Abstract
Four laboratories tested three isolates of Candida species and two isolates of Aspergillus fumigatus using 96-well plates containing combinations of amphotericin B, anidulafungin, caspofungin, micafungin, fluconazole, itraconazole, posaconazole, and voriconazole. The majority of summation fractional inhibitory concentration indices (ΣFICI) based on the Lowe additivity formula suggested indifferent drug interactions (ΣFICI > 0.5 and ≤4.0) and no instance of drug antagonism (ΣFICI > 4.0). The intra- and interlaboratory agreement rates were superior when MIC100 readings were used as endpoints (at a 99% confidence interval [CI]).
TEXT
The incidence of systemic fungal infections continues to rise and to cause high morbidity and mortality (1, 2, 3). In many instances, hospitalized patients still receive empirical antifungal therapy, which prolongs hospitalization and increases mortality risk (4). Antifungal susceptibility test results are crucial for making informed clinical decisions (5). Currently, a number of standardized methods and devices are available for antifungal susceptibility testing in clinical laboratories (6–11). However, there is no standard method for laboratory testing of antifungal combinations, despite emerging evidence for the efficacy of combination therapy for fungal infection (12, 13). The present study details results of multilaboratory testing of two antifungal drug combinations with the ultimate goal of standardizing a method suitable for routine use in the clinical laboratories.
Multidrug-resistant fungal isolates of Candida albicans 20464.007 (AMBR, FLCR, ITCR, VRCR), Aspergillus fumigatus 20684.002 (AMBR, ANDR, CSPR, FLCR, ITCR, MCFR) and 20684.007 (AMBR, ANDR, CSPR, FLCR, ITCR, MCFR) were obtained from the Department of Pathology, University of Iowa. Candida glabrata M1409 (AMBR, FLCR, ITCR, PSCR, VRCR) was selected from the Wadsworth Center Mycology Laboratory Culture Collection. The multidrug-resistant isolates were included because they are especially useful for evaluation of the efficacy of two-drug combinations due to their known resistance to at least one drug in the combination. Candida krusei ATCC 6258 was the quality control (QC) strain from participating sites. The 96-well plates in broth microdilution format were commercially prepared (Trek Diagnostics Systems, Cleveland, OH). One hundred microliters of amphotericin B (AMB; Sigma Chemical Co., St. Louis, MO), anidulafungin (AND; Pfizer, Inc., Groton, CT), caspofungin (CSP; Merck & Co., Rahway, NJ), micafungin (MCF; Astellas Pharma Inc., Tokyo, Japan), fluconazole (FLC; Sigma Chemical Co., St. Louis, MO), itraconazole (ITC; Sigma Chemical Co., St. Louis, MO), posaconazole (PSC; Schering-Plough Corp., Kenilworth, NJ), and voriconazole (VRC; Pfizer, Inc., Groton, CT) was dispensed singly or in pairwise combinations as detailed elsewhere (14, 15). One test set included 17 plates with 282 possible drug combinations. RPMI 1640 broth was used as a culture medium and for drug dilutions. The plates were shipped frozen by the manufacturer and were stored at −70°C. The test inoculum, setup, and reading followed CLSI guidelines M27-A3 for yeasts and M38-A2 for molds, with modifications (14, 15). All plates were read at 48 h for 50% and 100% growth inhibitions compared to growth control wells (MIC50 and MIC100). Drug combination interactions were determined by the summation of fractional inhibitory concentration indices (ΣFICI), which were calculated based on the Lowe additivity formula (16). The interpretations were as follows: ≤0.5, synergistic; >0.5 to ≤4.0, indifferent (no antagonism); >4.0, antagonistic (15, 17, 18). The interpretations of ΣFICI were categorized as A, B, and C, corresponding to synergy, indifference, and antagonism, respectively. If there was no ΣFICI value for a specific drug combination, there was no interpretation, which was categorized as D. Agreements and intra-/interclass correlation coefficients (ICCs) were used to examine the reliability of the results. Agreement was calculated as the percentage of the number of the most frequent categorized ΣFICI values divided by the number of all ΣFICI values. The agreement between all of the duplex experiment events for each laboratory was defined as the intralaboratory agreement rate. The agreement between four laboratories for each tested fungal isolate was defined as interlaboratory agreement rate. Intra- and interlaboratory ICCs (IaCC and IeCC, respectively) were calculated based on the ordered categorized ΣFICI values (19, 20).
The four participating laboratories collected about three thousand MIC readings for eight single drugs and 28 two-drug combinations. The single-drug MIC results for Candida species were in close agreement, while A. fumigatus isolates were reported to have a more diverse MIC range (data not shown). ΣFICI values were calculated antifungal drug combinations obtained from MIC50 and MIC100 readings (Table 1). The majority of the ΣFICI values were between 0.5 and 4.0, indicating indifference. A few ΣFICI values were greater than 4.0, which indicated antagonism. However, the antagonism was not seen consistently for any drug combination or isolate tested (Table 1). Mean ΣFICI50 values were in the range of 0.06 to 3.63 and mean ΣFICI100 values were in the range of 0.18 to 2.31 for all five isolates against all 28 drug combinations tested (Table 1). Interestingly, synergistic interactions of antifungals assessed by ΣFICI (≤0.5) at both 50% and 100% inhibition levels included CSP-PSC synergism against C. albicans 20464.007, AMB-PSC synergism against C. glabrata M1409, PSC-VRC synergism against A. fumigatus 20684.002, and AND-PSC synergism against A. fumigatus 20684.007 (see Table S1 in the supplemental material). Some synergistic drug interactions had a high percentage of agreement, and some had a low percentage of agreement.
TABLE 1.
ΣFICI values for 28 possible antifungal drug combinations obtained from MIC50 and MIC100 readingsa

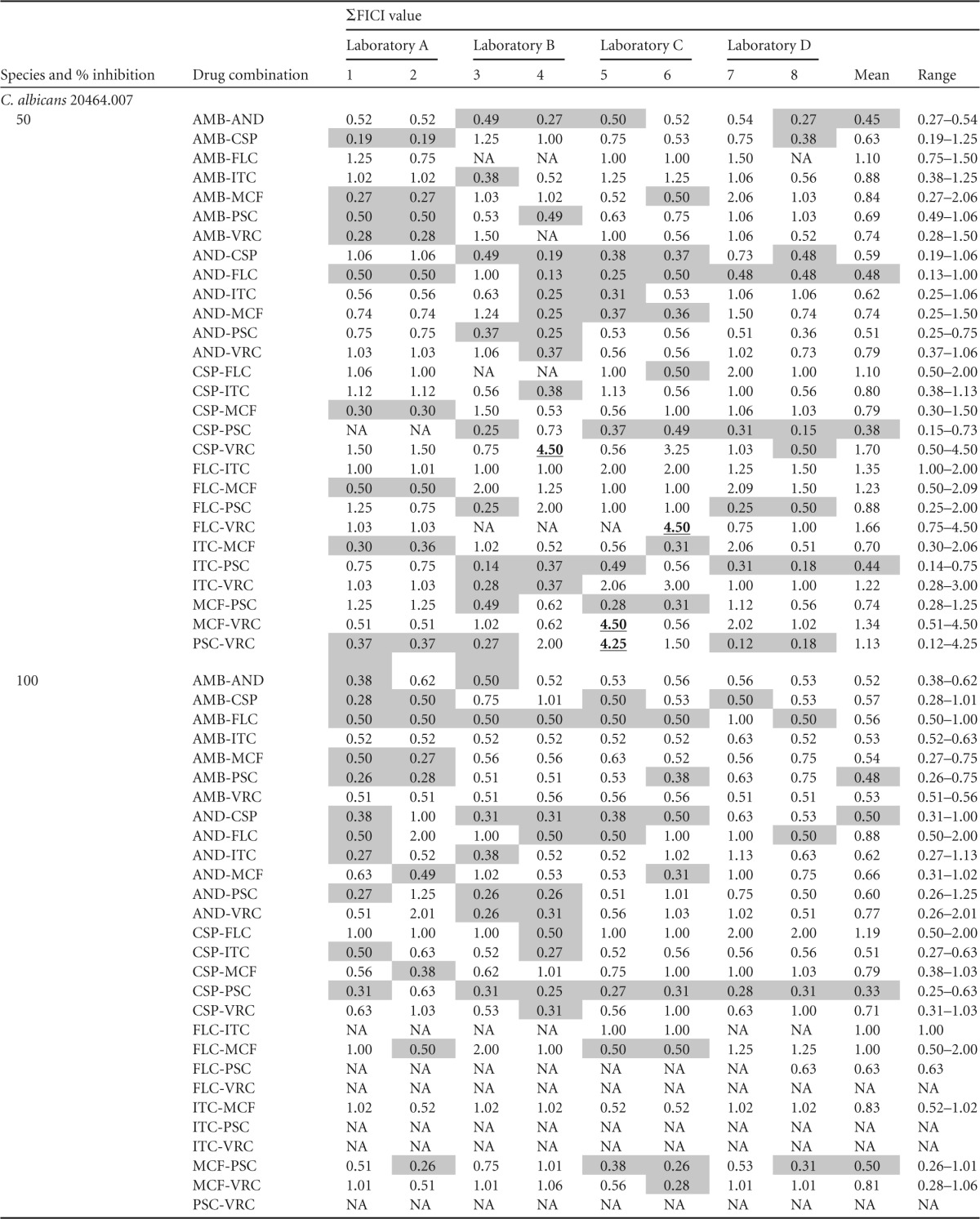


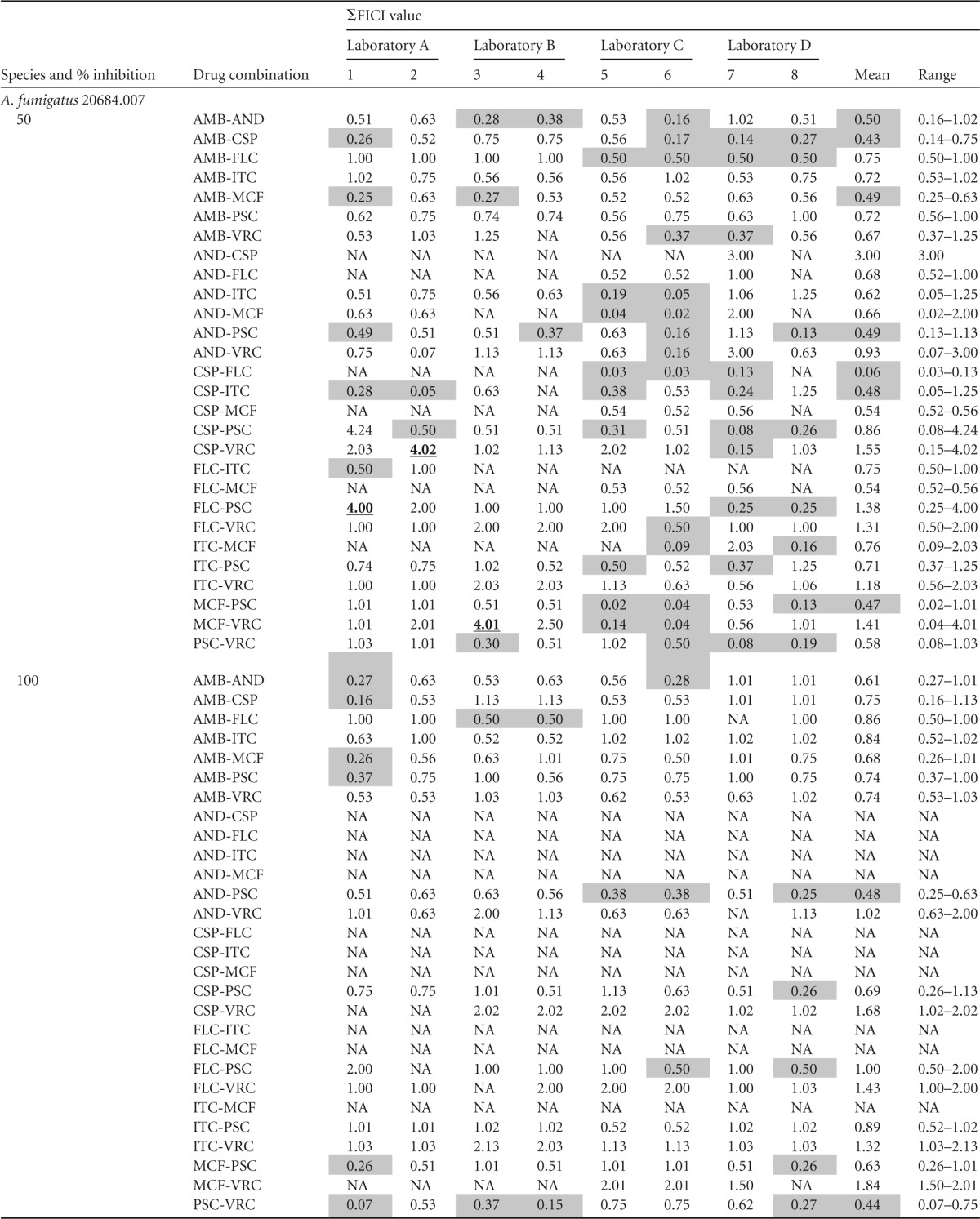
Shading indicates synergistic interactions (ΣFICI ≤ 0.5); boldface and underlining indicate antagonistic interactions. NA, not available; AMB, amphotericin B; AND, anidulafungin; CSP, caspofungin; FLC, fluconazole; ITC, itraconazole; MCF, micafungin; PSC, posaconazole; VRC, voriconazole.
The performance of each laboratory on two separate testing occasions was compared at different MIC reading levels against all 28 drug combinations using intralaboratory agreement rates and their correlation coefficients (IaCCs) (Table 2). IaCC was defined as the conformity between all the duplex events under the same experimental conditions. All four laboratories had over 80% intralaboratory agreement rates when MIC100 was used as the endpoint (Table 2). A comparison of intralaboratory agreement rates between MIC50 and MIC100 readings showed significant difference (P < 0.05). Furthermore, IaCCs were greater than 0.9 for all the laboratories at the MIC100 reading level, but one of the IaCCs at the MIC50 reading level was as low as 0.677. The performance between different laboratories for the yeasts or molds tested was compared at both the MIC50 and the MIC100 reading levels against 28 drug combinations by interlaboratory agreement rates and their correlation coefficients (IeCCs) (Table 3). IeCC aimed to examine the conformity between different laboratories for the experiment results on the same isolate. Again, the interlaboratory agreement rates were higher at the MIC100 reading level (86% ∼ 90%) than at the MIC50 reading level (73% ∼ 76%). The IeCCs at the MIC100 reading level were greater than 0.96 with a 99% confidence interval (CI) for both Candida species and A. fumigatus. The IeCCs were around 0.86 with 99% CI for different isolates at the MIC50 reading level.
TABLE 2.
Intralaboratory agreement rates and correlation coefficients (IaCCs) for each participating laboratory
| Laboratory | Value for MIC50/value for MIC100 |
|
|---|---|---|
| Agreement of ΣFICI interpretation (%)a,c | IaCCb | |
| A | 81/87 | 0.927/0.925 |
| B | 79/95 | 0.893/0.983 |
| C | 76/89 | 0.876/0.955 |
| D | 68/82 | 0.677/0.921 |
Agreement was calculated as the percentage of the number of the most frequent categorized ΣFICI values divided by the number of all ΣFICI values.
Calculated at a 99% confidence interval.
The agreement rates at the 100% inhibition level were statistically significantly higher than those at the 50% inhibition level (P < 0.05).
TABLE 3.
Interlaboratory agreement rates and correlation coefficients (IeCCs) for each yeast or mold tested
| Fungus | Value for MIC50/value for MIC100 |
|
|---|---|---|
| Agreement of ΣFICI interpretation (%)a,c | IeCCb | |
| Candida species | 76/86 | 0.868/0.962 |
| Aspergillus fumigatus | 73/90 | 0.861/0.978 |
Agreement was calculated as the percentage of the number of the most frequent categorized ΣFICI values divided by the number of all ΣFICI values.
Calculated at a 99% confidence interval.
The agreement rates at the 100% inhibition level were statistically significantly higher than those at the 50% inhibition level (P < 0.05).
This study marks another milestone in our quest to define a standard method for antifungal combination testing in clinical laboratories (14, 15). The superior intra- and interlaboratory agreements obtained using MIC100 endpoints were the highlight of this study. However, we also found that different drug combinations yielded variable interpretations for the same isolate, even when MIC100 endpoints were used. These results affirm earlier suggestions that the FIC index model is subjective and sensitive to experimental error (17). The checkerboard dilution method used for the calculation of ΣFICI assumes that all antifungal drugs interact with each other in a linear model, providing an all-or-none view, thus artificially creating ΣFICI values (21, 22). Notably, fixed concentrations or fixed ratios of antimicrobial combinations were found to yield more useful results, especially for antibacterial combination testing (20, 23, 24). A single study found fixed ratios to be a better approach for antifungal combination testing (25). Thus, our future multilaboratory studies will assess fixed-concentration or fixed-ratio antifungal combination testing by the microbroth method using MIC100 endpoints.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by funds from the Wadsworth Center Clinical Laboratory Reference System (V.C.). Additional support came in the form of investigative grants from Astellas USA, Merck & Co., Pfizer Inc., and Schering-Plough Research Institute (V.C.). Trek Diagnostic Systems partially subsidized the cost of custom 96-well plates.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04545-14.
REFERENCES
- 1.Azie N, Neofytos D, Pfaller MA, Meier-Kriesche HU, Quan SP, Horn D. 2012. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis 73:293–300. doi: 10.1016/j.diagmicrobio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Denning DW, Levitz SM. 2012. Tackling human fungal infections. Science 336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- 3.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 4.Zilberberg MD, Kollef MH, Arnold H, Labelle A, Micek ST, Kothari S, Shorr AF. 2010. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis 10:150. doi: 10.1186/1471-2334-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah DN, Yau R, Weston J, Lasco TM, Salazar M, Palmer HR, Garey KW. 2011. Evaluation of antifungal therapy in patients with candidaemia based on susceptibility testing results: implications for antimicrobial stewardship programmes. J Antimicrob Chemother 66:2146–2151. doi: 10.1093/jac/dkr244. [DOI] [PubMed] [Google Scholar]
- 6.CLSI. 2009. Method for antifungal disk diffusion susceptibility testing of yeasts. Approved guideline, 2nd ed CLSI document M44-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 7.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed CLSI document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.CLSI. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts. Fourth informational supplement. CLSI document M27-S4 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.EUCAST. 2008. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. EUCAST definitive document E.DEF 9.1. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID, European Committee for Antimicrobial Susceptibility Testing (EUCAST). [Google Scholar]
- 11.EUCAST. 2012. Method for the determination of broth dilution of antifungal agents for fermentative yeasts; revised. EUCAST definitive document EDef 7.2, revision. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID, European Committee for Antimicrobial Susceptibility Testing (EUCAST). [DOI] [PubMed] [Google Scholar]
- 12.Candoni A, Caira M, Cesaro S, Busca A, Giacchino M, Fanci R, Delia M, Nosari A, Bonini A, Cattaneo C, Melillo L, Caramatti C, Milone G, Scime R, Picardi M, Fanin R, Pagano L. 2014. Multicentre surveillance study on feasibility, safety and efficacy of antifungal combination therapy for proven or probable invasive fungal diseases in haematological patients: the SEIFEM real-life combo study. Mycoses 57:342–350. doi: 10.1111/myc.12161. [DOI] [PubMed] [Google Scholar]
- 13.Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ, Thai le H, Chuong LV, Sinh DX, Duong VA, Hoang TN, Diep PT, Campbell JI, Sieu TP, Baker SG, Chau NV, Hien TT, Lalloo DG, Farrar JJ. 2013. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi V, Ramani R, Andes D, Diekema DJ, Pfaller MA, Ghannoum MA, Knapp C, Lockhart SR, Ostrosky-Zeichner L, Walsh TJ, Marchillo K, Messer S, Welshenbaugh AR, Bastulli C, Iqbal N, Paetznick VL, Rodriguez J, Sein T. 2011. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candida glabrata, and Candida parapsilosis. Antimicrob Agents Chemother 55:1543–1548. doi: 10.1128/AAC.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi V, Ramani R, Ghannoum MA, Killian SB, Holliday N, Knapp C, Ostrosky-Zeichner L, Messer SM, Pfaller MA, Iqbal NJ, Arthington-Skaggs BA, Vazquez JA, Sein T, Rex JH, Walsh TJ. 2008. Multilaboratory testing of antifungal combinations against a quality control isolate of Candida krusei. Antimicrob Agents Chemother 52:1500–1502. doi: 10.1128/AAC.00574-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isenberg HD. (ed). 1992. Synergism testing: broth microdilution checkerboard and broth macrodilution methods. American Society for Microbiology, Washington, DC. [Google Scholar]
- 17.Meletiadis J, Verweij PE, Te Dorsthorst DT, Meis JF, Mouton JW. 2005. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol 43:133–152. doi: 10.1080/13693780410001731547. [DOI] [PubMed] [Google Scholar]
- 18.Te Dorsthorst DT, Verweij PE, Meletiadis J, Bergervoet M, Punt NC, Meis JF, Mouton JW. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob Agents Chemother 46:2982–2989. doi: 10.1128/AAC.46.9.2982-2989.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGraw KO, Wong SP. 1996. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1:30–46. doi: 10.1037/1082-989X.1.1.30. [DOI] [Google Scholar]
- 20.Milan-Segovia RC, Dominguez-Ramirez AM, Jung-Cook H, Magana-Aquino M, Romero-Mendez MC, Medellin-Garibay SE, Vigna-Perez M, Romano-Moreno S. 2010. Relative bioavailability of rifampicin in a three-drug fixed-dose combination formulation. Int J Tuberc Lung Dis 14:1454–1460. [PubMed] [Google Scholar]
- 21.Cuenca-Estrella M. 2004. Combinations of antifungal agents in therapy—what value are they? J Antimicrob Chemother 54:854–869. doi: 10.1093/jac/dkh434. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob Agents Chemother 48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agrawal S, Kaur KJ, Singh I, Bhade SR, Kaul CL, Panchagnula R. 2002. Assessment of bioequivalence of rifampicin, isoniazid and pyrazinamide in a four drug fixed dose combination with separate formulations at the same dose levels. Int J Pharm 233:169–177. doi: 10.1016/S0378-5173(01)00939-5. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller MA, Barry AL, Fuchs PC, Gerlach EH, Hardy DJ, McLaughlin JC. 1993. Comparison of fixed concentration and fixed ratio options for dilution susceptibility testing of gram-negative bacilli to ampicillin and ampicillin/sulbactam. Eur J Clin Microbiol Infect Dis 12:356–362. doi: 10.1007/BF01964434. [DOI] [PubMed] [Google Scholar]
- 25.Jones RN, Castanheira M, Pfaller MA. 2010. Fixed-ratio combination testing of an echinocandin, anidulafungin, and an azole, voriconazole, against 1,467 Candida species isolates. Antimicrob Agents Chemother 54:4041–4043. doi: 10.1128/AAC.00330-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


