Abstract
The influence of hypoxia on the in vitro activities of amphotericin B, azoles, and echinocandins against Aspergillus spp. was evaluated by comparing MICs, minimal fungicidal concentrations (MFCs), and epidemiological cutoffs (ECOFFs). Changes of MIC distributions due to hypoxia largely depend on the method, the species, and the growth ability under hypoxia. The activities of antifungals were not significantly altered under hypoxia, except for Aspergillus terreus, for which the activity changed from fungicidal to fungistatic.
TEXT
At sites of infection, microenvironmental factors influence the growth of fungal pathogens and most likely also the efficacy of antifungal drugs (1). Hypoxia is one microenvironmental stress that occurs during pulmonary fungal infections in vivo (2) and has a significant impact on antifungal targets such as ergosterol biosynthesis or β-glucan in Aspergillus fumigatus (3, 4). Simulating the host environment in in vitro susceptibility testing will contribute to a better understanding of how these conditions influence antifungal activity.
In this study, the in vitro activities of amphotericin B, triazoles, and echinocandins against Aspergillus spp. under hypoxia were evaluated by using the Etest (bioMérieux, France) and broth microdilution method according to EUCAST guideline 9.2 (5). Epidemiological cutoff values (ECOFFs) were established and set two dilution steps higher than the modal MIC (6). Both methods were chosen to verify the different impacts of oxygen on surface (exposure to 1%) or liquid cultures, where the oxygen concentration might also vary in normoxic cultures. Putative changes from fungicidal to fungistatic activity were determined based on minimal fungicidal concentrations (MFCs) (7), defined as the lowest drug concentration resulting in 99.9% killing.
All clinical isolates tested (n = 49) were identified by internal transcribed spacer sequencing, according to the methods of White et al. (8). The strain set comprised A. fumigatus (n = 25), including five azole-resistant isolates with a mutation in cyp51A (9), A. terreus (n = 16), and A. flavus (n = 8). Hypoxic conditions were set to 1% O2, 5% CO2, 94% N2 (C-Chamber and Pro-Ox, Pro-CO2 controller; Biospherics), and all experiments were done in parallel under normoxia (∼21% O2). To check for a normal distribution, the D'Agostino and Pearson omnibus normality test was performed. The Kruskal-Wallis test was applied, since data were not normally distributed. P values of ≤0.05 were regarded as statistically significant. For supplemented media, ergosterol or cholesterol (25 μM) was mixed with coenzyme Q10 (5 μM) and added to RPMI agar. Additionally, Etests were conducted on blood agar (25% [vol/vol]). To compare fungal growth under both oxygen conditions, radial growth assays were performed according to methods described previously (10).
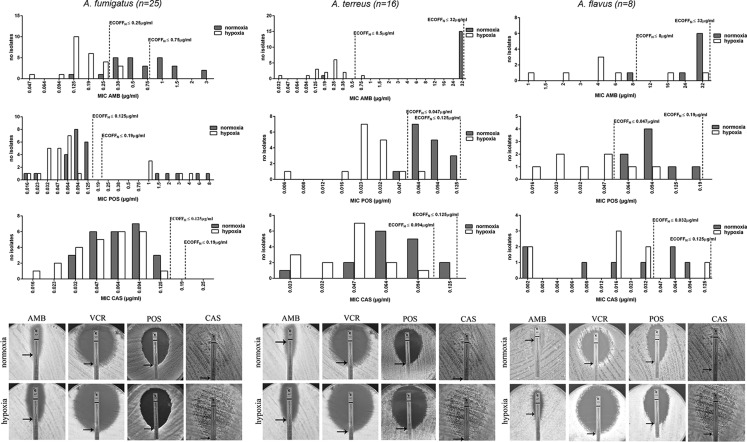
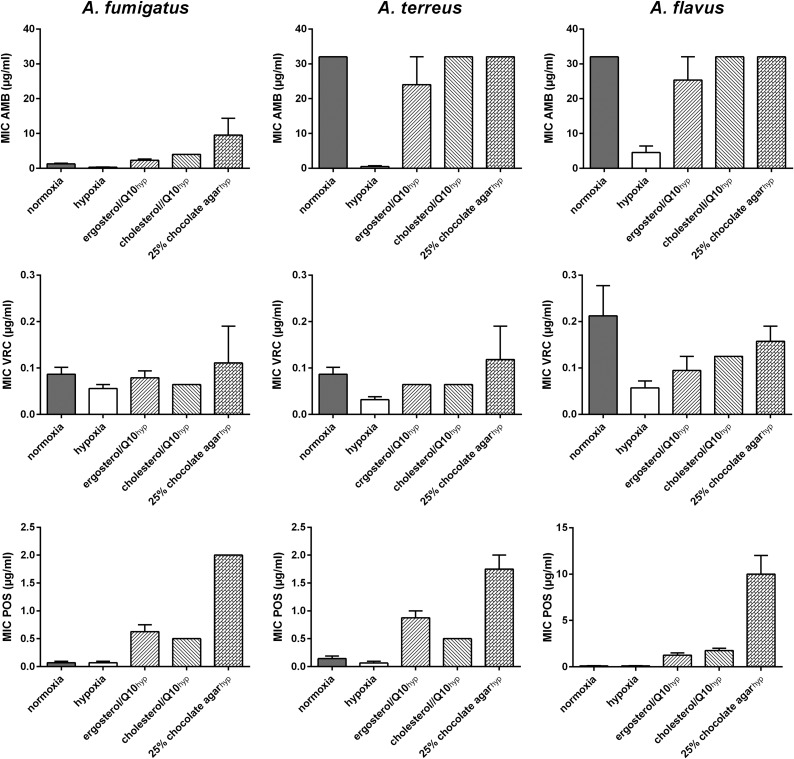
With the Etest, the influence of hypoxia on the susceptibility profile demonstrated a species- and drug-dependent manner (Fig. 1; Table 1). Among all Aspergillus spp. tested, A. fumigatus isolates exhibited the lowest oxygen-dependent changes in MICs for all antifungals tested. A significant reduction of the MIC distribution was observed for amphotericin B, while no alterations in MICs for azoles and echinocandins were detected. A. fumigatus strains carrying a mutation in the cyp51A gene did not show differences in azole susceptibility. Aspergillus terreus isolates, a species that is intrinsically resistant to amphotericin B (11, 12), exhibited susceptibility under hypoxia, with a significant decrease in the MIC distribution (12 log2 dilutions). Lower MICs were mainly due to the missing mycelium sterilium zone (Fig. 1). For the azoles, a significant reduction in the MIC distribution was observed under hypoxia while, as for A. fumigatus, no alteration was detected for echinocandins. The same results were found for A. flavus. Reductions in MICs under hypoxia were abrogated by addition of ergosterol, cholesterol, or whole blood to the medium (Fig. 2). MIC changes under hypoxia correlated with impaired growth under hypoxia; the in vitro susceptibilities of fungi that were less sensitive to low oxygen concentrations were less affected (Fig. 3).
FIG 1.
MIC distributions for amphotericin B (AMB), posaconazole (POS), and caspofungin (CAS) for A. fumigatus (left column), A. terreus (middle column), and A. flavus (right column) strains under normoxic (gray) and hypoxic (white) growth conditions. Antifungal susceptibility testing was performed via the Etest method, MICs were determined under both oxygen conditions after 48 h at 37°C, and ECOFFs were established for both conditions. Images present results for one representative strain of the species group.
TABLE 1.
In vitro susceptibilities to amphotericin B, azoles, and echinocandins of Aspergillus species, determined by Etesta
| Agentb |
A. fumigatus isolates (n = 26) |
A. terreus isolates (n = 14) |
A. flavus isolates (n = 8) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normoxia |
Hypoxia |
Normoxia |
Hypoxia |
Normoxia |
Hypoxia |
|||||||||||||
| MIC range | MIC50 | ECOFF | MIC range | MIC50 | ECOFF | MIC range | MIC50 | ECOFF | MIC range | MIC50 | ECOFF | MIC range | MIC50 | ECOFF | MIC range | MIC50 | ECOFF | |
| AMB | 0.125–3 | 0.75 | 0.75 | 0.047–0.38 | 0.19 | 0.25 | 0.19 to >32 | >32 | >32 | 0032–0.75 | 0.25 | 0.5 | 8 to >32 | 32 | >32 | 1 to >32 | 4 | 8 |
| VRC | 0.064–8 | 0.125 | 0.19 | 0.032–4 | 0.064 | 0.094 | 0.032–0.125 | 0.064 | 0.125 | 0.008–0.032 | 0.016 | 0.032 | 0.19–0.38 | 0.25 | 0.5 | 0.016–0.064 | 0.047 | 0.094 |
| POS | 0.016–8 | 0.094 | 0.19 | 0.016–4 | 0.064 | 0.125 | 0.047–0.125 | 0.064 | 0.125 | 0.006–0.064 | 0.023 | 0.047 | 0.064–0.19 | 0.094 | 0.19 | 0.016–0.094 | 0.023 | 0.047 |
| ITR | 0.064–4 | 0.5 | 1 | 0.047–4 | 0.25 | 0.5 | 0.25–0.5 | 0.25 | 0.5 | 0.023–0.125 | 0.094 | 0.19 | 0.5–1 | 0.75 | 1.5 | 0.047–0.75 | 0.38 | 0.75 |
| CAS | 0.032–0.125 | 0.064 | 0.19 | 0.016–0.125 | 0.064 | 0.125 | 0.023–0.125 | 0.064 | 0.125 | 0.023–0.094 | 0.047 | 0.094 | 0.002–0.094 | 0.016 | 0.125 | 0.002–0.125 | 0.016 | 0.032 |
| AND | 0.002–0.047 | 0.016 | 0.032 | 0.004–0.032 | 0.016 | 0.032 | 0.002–0.006 | 0.002 | 0.004 | 0.002–0.006 | 0.002 | 0.004 | 0.002–0.002 | 0.002 | 0.006 | 0.002–0.002 | 0.002 | 0.004 |
| MYC | 0.002–0.19 | 0.002 | 0.004 | 0.002–0.094 | 0.002 | 0.004 | 0.002–0.008 | 0.004 | 0.008 | 0.002–0.016 | 0.006 | 0.012 | 0.002–0.19 | 0.002 | 0.006 | 0.002–0.125 | 0.002 | 0.004 |
MICs (in μg/ml) were determined after growth for 48 h at 37°C under normal oxygen conditions or hypoxic growth conditions.
AMB, amphotericin B; VRC, voriconazole; POS, posaconazole; ITR, itraconazole; CAS, caspofungin; AND, anidulafungin; MYC, micafungin.
FIG 2.
Supplementation of ergosterol/coenzyme Q10 (Q10), cholesterol/Q10, and blood enhances antifungal susceptibilities to amphotericin B (AMB), voriconazole (VRC), and posaconazole (POS) of Aspergillus spp. in hypoxia. Final concentrations of ergosterol or cholesterol and Q10 (Sigma-Aldrich, Germany) in RPMI 1640 agar were 25 µM for the sterols and 5 µM for Q10, respectively. Chocolate agar consisted of 25% (vol/vol) whole blood added to water agar, cooked for 30 min at 80°C. Bars represent MICs of one representative isolate of A. fumigatus, A. fumigatus, and A. flavus.
FIG 3.
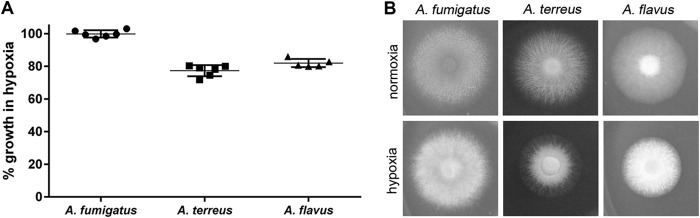
Hypoxia influences the growth of Aspergillus species. A total of 1 × 104 conidia were point inoculated on RPMI 1640 plates and incubated for 48 h at 37°C under normal oxygen and hypoxic growth conditions before colony diameter was determined. (A) Percentage of radial growth of hypoxic cultures normalized to normoxic growth (= 100%). (B) One representative example of six parallels.
In broth microdilution assays, MICs of voriconazole and posaconazole were not altered under hypoxia for all Aspergillus spp. tested (Table 2). For only amphotericin B was a stepwise decrease in MICs (≤2 log2 dilutions) under hypoxia prominent for A. terreus and A. flavus strains, while no difference was detected for A. fumigatus strains. Minimal effective concentrations (MECs) for caspofungin were not significantly influenced by hypoxia.
TABLE 2.
In vitro susceptibilities of Aspergillus spp., determined by the EUCAST methoda
| Species (n) | Agent | Normoxia |
Hypoxia |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | ECOFF | MFC range | MFC | MIC range | MIC50 | MIC90 | ECOFF | MFC range | MFC | ||
| A. fumigatus (25) | AMB | 0.5–1 | 1 | 1 | 4 | 1–2 | 2 | 0.25–1 | 0.5 | 1 | 2 | 1–2 | 2 |
| VRC | 0.125–8 | 0.5 | 6 | 2 | 0.25–1 | 0.5 | 0.125–4 | 0.25 | 2 | 1 | 0.25–0.5 | 0.25 | |
| POS | 0.25–8 | 0.5 | 6 | 2 | 0.5–1 | 1 | 0.125–8 | 0.25 | 6 | 1 | 0.125–0.5 | 0.5 | |
| CASb | 0.125–0.5 | 0.25 | 0.5 | 1 | 16 | >16 | 0.125–0.5 | 0.25 | 0.5 | 1 | 16 | >16 | |
| A. terreus (16) | AMB | 1–8 | 4 | 8 | 16 | 4–16 | >16 | 0.5–2 | 1 | 2 | 4 | 16 | >16 |
| VRC | 0.25–0.75 | 0.5 | 0.5 | 1 | 4–8 | 8 | 0.125–0.25 | 0.25 | 0.25 | 0.5 | 8–16 | >16 | |
| POS | 0.25–0.5 | 0.5 | 0.5 | 2 | 0.25–4 | 4 | 0.25–0.5 | 0.25 | 0.5 | 1 | 4–16 | >16 | |
| CASb | 0.06–0.5 | 0.125 | 0.25 | 0.5 | 16 | >16 | 0.03–0.5 | 0.125 | 0.25 | 0.5 | 16 | >16 | |
| A. flavus (8) | AMB | 1–4 | 2 | 4 | 8 | 1–4 | 2 | 1–2 | 1 | 2 | 4 | 2–16 | 2 |
| VRC | 0.5–1 | 1 | 1 | 4 | 0.5–2 | 1 | 0.25–1 | 0.5 | 0.5 | 2 | 0.5–1 | 1 | |
| POS | 0.25–0.5 | 0.5 | 0.5 | 2 | 0.25–1 | 0.5 | 0.125–0.5 | 0.25 | 0.5 | 1 | 0.25–0.5 | 0.5 | |
| CASb | 0.06–0.5 | 0.25 | 0.25 | 1 | 16 | >16 | 0.03–0.25 | 0.125 | 0.125 | 0.5 | 16 | >16 | |
MIC/MEC, MFC, and ECOFF values for amphotericin B (AMB), voriconazole (VRC), posaconazole (POS), and caspofungin (CAS) (in μg/ml) were determined under normal oxygen conditions and hypoxic conditions.
Data for caspofungin are MEC (minimal effective concentration) values rather than MIC values.
MFCs demonstrated no alterations between hypoxic and normoxic conditions for A. fumigatus and A. flavus strains (Table 2), and this correlated with already published data (7). For A. terreus strains, either increased or no MFCs were detected for azoles under hypoxia. Similarly, significantly more colonies were able to recover from cultures treated with amphotericin B under hypoxia, although no MFCs could be determined under either oxygen condition.
So far, only a few studies have investigated the effect of hypoxia on antifungal susceptibility of Aspergillus spp., focusing either on some antifungal agents (13) or on one standard in vitro test method (7). Similar to what was shown for anidulafungin (13), MIC/MEC readings were much easier to obtain under hypoxia, as typical “trailing” (microcolonies within the inhibition zone [14]) was less pronounced for echinocandins. The observed reductions in the MICs, being more pronounced with the agar-based method than in liquid assays, matched results obtained by Warn et al. (7) and might have been due to oxygen depletion in microtiter plates, even under normoxia. Increased susceptibility to antifungals that target ergosterol itself or its biosynthesis (oxygen-dependent pathway [3]) indicated that the fungus has to cope with two stressors: antifungal pressure and maintenance of membrane stability, despite lacking oxygen as a cofactor for ergosterol biosynthetic enzymes. Additionally, MICs under hypoxia rose to the levels of those under normoxia when membrane compounds were available. Xiong et al. (15) demonstrated that cholesterol is integrated into fungal membranes to compensate for ergosterol depletion during azole treatment. Further, cholesterol can be used as a putative carbon source in filamentous fungi (16) and thereby enhance growth.
Except for A. terreus, MFCs were less influenced by oxygen than were MICs of surface cultures. This may even better reflect the actual situation in the host, as Rex et al. (17) already suggested that MFCs are more relevant for predicting the clinical outcome. For A. terreus, no MFCs were detectable, suggesting a shift to fungistatic activity under low-oxygen conditions. Slesonia et al. (18) showed that A. terreus is able to persist and survive without germination within acidified phagolysosomes due to the resistance against microbicidal enzymes. Also, conidia are more resistant to environmental conditions than are hyphae (19). Therefore, delayed germination, especially after diluting the antifungal agent by plating on agar, could contribute to enhanced resistance against antifungal drugs under hypoxia.
In conclusion, hypoxia influenced in vitro antifungal susceptibilities of Aspergillus spp. marginally, and observed differences were most pronounced with the Etest. Importantly, changes in antifungal activities against A. terreus strains under hypoxia might partially explain the high failure rate of antifungal therapy in vivo (12, 20).
ACKNOWLEDGMENTS
We thank Caroline Hörtnagl for technical assistance.
This work is supported by the Austrian Science Foundation (FWF), within the coordinated action of ERA-NET PathoGenoMics (ZFI006610) to C.L.F.
In the past 5 years, C.L.F. has received grant support from the Austrian Science Foundation, Astellas Pharma, Gilead Sciences, Pfizer, Schering Plough, and Merck Sharp & Dohme. She has been an advisor/consultant to Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. She has received honoraria for talks and travel costs from Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas Pharma, and Schering Plough. The other authors have no conflicts of interest to declare.
REFERENCES
- 1.Bartizal C, Odds FC. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrob Agents Chemother 47:2100–2107. doi: 10.1128/AAC.47.7.2100-2107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, Cramer RA. 2011. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog 7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker BM, Kroll K, Vodisch M, Mazurie A, Kniemeyer O, Cramer RA. 2012. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC Genomics 13:62. doi: 10.1186/1471-2164-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepardson KM, Ngo LY, Aimanianda V, Latge JP, Barker BM, Blosser SJ, Iwakura Y, Hohl TM, Cramer RA. 2013. Hypoxia enhances innate immune activation to Aspergillus fumigatus through cell wall modulation. Microbes Infect 15:259–269. doi: 10.1016/j.micinf.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendrup M. C. C-EM, Lass-Flörl C., Hope W., Howard S.J. and Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committeefor Antimicrobial Susceptibility Testing (EUCAST) . 2014. EUCAST definitive document EDef 9.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. EUCAST, Växjö, Sweden. [Google Scholar]
- 6.Rodriguez-Tudela JL, Hope W, Cuenca-Estrella M, Donnelly JP, Lass-Flörl C, Arendrup MC. 2011. Can we achieve clinical breakpoints for the triazoles in aspergillosis? Curr Fungal Infect Rep 5:128–134. doi: 10.1007/s12281-011-0058-6. [DOI] [Google Scholar]
- 7.Warn PA, Sharp A, Guinea J, Denning DW. 2004. Effect of hypoxic conditions on in vitro susceptibility testing of amphotericin B, itraconazole and micafungin against Aspergillus and Candida. J Antimicrob Chemother 53:743–749. doi: 10.1093/jac/dkh153. [DOI] [PubMed] [Google Scholar]
- 8.White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Immis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, New York, NY. [Google Scholar]
- 9.Van der Linden J. 2011. Prospective international surveillance of azole resistance (AR) in Aspergillus fumigatus (Af) (SCARE-Network). Abstr 51st Intersci Conf Antimicrob Agents Chemother, Chicago, IL, abstr M-490. American Society for Microbiology, Washington, DC. [Google Scholar]
- 10.Binder U, Oberparleiter C, Meyer V, Marx F. 2010. The antifungal protein PAF interferes with PKC/MPK and cAMP/PKA signalling of Aspergillus nidulans. Mol Microbiol 75:294–307. doi: 10.1111/j.1365-2958.2009.06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara KS, Ryu JH, Lie JT, Roberts GD. 1989. Disseminated Aspergillus terreus infection in immunocompromised hosts. Mayo Clin Proc 64:770–775. doi: 10.1016/S0025-6196(12)61749-2. [DOI] [PubMed] [Google Scholar]
- 12.Steinbach WJ, Benjamin DK Jr, Kontoyiannis DP, Perfect JR, Lutsar I, Marr KA, Lionakis MS, Torres HA, Jafri H, Walsh TJ. 2004. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis 39:192–198. doi: 10.1086/421950. [DOI] [PubMed] [Google Scholar]
- 13.Perkhofer S, Jost D, Dierich MP, Lass-Florl C. 2008. Susceptibility testing of anidulafungin and voriconazole alone and in combination against conidia and hyphae of Aspergillus spp. under hypoxic conditions. Antimicrob Agents Chemother 52:1873–1875. doi: 10.1128/AAC.01572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morace G, Borghi E, Iatta R, Montagna MT. 2009. Anidulafungin, a new echinocandin: in vitro activity. Drugs 69(Suppl 1):S91–S94. doi: 10.2165/11315560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Xiong Q, Hassan SA, Wilson WK, Han XY, May GS, Tarrand JJ, Matsuda SP. 2005. Cholesterol import by Aspergillus fumigatus and its influence on antifungal potency of sterol biosynthesis inhibitors. Antimicrob Agents Chemother 49:518–524. doi: 10.1128/AAC.49.2.518-524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.al Musallam AA, Radwan SS. 1990. Wool-colonizing micro-organisms capable of utilizing wool-lipids and fatty acids as sole sources of carbon and energy. J Appl Bacteriol 69:806–813. doi: 10.1111/j.1365-2672.1990.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 17.Rex JH, Pfaller MA, Walsh TJ, Chaturvedi V, Espinel-Ingroff A, Ghannoum MA, Gosey LL, Odds FC, Rinaldi MG, Sheehan DJ, Warnock DW. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin Microbiol Rev 14:643–658. doi: 10.1128/CMR.14.4.643-658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slesiona S, Gressler M, Mihlan M, Zaehle C, Schaller M, Barz D, Hube B, Jacobsen ID, Brock M. 2012. Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages. PLoS One 7:e31223. doi: 10.1371/journal.pone.0031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond RD. 1988. Fungal surfaces: effects of interactions with phagocytic cells. Rev Infect Dis 10(Suppl 2):S428–S431. doi: 10.1093/cid/10.Supplement_2.S428. [DOI] [PubMed] [Google Scholar]
- 20.Lass-Florl C, Griff K, Mayr A, Petzer A, Gastl G, Bonatti H, Freund M, Kropshofer G, Dierich MP, Nachbaur D. 2005. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br J Haematol 131:201–207. doi: 10.1111/j.1365-2141.2005.05763.x. [DOI] [PubMed] [Google Scholar]