Abstract
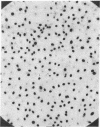
Of all the components of the culture medium, only CaCl2 induces DNA replication when added to resting cultures of Balb/c 3T3 cells. The effect is present even in a serum-free medium. Increasing the Ca++ concentration above the standard 1.8 mM in the medium of a new culture increases the total number of cells ultimately produced, without affecting the initial cell growth rate. This effect is synergistic with that of serum. The elevated Ca++ concentration also induces striking morphological changes. The Ca++ effect could not be reproduced by a Ca++ ionophore. These observations afford a new tool for studying how the various intracellular events following the addition of growth factors to resting cultures are involved in the control of cellular growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. D. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Feb;68(2):271–275. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Morton H. J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974 Jul-Aug;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Conditions limiting multiplication of fibroblastic and epithelial cells in dense cultures. Nature. 1973 Nov 23;246(5430):197–199. doi: 10.1038/246197a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A. Control of growth of a tumor cell by linoleic acid. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3976–3978. doi: 10.1073/pnas.71.10.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maino V. C., Green N. M., Crumpton M. J. The role of calcium ions in initiating transformation of lymphocytes. Nature. 1974 Sep 27;251(5473):324–327. doi: 10.1038/251324b0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S., Gospodarowicz D., Seifert W. Activation of guanyl cyclase and intracellular cyclic GMP by fibroblast growth factor. Nature. 1974 Aug 30;250(5469):741-2, 773-4. doi: 10.1038/250741a0. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Seifert W., Gospodarowicz D. Growth control in cultured mouse fibroblasts: induction of the pleiotypic and mitogenic responses by a purified growth factor. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2600–2604. doi: 10.1073/pnas.71.7.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- Stoker M. G. Role of diffusion boundary layer in contact inhibition of growth. Nature. 1973 Nov 23;246(5430):200–203. doi: 10.1038/246200a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., Piggott D. Shaking 3T3 cells: further studies on diffusion boundary effects. Cell. 1974 Nov;3(3):207–215. doi: 10.1016/0092-8674(74)90133-0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Boynton A. L., Gillan D. J., Isaacs R. J. Concanavalin A and the initiation of thymic lymphoblast DNA synthesis and proliferation by a calcium-dependent increase in cyclic GMP level. J Cell Physiol. 1974 Dec;84(3):445–458. doi: 10.1002/jcp.1040840312. [DOI] [PubMed] [Google Scholar]