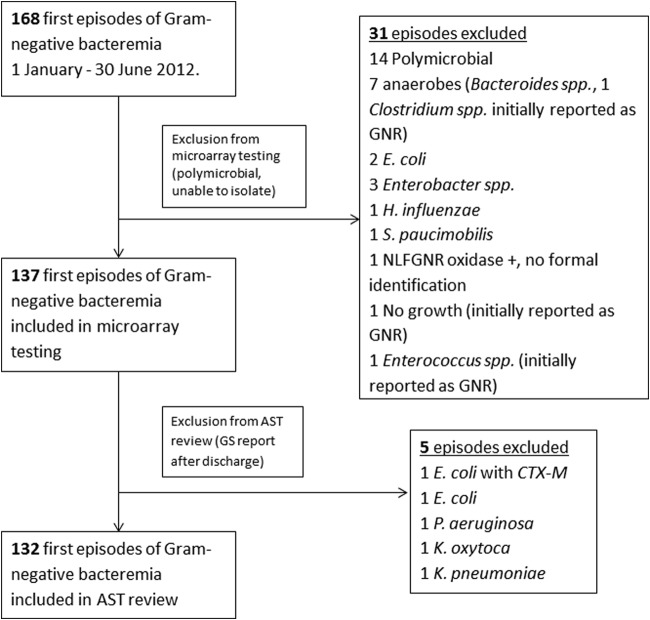
Between 1 January 2012 and 30 June 2012, 168 subjects had a first episode of GN bacteremia, of which 14 were excluded due to polymicrobial cultures. An additional 17 were excluded due to difficulty isolating the organism (i.e., not recovered from a frozen clinical isolate) (Fig. 1). A total of 137 isolates were tested using BC-GN, with the following organism characteristics: K. pneumoniae, 36 (26%); E. coli, 35 (26%); P. aeruginosa, 21 (15%); Enterobacter spp., 9 (6.5%); Acinetobacter spp., 7 (5.1%); K. oxytoca, 5 (3.6%); Proteus spp., 4 (2.9%); Citrobacter spp., 2 (1.5%); and nontarget, 18 (13%) (Serratia marcescens, 7; Morganella morganii, 3; Providencia spp., 2; Salmonella spp., 2; Hafnia alvei, 1; Raoultella ornithinolytica, 1; Burkholderia cepacia, 1; and Sphingomonas paucimobilis, 1).
FIG 1.
Selection of patients. NLFGNR, non-lactose-fermenting Gram-negative rod; GS, Gram stain; GNR, Gram-negative rod.
Of the 137 isolates, 5 were from patients who either were discharged or died before the Gram stain report and thus were excluded from the time comparison analysis (Fig. 1). In the 132 cases included in the time comparison, the mean age of patients was 55 ± 16.3 years, 55% of the patients were male, and malignancy (23%) was the most common comorbidity. The plurality of cases had no known source of bacteremia (39%), and the majority of cases were hospital acquired (59%). Overall inpatient mortality and mean length of hospitalization were 18% and 33.4 ± 46.5 days, respectively (Table 1).
TABLE 1.
Clinical characteristics of AST-reviewed Gram-negative bloodstream infection cohort (n = 132)
| Clinical characteristica | Value |
|---|---|
| Age, yr, mean ± SD | 55 ± 16.3 |
| Male, no. (%) | 72 (55) |
| Comorbidities, no. (%) | |
| Diabetes mellitus | 12 (9) |
| Heart disease | 20 (15) |
| Lung disease | 7 (5) |
| Liver disease | 14 (11) |
| Kidney disease | 16 (12) |
| Vascular disease | 6 (5) |
| Stroke/TBI | 17 (13) |
| Malignancy | 30 (23) |
| BMT | 3 (2) |
| Solid-organ transplant | 15 (11) |
| HIV | 11 (8) |
| Allergy, no. (%) | 40 (30) |
| Beta-lactam | 22 (17) |
| Service, no. (%) | |
| Medicine | 32 (24) |
| Surgery | 35 (27) |
| Trauma | 20 (15) |
| MICU/SICU | 26 (20) |
| Cancer | 13 (10) |
| Otherb | 6 (5) |
| Location, no. (%) | |
| ED | 30 (23) |
| Med-Surg | 42 (32) |
| ICU | 36 (27) |
| Trauma | 19 (14) |
| Otherc | 5 (4) |
| ID consult, no. (%) | 109 (83) |
| Source of bacteremia, no. (%) | |
| Primary | 51 (39) |
| Catheter related | 12 (9) |
| Lung | 13 (10) |
| Urine | 34 (26) |
| Intra-abdominal | 10 (8) |
| VAD | 5 (4) |
| SSTI | 3 (2) |
| Otherd | 4 (3) |
| Type of acquisition, no. (%) | |
| Hospital acquired, >48 h | 78 (59) |
| Community acquired, <48 h | 54 (41) |
| Health care associatede | 30 (23) |
| Length of hospitalization, days, mean ± SD | 33.4 ± 46.5 |
| ICU stay, no. (%) | 78 (59) |
| Length of ICU stay, d, mean ± SD | 18.2 ± 38 |
| Inpatient mortality, no. (%) | 24 (18) |
Abbreviations: TBI, traumatic brain injury; HIV, human immunodeficiency virus; VAD, ventricular assist device; SSTI, skin and soft tissue infection; BMT, bone marrow transplant.
Neurology, pediatrics, and obstetrics.
BMT, pediatric ward, and postpartum ward.
Central nervous system, mediastinitis, and vascular graft.
Criteria: from another hospital or facility, hospitalization of <3 months, indwelling device (VAD, line), hemodialysis.
Verigene BC-GN performance.
For organism detection, BC-GN correctly identified 95.6% (131/137), with a sensitivity of 97.1% (95% CI, 90.7 to 98.4%) and a specificity of 99.5% (95% CI, 98.8 to 99.8%). At the organism level, K. pneumoniae demonstrated a sensitivity of 86.1%. Enterobacter spp. and K. oxytoca demonstrated a specificity of 97.7% and 99.2%, respectively. The nontarget organisms collectively had a sensitivity and specificity of 94.4% and 98.3%, respectively. Of 5 K. pneumoniae isolates, 2 were misidentified as Enterobacter spp., 1 was misidentified as K. oxytoca, and 2 were “not detected.” R. ornithinolytica was misidentified as Enterobacter spp. (Table 2).
TABLE 2.
Performance of Verigene BC-GN for organism identification
| Organism | No. (%) of isolates |
Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| Total | Correctly identified | Not detected | Misidentified | |||||
| Klebsiella pneumoniae | 36 (26) | 31 (86) | 2 | 3a | 86.1 | 70.5–95.3 | 100 | 96.4–100 |
| Escherichia coli | 35 (26) | 35 (100) | 100 | 90.0–100 | 100 | 96.5–100 | ||
| Pseudomonas aeruginosa | 21 (15) | 21 (100) | 100 | 83.9–100 | 100 | 96.9–100 | ||
| Enterobacter spp. | 9 (6.5) | 9 (100) | 100 | 66.4–100 | 97.7 | 93.3–99.5 | ||
| Acinetobacter spp. | 7 (5.1) | 7 (100) | 100 | 59.0–100 | 100 | 97.2–100 | ||
| Klebsiella oxytoca | 5 (3.6) | 5 (100) | 100 | 47.8–100 | 99.2 | 95.9–99.9 | ||
| Proteus spp. | 4 (2.9) | 4 (100) | 100 | 39.8–100 | 100 | 97.3–100 | ||
| Citrobacter spp. | 2 (1.5) | 2 (100) | 100 | 15.8–100 | 100 | 97.3–100 | ||
| Nontarget | 18 (13) | 17 (94) | 1 | 94.4 | 72.7–99.9 | 98.3 | 94.1–99.8 | |
| Serratia marcescens | 7 | 7 | ||||||
| Morganella morganii | 3 | 3 | ||||||
| Providencia spp. | 2 | 2 | ||||||
| Salmonella spp. | 2 | 2 | ||||||
| Hafnia alvei | 1 | 1 | ||||||
| Raoultella ornithinolyticac | 1 | 1b | ||||||
| Burkholderia cepacia | 1 | 1 | ||||||
| Sphingomonas paucimobilis | 1 | 1 | ||||||
| Total | 137 | 114 | 19 | 4 | 97.1 | (90.7–98.4) | 99.5 | (98.8–99.8) |
Two as Enterobacter spp. and one as K. oxytoca.
Enterobacter spp.
Formerly Klebsiella spp.
BC-GN reported invalid (“No Call”) results for 2/132 (1.5%) of the clinical blood cultures, all due to the reader being unable to obtain test results because of high variability in target-specific signals. Both repeated tests yielded “not detected,” corresponding to Vitek 2-identified M. morganii (nontarget) and K. pneumoniae (discordant result).
Based on susceptibility testing, 13/91 (14%) and 16/91 (18%) Enterobacteriaceae isolates were ceftriaxone resistant and piperacillin-tazobactam resistant, respectively; carbapenem resistance was present in 6/21 (29%) and 7/7 (100%) isolates of P. aeruginosa and Acinetobacter baumannii, respectively. There were no carbapenem-resistant Enterobacteriaceae. Using BC-GN, 5/13 (38%) Enterobacteriaceae isolates that were resistant or intermediate to ceftriaxone were detected as having CTX-M and 7/7 (100%) of the MDR A. baumannii were detected as having OXA. However, none of the P. aeruginosa isolates (0/6) that were carbapenem resistant had associated resistance targets (Table 3). PCR testing confirmed the presence or absence of resistance targets detected by BC-GN in which the isolates were resistant to ceftriaxone or a carbapenem. The PCR results for all the samples correlated with the BC-GN results, except in the case of one isolate of A. baumannii which had both blaOXA-23 and blaCTX-M1 by PCR but only OXA detected by BC-GN.
TABLE 3.
Resistant organisms in total Gram-negative bloodstream infection cohort (n = 137)a
| Isolate group | Profile no. | Organism | Resistance profile |
Verigene BC-GN result |
ABX at GS | ABX recommendation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | SAM | FEP | CTX | CIP | GEN | IPM | TZP | TGC | ERT | ATM | PMB | Organism | RT | |||||
| MDR Acinetobacter | 22 | A. baumannii | S | S | R | R | R | S | R | R | I | S | A. baumannii | OXA | FEP | SAM/CST | ||
| 56 | A. baumannii | S | R | R | R | R | S | R | R | I | R | S | A. baumannii | OXA | MEM/CST | SAM/CST | ||
| 88 | A. baumannii | S | R | R | R | R | R | R | R | R | R | S | A. baumannii | OXA | FEP | SAM/CST | ||
| 95 | A. baumannii | S | R | R | R | R | S | R | R | I | R | R | S | A. baumannii | OXA | FEP | SAM/CST | |
| 117 | A. baumannii | S | R | R | R | R | S | R | R | S | A. baumannii | OXA | MEM/CST | SAM/CST | ||||
| 145 | A. baumannii | I | R | R | R | R | S | R | R | I | S | A. baumannii | OXA | TZP | SAM/CST | |||
| 146 | A. baumannii | S | I | I | I | R | R | R | R | I | R | S | A. baumannii | OXA | SAM | SAM/CST | ||
| Ceftriaxone-resistant Enterobacteriaceae | 12 | Citrobacter spp. | S | R | S | I | S | S | S | S | S | S | Citrobacter | ND | TZP | TZP | ||
| 121 | E. aerogene | S | R | S | I | S | S | S | S | S | S | Enterobacter | ND | TZP | FEP | |||
| 68 | E. cloacae | S | R | S | R | S | R | S | I | S | S | Enterobacter | ND | TZP | FEP | |||
| 104 | E. cloacae | S | I | S | R | S | S | S | S | S | S | Enterobacter | ND | TZP | FEP | |||
| 110 | E. cloacae | S | R | S | R | S | S | S | S | I | S | Enterobacter | ND | CIP | FEP | |||
| 6 | E. coli | S | R | S | R | R | S | R | S | S | E. coli | ND | TGC | MEM/ERT | ||||
| 66 | E. coli | S | R | S | R | R | R | S | I | S | S | E. coli | ND | TZP | CTX | |||
| 114 | E. coli | S | S | I | R | R | S | S | S | S | S | E. coli | CTX-M | NA | NA | |||
| 52 | K. pneumoniae | S | R | I | R | R | R | S | S | S | S | K. pneumoniae | CTX-M | TZP | MEM/ERT | |||
| 79 | K. pneumoniae | S | R | I | R | R | R | S | I | S | S | K. pneumoniae | CTX-M | CIP | MEM/ERT | |||
| 111 | K. pneumoniae | I | R | R | R | R | R | S | R | S | S | S | K. pneumoniae | CTX-M | TZP | MEM/ERT | ||
| 119 | K. pneumoniae | S | I | S | R | S | S | S | S | S | S | K. oxytoca | ND | MEM/AMK | MEM/ERT | |||
| 143 | K. pneumoniae | R | R | R | R | R | R | S | R | S | S | K. pneumoniae | CTX-M | TZP | MEM/ERT | |||
| Carbapenem-resistant Pseudomonas | 49 | P. aeruginosa | S | R | R | S | R | R | S | P. aeruginosa | ND | MEM/CST | MEM/CST | |||||
| 54 | P. aeruginosa | S | S | S | S | R | S | I | S | P. aeruginosa | ND | FEP | FEP | |||||
| 75 | P. aeruginosa | S | R | R | S | R | R | R | S | P. aeruginosa | ND | TZP | FEP/CST | |||||
| 109 | P. aeruginosa | S | I | S | S | R | R | S | P. aeruginosa | ND | CIP | MEM/AMK | ||||||
| 159 | P. aeruginosa | S | R | R | R | R | R | R | S | P. aeruginosa | ND | NA | NA | |||||
| 164 | P. aeruginosa | S | S | R | R | R | S | S | S | P. aeruginosa | ND | MEM | FEP | |||||
Abbreviations: RT, resistance target; R resistant; I, intermediate; S, susceptible; ABX, antibiotic; GS Gram stain; CTX, ceftriazone; FEP, cefepime; SAM, ampicillin-sulbactam; CST, colistin; TZP, piperacillin-tazobactam; MEM, meropenem; ERT, ertapenem; IPM, imipenem; AMK, amikacin; TGC, tigecycline; ATM, aztreonam; PMB, polymyxin B; NA, not applicable (not included in chart review); ND, not detected.
Antibiotic therapy outcomes.
There were 91/132 (69%) changes in antibiotic therapy based on BC-GN result and AST review in the theoretical intervention group. For the effective antibiotic therapy outcome, there were no cases with inappropriate de-escalation, 16/91 (18%) demonstrated appropriate escalation, and in 75/91 (82%) no difference was observed. Some of these antibiotic changes are highlighted in Table 4. For the optimal antibiotic therapy outcome, 61/91 (67%) demonstrated appropriate de-escalation, 10/91 (11%) demonstrated inappropriate escalation, and in 20/91 (22%) no difference was observed.
TABLE 4.
Sensitivity analysis using proportional hazards regression and paired sample t test for time to effective and optimal antibiotic therapies
| Time of BC-GN report | Effective antibiotic therapy |
Optimal antibiotic therapy |
||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI (P value)a | Mean difference (SD) | 95% CI (P value) | Hazard ratio | 95% CI (P value) | Mean difference (SD) | 95% CI (P value) | |
| 3 h | 1.33 | 1.11–1.60 (0.002) | 4.8 h (16.5) | 2.0–7.6 (0.001) | 2.5 | 2.00–3.12 (<0.001) | 22.5 h (32.9) | 16.8–28.1 (<0.001) |
| 6 h | 1.29 | 1.09–1.54 (0.004) | 4.4 h (15.7) | 1.7–7.1 (0.002) | 2.43 | 1.95–3.04 (<0.001) | 21.1 h (31.6) | 15.7–26.6 (<0.001) |
| 12 h | 1.14 | 0.98–1.33 (0.08) | 3.7 h (14.3) | 1.3–6.2 (0.003) | 2.32 | 1.86–2.89 (<0.001) | 18.3 h (29.2) | 13.3–23.4 (<0.001) |
| 18 h | 2.9 h (13.0) | 0.8–5.2 (0.009) | 2.30 | 1.83–2.87 (<0.001) | 15.6 h (26.8) | 10.9–20.2 (<0.001) | ||
| 24 h | 2.3 h (11.8) | 0.2–4.3 (0.03) | 2.30 | 1.83–2.87 (<0.001) | 12.8 h (24.6) | 8.5–17.0 (<0.001) | ||
The statistically significant cutoff for all P values was <0.05.
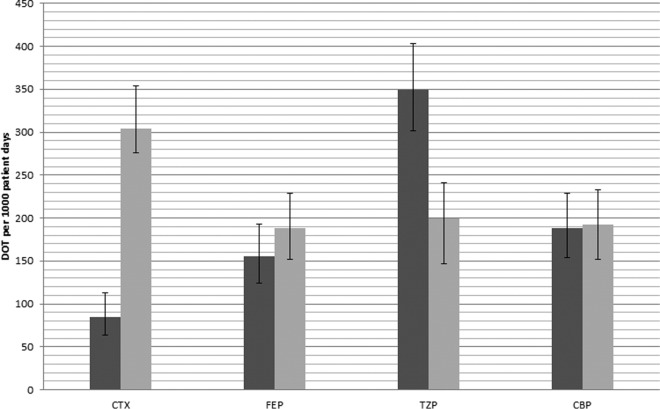
Evaluating total antibiotic utilization between the control and intervention groups, piperacillin-tazobactam use decreased from 349.8/1,000 patient days (95% CI, 302.0 to 403.1) to 199.6/1,000 patient days (95% CI, 163.9 to 240.8) and ceftriaxone use increased from 84.2/1,000 patient days (95% CI, 63.6 to 112.5) to 304/1,000 patient days (95% CI, 259.5 to 254.0), both statistically significant (Fig. 2). There was no significant change in carbapenem use (188.6/1,000 patient days [95% CI, 154.0 to 228.8] versus 192.3/1,000 patient days [95% CI, 163.9 to 240.8]).
FIG 2.
Days of therapy (DOT) per 1,000 patient days from the Gram stain report until 24 h after the susceptibility report. Dark gray bars, control; light gray bars, intervention. For the intervention group, the antibiotic is considered to be administered 3 h from the Gram stain report. Abbreviations: CTX, ceftriaxone; FEP, cefepime; TZP, piperacillin-tazobactam; CBP, carbapenem.
Sensitivity analysis of selected time intervals demonstrated that the cutoff time for a statistically significant difference in time to effective antibiotic administration for BC-GN reporting was 12 h from the Gram stain report, while the difference in time to optimal antibiotic administration remained statistically significant even up to 24 h from the Gram stain report (Table 4). Allowing 12 h for antibiotic changes to be made based on BC-GN and AST, effective antibiotic therapy could be achieved an average of 3.7 h earlier (95% CI, 1.3 to 6.2; P < 0.01) and optimal antibiotic therapy could be achieved an average of 18.3 h earlier (95% CI, 13.3 to 23.4; P < 0.01) in the intervention group than in the controls (Fig. 3). Using proportional hazards regression, we also found that the intervention group was significantly more likely to receive optimal antibiotic therapy earlier than the control group (hazard ratio, 2.32; 95% CI, 1.86 to 2.89; P < 0.01).
FIG 3.
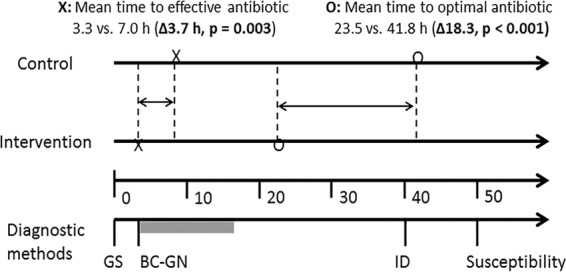
Timeline of events (hours). Abbreviations: GS, Gram stain; ID, identification.
DISCUSSION
In this study, we confirmed the accuracy of BC-GN for identifying GN bacteria and resistance markers in BSI. We found a significant difference in potential times to both effective and optimal antibiotic therapies in treatment of GN BSI using a theoretical BC-GN result combined with AST approach compared to standard management. Further, our sensitivity analysis suggested that this approach could be beneficial even when implemented up to 12 to 18 h after the Gram stain report.
There are competing, commercially available, rapid diagnostic modalities that have demonstrated improved clinical outcomes, such as time to appropriate antibiotic therapy, length of stay, and mortality. These modalities include peptide nucleic acid fluorescent in situ hybridization (PNA-FISH) (9, 16), MALDI-TOF (15), and real-time PCR for Staphylococcus aureus bacteremia (17). The multiplex PCR FilmArray system (18) and Verigene BC-GP and BC-GN have yet to be comprehensively evaluated for their impact on patient outcomes.
In our study, the overall performance of Verigene BC-GN was similar to that in the recent reports (3–7). Several important differences include the inclusion of S. marscesens (removed from the final FDA-approved panel) (3–5) and use of a combination of real-time clinical samples and contrived blood cultures. In addition, Sullivan et al. primarily looked at a pediatric population (5). The total concordance rate for organism identification ranged from 89 to 100%. K. pneumoniae consistently had a lower agreement (∼86%), with isolates either not detected or misidentified as Enterobacter spp. (1 isolate) (4).
Our discrepant isolates included K. pneumoniae (2 misidentified as Enterobacter spp., 1 misidentified as K. oxytoca, and 2 not detected) and R. ornithinolytica (misidentified as Enterobacter). A recognized limitation of the test is the cross-reactivity of K. pneumoniae with Enterobacter spp. (2), which can explain the discordance. The R. ornithinolytica isolate that was detected as Enterobacter was also identified as Enterobacter by MALDI-TOF. The K. pneumoniae isolate that was detected as K. oxytoca was identified by sequencing (94% identity) as R. ornithinolytica, which is also known to cross-react with K. oxytoca (2). K. pneumoniae isolates that were reported as not detected may actually be Klebsiella variicola, a recently identified Klebsiella sp. that closely resembles K. pneumoniae (19) and is not detected by BC-GN.
The resistance marker detection capability of BC-GN, while extensive for carbapenemases, is limited to only CTX-M among the extended-spectrum (TEM and SHV) and AmpC-producing beta-lactamases. Based on recent surveillance data, the prevalence of CTX-M in cephalosporin-resistant Enterobacteriaceae bacteremia isolates in U.S. hospitals is increasing (26.6% in 2007 to 43.8% in 2010) and is thus a relevant marker for early detection (20). However, the prevalences of TEM (51%), SHV (16%), and AmpC (10%) from the same surveillance data are not insignificant, and the lack of these markers remains a potential limitation of this platform. In our study, we had a CTX-M prevalence of 38% (5/13), with unknown resistance mechanisms for the remaining 8 isolates. Having these additional resistant mechanisms would be helpful. However, due to the high prevalence of broad-spectrum beta-lactamases (TEM-1, SHV-11, and SHV-1), one potential consequence would be the overuse of broad-spectrum antibiotics, such as carbapenems.
Conversely, there are theoretical concerns of harmful de-escalation because of the decreased sensitivity of K. pneumoniae. For example, in the case of a Klebsiella sp. harboring a KPC that is not detected, no organism or resistance gene is reported and effective antibiotic therapy (i.e., colistin) may be discontinued inappropriately. Due to the limitation that we did not observe any CRE BSI in our study, the magnitude of this discrepancy and clinical impact needs to be further evaluated.
In the analysis of aggregate antimicrobial use, we found an inverse relationship between piperacillin-tazobactam and ceftriaxone use: BC-GN application was associated with a significant decrease in piperacillin-tazobactam and a significant increase in ceftriaxone use, and it was not associated with any inappropriate de-escalation. Therefore, the strategy of BC-GN results along with AST guidance has the potential to safely limit use of broad-spectrum antibiotics earlier in the course of therapy. Of the 10 cases that were described as inappropriate escalation, 9/10 involved ICU patients, which may reflect a shortcoming in our algorithm. For example, the antibiogram profile in the ICUs for K. pneumoniae has a lower susceptibility to first-line agents like ceftriaxone (79%) and piperacillin-tazobactam (75%); thus, a carbapenem (97%) is recommended upfront.
Nonetheless, a strong component of our study is the development of algorithms to guide ASTs and/or prescribers with the clinical application of the BC-GN. The algorithms were compiled by thorough review of evidence-based literature and expert opinion. However, there are institution (i.e., antibiogram) and patient (i.e., colonization) considerations that need to be incorporated. One treatment specific controversy we encountered was addressing the Enterobacteriaceae likely harboring AmpC-producing β-lactamases (Enterobacter, Citrobacter, and Proteus), for which cefepime and/or piperacillin-tazobactam (as opposed to carbapenem) was the recommended antibiotic, as new data suggest that these antibiotics may be acceptable since they are both low-level inducers (21, 22). However, whether the application of these algorithms to BC-GN result will be effective in institutions that lack active ASTs needs to be studied.
A major limitation of the experimental portion of our study is the retrospective design with evaluation of only theoretical therapy-related outcomes. Also, it is assumed that 100% of the AST recommendations would be followed; however, a 90% acceptance rate is more likely (15). Although “effective antibiotic” is a clear, indisputable term, optimal antibiotic choice is less objective and could be perceived as a less reliable outcome measure. Another recognized limitation of our study is exclusion of polymicrobial infections, which were present in 14/168 (8%) of the initial samples. Accurate identification of polymicrobial samples is a known limitation of BC-GN, as it is in other modalities.
In conclusion, our study demonstrated a potential decreased time to both effective and optimal antibiotic therapies in GN BSI using a combined intervention of rapid testing with Verigene BC-GN and AST recommendation. In order to maximize the impact, the results reported by the clinical microbiology laboratory and AST recommendation need to be efficiently coordinated. In addition, the incorporation of treatment algorithms into AST practice will help bridge the communication of advancing technology in the clinical microbiology laboratory with patient care. Although these results are promising, prospective studies are needed to further validate this strategy.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by Nanosphere, Inc.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04259-14.
REFERENCES
- 1.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanosphere, Inc. February 2014. Verigene Gram-negative blood culture nucleic acid test (BC-GN) package insert 027-00039-01, Rev B. Nanosphere Inc, Northbrook, IL. [Google Scholar]
- 3.Mancini N, Infurnari L, Ghidoli N, Valzano G, Clementi N, Burioni R, Clementi M. 2014. Potential impact of a microarray-based nucleic acid assay for rapid detection of Gram-negative bacteria and resistance markers in positive blood cultures. J Clin Microbiol 52:1242–1245. doi: 10.1128/JCM.00142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tojo M, Fujita T, Ainoda Y, Nagamatsu M, Hayakawa K, Mezaki K, Sakurai A, Masui Y, Yazaki H, Takahashi H, Miyoshi-Akiyama T, Totsuka K, Kirikae T, Ohmagari N. 2014. Evaluation of an automated rapid diagnostic assay for detection of gram-negative bacteria and their drug-resistance genes in positive blood cultures. PLoS One 9:e9406. doi: 10.1371/journal.pone.0094064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan KV, Deburger B, Roundtree SS, Ventrola CA, Blecker-Shelly DL, Mortensen JE. 2014. Pediatric multicenter evaluation of the Verigene Gram-negative blood culture test for rapid detection of inpatient bacteremia involving Gram-negative organisms, extended-spectrum beta-lactamases, and carbapenemases. J Clin Microbiol 52:2416–2421. doi: 10.1128/JCM.00737-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodémont M, De Mendonca R, Nonhoff C, Roisin S, Denis O. 2014. Performance of Verigene Gram-negative blood culture assay for rapid detection of bacteria and resistance determinants. J Clin Microbiol 52:3085–3087. doi: 10.1128/JCM.01099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill JT, Tran KD, Barton KL, Labreche MJ, Sharp SE. 13 August 2014. Evaluation of the Nanosphere-Verigene BC-GN for the direct identification of Gram negative bacilli and antibiotic resistance markers from positive blood cultures and the potential impact for more rapid antibiotic interventions. J Clin Microbiol doi: 10.1128/JCM.01537-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kothari A, Morgan M, Haake D. 2014. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis 59:272–278. doi: 10.1093/cid/ciu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrest GN, Mehta S, Weekes E, Lincalis DP, Johnson JK, Venezia RA. 2006. Impact of rapid in situ hybridization testing on coagulase-negative staphylococci positive blood cultures. J Antimicrob Chemother 58:154–158. doi: 10.1093/jac/dkl146. [DOI] [PubMed] [Google Scholar]
- 10.Holtzman C, Whitney D, Barlam T, Miller NS. 2011. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol 49:1581–1582. doi: 10.1128/JCM.02461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement. M100-S22 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SG, Livermore DM. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Woodford N, Fagan EJ, Ellington MJ. 2006. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J Antimicrob Chemother 57:154–155. doi: 10.1128/JCM.06382-11. [DOI] [PubMed] [Google Scholar]
- 15.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 57:1237–1245. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 16.Forrest GN, Mankes K, Jabra-Rizk MA, Weekes E, Johnson JK, Lincalis DP, Venezia RA. 2006. Peptide nucleic acid fluorescence in situ hybridization-based identification of Candida albicans and its impact on mortality and antifungal therapy costs. J Clin Microbiol 44:3381–3383. doi: 10.1128/JCM.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye AM, Baker CA, Rustvold DL, Heath KA, Hunt J, Leggett JE, Oethinger M. 2012. Clinical impact of a real-time PCR assay for rapid identification of staphylococcal bacteremia. J Clin Microbiol 50:127–133. doi: 10.1128/JCM.06169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaschke AJ, Heyrend C, Byington CL, Fisher MA, Barker E, Garrone NF, Thatcher SA, Pavia AT, Barney T, Alger GD, Daly JA, Ririe KM, Ota I, Poritz MA. 2012. Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis 74:349–355. doi: 10.1016/j.diagmicrobio.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenblueth M, Martinez L, Silva J, Martinez-Romero E. 2004. Klebsiella variicola, a novel species with clinical and plant-associated isolates. Syst Appl Microbiol 27:27–35. doi: 10.1078/0723-2020-00261. [DOI] [PubMed] [Google Scholar]
- 20.Castanheira M, Farrell SE, Deshpande LM, Mendes RE, Jones RN. 2013. Prevalence of beta-lactamase-encoding genes among Enterobacteriaceae bacteremia isolates collected in 26 U.S. hospitals: report from the SENTRY Antimicrobial Surveillance Program (2010). Antimicrob Agents Chemother 57:3012–3020. doi: 10.1128/AAC.02252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamma PD, Girdwood SC, Gopaul R, Tekle T, Roberts AA, Harris AD, Cosgrove SE, Carroll KC. 2013. The use of cefepime for treating AmpC beta-lactamase-producing Enterobacteriaceae. Clin Infect Dis 57:781–788. doi: 10.1093/cid/cit395. [DOI] [PubMed] [Google Scholar]
- 22.Siedner MJ, Galar A, Guzman-Suarez BB, Kubiak DW, Baghdady N, Ferraro MJ, Hooper DC, O'Brien TF, Marty FM. 2014. Cefepime vs other antibacterial agents for the treatment of Enterobacter species bacteremia. Clin Infect Dis 58:1554–1563. doi: 10.1093/cid/ciu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.