Abstract
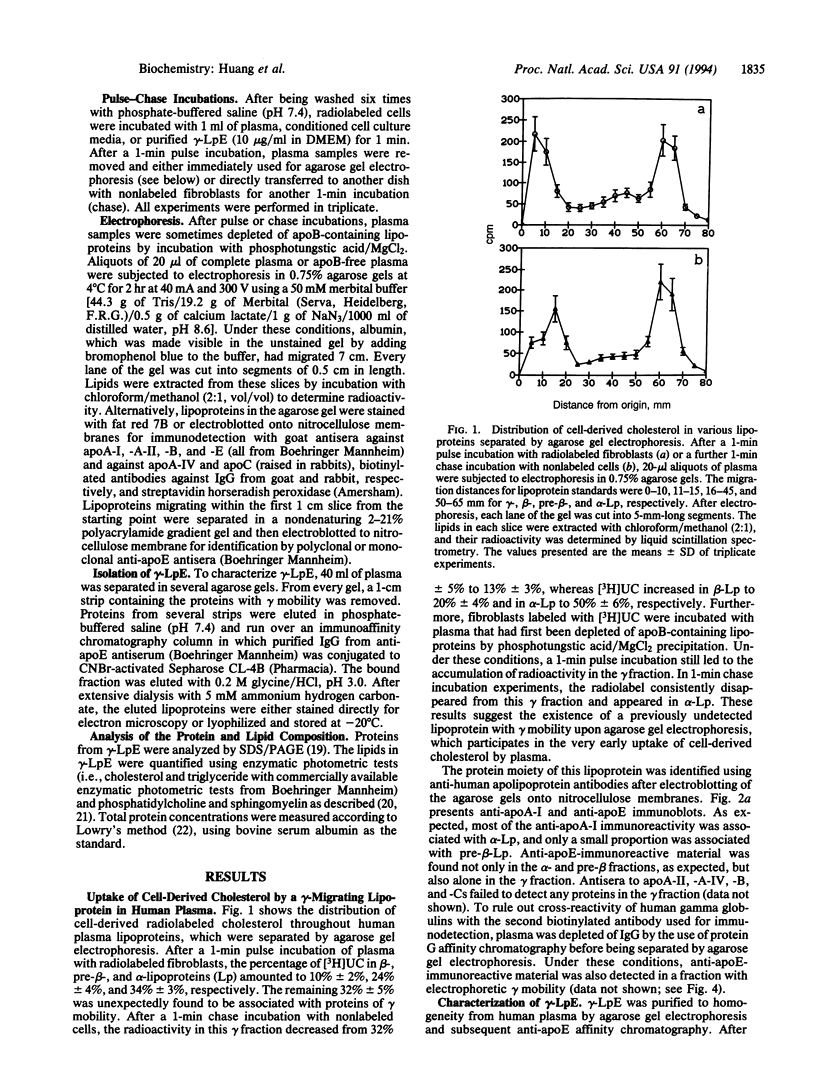
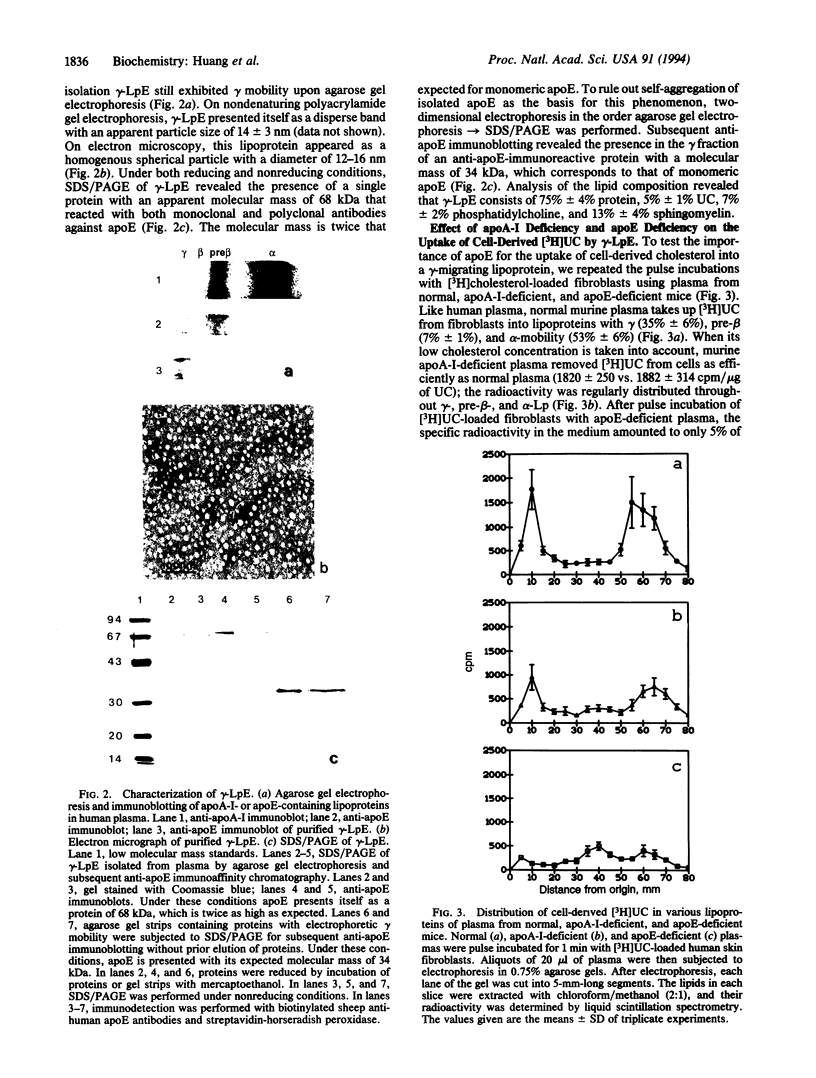
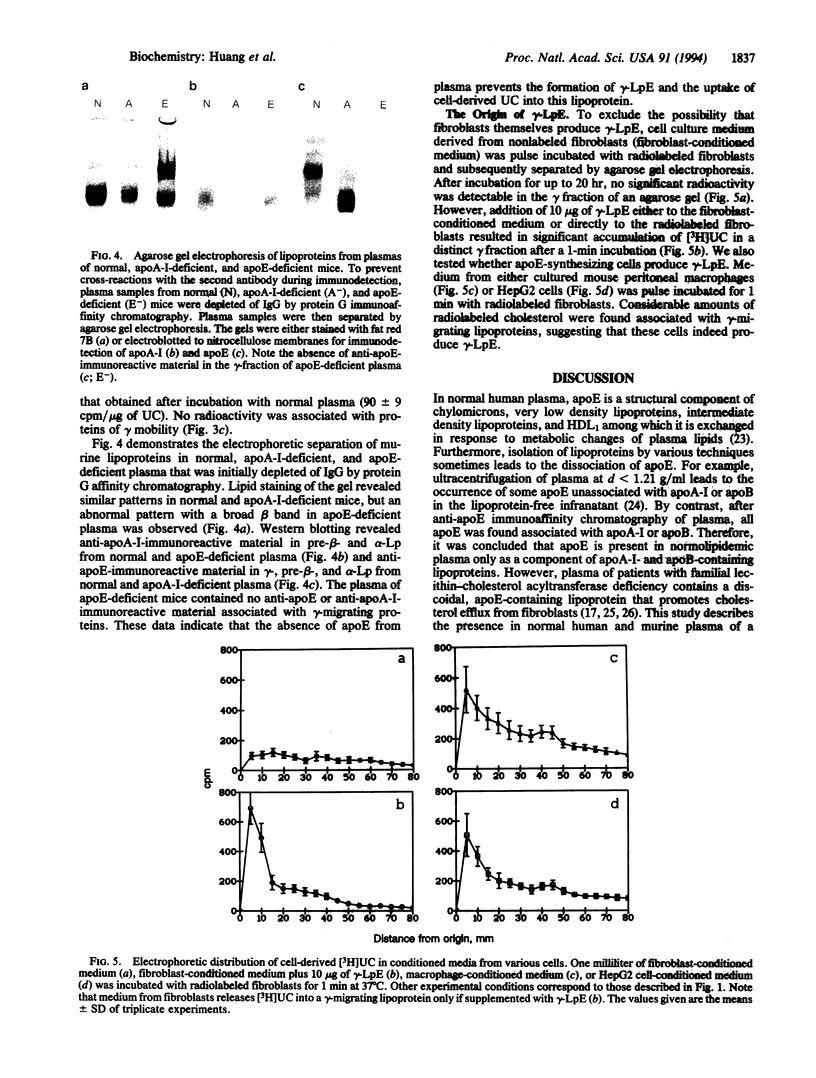
Previous studies have identified lipid-poor high density lipoproteins with electrophoretic pre-beta mobility as the initial acceptors of cell-derived cholesterol in human plasma. These lipoproteins contain apolipoprotein A-I (apo A-I) as their sole apolipoprotein. In the present study, incubation of human plasma with [3H]cholesterol-laden skin fibroblasts has led to the identification of another lipoprotein that serves as a potent initial acceptor of cell-derived cholesterol. This lipoprotein, which we term gamma-LpE, exhibits gamma mobility on agarose gel electrophoresis. As determined by nondenaturing PAGE and by electron microscopy, the size of the spherical particle ranges between 12 and 16 nm. SDS/PAGE and subsequent immunoblotting identified apoE as its sole apolipoprotein. Plasma from normal and apoA-I-deficient mice, but not from apoE-deficient mice, released [3H]cholesterol from fibroblasts into a gamma-migrating lipoprotein. Cell culture media from hepatoma cells or mouse peritoneal macrophages, both of which contain apoE of cellular origin, also promoted efflux of [3H]cholesterol from fibroblasts into a gamma-migrating fraction. This was not observed with cell culture medium from fibroblasts alone. In conclusion, our results strongly indicate the presence in human plasma of a lipoprotein containing only apoE, gamma-LpE, which is secreted by peripheral cells and is a potent acceptor of cell-derived cholesterol.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Castro G. R., Fielding C. J. Early incorporation of cell-derived cholesterol into pre-beta-migrating high-density lipoprotein. Biochemistry. 1988 Jan 12;27(1):25–29. doi: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- Castro G. R., Fielding C. J. Evidence for the distribution of apolipoprotein E between lipoprotein classes in human normocholesterolemic plasma and for the origin of unassociated apolipoprotein E (Lp-E). J Lipid Res. 1984 Jan;25(1):58–67. [PubMed] [Google Scholar]
- Dory L. Regulation of apolipoprotein E secretion by high density lipoprotein 3 in mouse macrophages. J Lipid Res. 1991 May;32(5):783–792. [PubMed] [Google Scholar]
- Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984 Oct;25(10):1017–1058. [PubMed] [Google Scholar]
- Elshourbagy N. A., Liao W. S., Mahley R. W., Taylor J. M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Frohlich J., Moser K., Fielding P. E. Promotion of sterol efflux and net transport by apolipoprotein E in lecithin:cholesterol acyltransferase deficiency. Metabolism. 1982 Oct;31(10):1023–1028. doi: 10.1016/0026-0495(82)90146-9. [DOI] [PubMed] [Google Scholar]
- Forte T., Norum K. R., Glomset J. A., Nichols A. V. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: structure of low and high density lipoproteins as revealed by elctron microscopy. J Clin Invest. 1971 May;50(5):1141–1148. doi: 10.1172/JCI106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke H., von Eckardstein A., Pritchard P. H., Albers J. J., Kastelein J. J., Droste C., Assmann G. A molecular defect causing fish eye disease: an amino acid exchange in lecithin-cholesterol acyltransferase (LCAT) leads to the selective loss of alpha-LCAT activity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4855–4859. doi: 10.1073/pnas.88.11.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke H., von Eckardstein A., Pritchard P. H., Hornby A. E., Wiebusch H., Motti C., Hayden M. R., Dachet C., Jacotot B., Gerdes U. Genetic and phenotypic heterogeneity in familial lecithin: cholesterol acyltransferase (LCAT) deficiency. Six newly identified defective alleles further contribute to the structural heterogeneity in this disease. J Clin Invest. 1993 Feb;91(2):677–683. doi: 10.1172/JCI116248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke H., von Eckardstein A., Pritchard P. H., Karas M., Albers J. J., Assmann G. A frameshift mutation in the human apolipoprotein A-I gene causes high density lipoprotein deficiency, partial lecithin: cholesterol-acyltransferase deficiency, and corneal opacities. J Clin Invest. 1991 Jan;87(1):371–376. doi: 10.1172/JCI114997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli G., Schaefer E. J., Gascon P., Breser H. B., Jr Type III hyperlipoproteinemia associated with apolipoprotein E deficiency. Science. 1981 Dec 11;214(4526):1239–1241. doi: 10.1126/science.6795720. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Rifkind B. M. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989 Nov 9;321(19):1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- Gotoda T., Yamada N., Murase T., Sakuma M., Murayama N., Shimano H., Kozaki K., Albers J. J., Yazaki Y., Akanuma Y. Differential phenotypic expression by three mutant alleles in familial lecithin:cholesterol acyltransferase deficiency. Lancet. 1991 Sep 28;338(8770):778–781. doi: 10.1016/0140-6736(91)90665-c. [DOI] [PubMed] [Google Scholar]
- Gualandri V., Franceschini G., Sirtori C. R., Gianfranceschi G., Orsini G. B., Cerrone A., Menotti A. AIMilano apoprotein identification of the complete kindred and evidence of a dominant genetic transmission. Am J Hum Genet. 1985 Nov;37(6):1083–1097. [PMC free article] [PubMed] [Google Scholar]
- Huang Y., von Eckardstein A., Assmann G. Cell-derived unesterified cholesterol cycles between different HDLs and LDL for its effective esterification in plasma. Arterioscler Thromb. 1993 Mar;13(3):445–458. doi: 10.1161/01.atv.13.3.445. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Mahlberg F. H., Rothblat G. H., Phillips M. C. Cholesterol transport between cells and high-density lipoproteins. Biochim Biophys Acta. 1991 Oct 1;1085(3):273–298. doi: 10.1016/0005-2760(91)90132-2. [DOI] [PubMed] [Google Scholar]
- Klein H. G., Lohse P., Pritchard P. H., Bojanovski D., Schmidt H., Brewer H. B., Jr Two different allelic mutations in the lecithin-cholesterol acyltransferase gene associated with the fish eye syndrome. Lecithin-cholesterol acyltransferase (Thr123----Ile) and lecithin-cholesterol acyltransferase (Thr347----Met). J Clin Invest. 1992 Feb;89(2):499–506. doi: 10.1172/JCI115612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka D., Teramoto T., Matsushima T., Yokoyama T., Yamada A., Aikawa T., Miyamoto Y., Kurokawa K. Apolipoprotein E deficiency with a depressed mRNA of normal size. Atherosclerosis. 1991 May;88(1):15–20. doi: 10.1016/0021-9150(91)90252-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mabuchi H., Itoh H., Takeda M., Kajinami K., Wakasugi T., Koizumi J., Takeda R., Asagami C. A young type III hyperlipoproteinemic patient associated with apolipoprotein E deficiency. Metabolism. 1989 Feb;38(2):115–119. doi: 10.1016/0026-0495(89)90249-7. [DOI] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mazzone T., Basheeruddin K., Poulos C. Regulation of macrophage apolipoprotein E gene expression by cholesterol. J Lipid Res. 1989 Jul;30(7):1055–1064. [PubMed] [Google Scholar]
- Norum K. R., Glomset J. A., Nichols A. V., Forte T. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: physical and chemical studies of low and high density lipoproteins. J Clin Invest. 1971 May;50(5):1131–1140. doi: 10.1172/JCI106585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Schriewer H., Jabs H. U., Assmann G. Zur enzymatischen Analytik des HDL-Sphingomyelin. J Clin Chem Clin Biochem. 1982 May;20(5):305–312. [PubMed] [Google Scholar]
- Schriewer H., Jung G., Emke F., Assmann G. An economical assay for HDL phosphatidyl choline. J Clin Chem Clin Biochem. 1983 Oct;21(10):611–614. doi: 10.1515/cclm.1983.21.10.611. [DOI] [PubMed] [Google Scholar]
- Tall A. R. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest. 1990 Aug;86(2):379–384. doi: 10.1172/JCI114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Lee D., Hagaman J., Maeda N. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7134–7138. doi: 10.1073/pnas.89.15.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Inoue I., Kawamura M., Harada K., Watanabe Y., Shimano H., Gotoda T., Shimada M., Kohzaki K., Tsukada T. Apolipoprotein E prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbits. J Clin Invest. 1992 Feb;89(2):706–711. doi: 10.1172/JCI115639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- von Eckardstein A., Funke H., Henke A., Altland K., Benninghoven A., Assmann G. Apolipoprotein A-I variants. Naturally occurring substitutions of proline residues affect plasma concentration of apolipoprotein A-I. J Clin Invest. 1989 Dec;84(6):1722–1730. doi: 10.1172/JCI114355. [DOI] [PMC free article] [PubMed] [Google Scholar]




