Abstract
Esophageal candidiasis is a frequent cause of morbidity in immunocompromised patients. Isavuconazole is a novel, broad-spectrum antifungal developed for the treatment of opportunistic fungal infections. This phase 2 trial compared the efficacy and safety of three oral dosing regimens of isavuconazole with an oral fluconazole regimen in the primary treatment of uncomplicated esophageal candidiasis. The isavuconazole regimens were as follows: 200 mg on day 1 and then 50 mg once daily (arm A), 400 mg on day 1 and then 400 mg once-weekly (arm B), and 400 mg on day 1 and then 100 mg once daily (arm C). Patients in arm D received fluconazole at 200 mg on day 1 and then 100 mg once daily. The minimum treatment duration was 14 days. The primary endpoint was the rate of endoscopically confirmed clinical response at end of therapy. Safety and tolerability were also assessed. Efficacy was evaluated in 153 of 160 enrolled patients. Overall, 146 (95.4%) achieved endoscopically confirmed clinical success. Each of the isavuconazole regimens was shown to be not inferior to fluconazole, i.e., arm A versus D, −0.5% (95% confidence interval [CI] −10.0 to 9.4), arm B versus D, 3.5% (95% CI, −5.6 to 12.7), and arm C versus D, −0.2% (95% CI, −9.8 to 9.4). The frequency of adverse events was similar in arm A (n = 22; 55%), arm B (n = 18; 45%), and arm D (n = 22; 58%), but higher in arm C (n = 29; 71%). In summary, efficacy and safety of once-daily and once-weekly isavuconazole were comparable with once-daily fluconazole in the primary treatment of uncomplicated esophageal candidiasis.
INTRODUCTION
Esophageal candidiasis is an opportunistic fungal infection that commonly occurs in immunocompromised patients. Individuals with HIV infection are particularly at risk (1), even in the era of antiretroviral therapy (2). Infections are predominantly caused by Candida albicans; however, infections with other non-albicans Candida species such as C. glabrata, C. krusei, and C. parapsilosis have also been reported (3–6). Although seldom fatal, esophageal candidiasis is associated with significant morbidity, causing dysphagia, odynophagia, and retrosternal pain (1).
Current guidelines recommend fluconazole, an echinocandin, or amphotericin B for the primary treatment of esophageal candidiasis (7). Recommended alternatives include itraconazole, posaconazole, and voriconazole (7). Candida albicans is usually susceptible to these commonly used antifungal agents; however, extended periods of antifungal treatment may lead to the development of microbiological resistance (7). Resistance to currently approved triazole medications, particularly fluconazole, is now well described in patients with HIV (8–12). Resistance to the echinocandins, such as caspofungin, is also starting to emerge (13). In addition, prolonged treatment may give rise to unwanted safety and tolerability effects. Thus, there is a therapeutic need for more, well-tolerated antifungal agents that are effective against emerging resistant Candida spp.
Isavuconazole is a novel, broad-spectrum, triazole, antifungal agent in development for the treatment of invasive fungal infections. The prodrug, isavuconazonium sulfate, is available in oral and intravenous formulations. After administration, it is immediately and completely converted to the active agent by esterases (14). The active agent is an inhibitor of sterol 14α-demethylase, which is required for biosynthesis of ergosterol, an essential component of fungal cell membranes (14). Isavuconazole displays concentration-dependent activity, pharmacodynamics that are related to its area under the concentration-time curve/MIC ratio, and a long half-life (14, 15).
In preclinical studies, isavuconazole has demonstrated potent activity in vitro against most clinically relevant fungal spp. including Candida spp., Aspergillus spp., Cryptococcus spp., and mucormycetes (16–19). The results of animal model studies have also shown that isavuconazole is effective in the treatment of invasive candidiasis, invasive aspergillosis, and mucormycosis (15, 20–23). Moreover, in a recent phase 2 study, isavuconazole was shown to be well tolerated as prophylaxis in neutropenic patients with acute myeloid leukemia (31).
The aim of this phase 2 study was to evaluate the safety and efficacy of three different dosing regimens of isavuconazole, compared to an approved once-daily fluconazole regimen, for the treatment of uncomplicated esophageal candidiasis.
MATERIALS AND METHODS
Patients.
All patients signed an Independent Ethics Committee-approved written, informed consent form prior to initiation of any study procedures. The study protocol was reviewed by the Independent Ethics Committee, and the study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki Good Clinical Practice, International Conference on Harmonization guidelines, and local applicable laws and regulations. This trial was initiated in December 2004 prior to introduction of the 2007 FDAAA 801 requirements for clinical trial reporting and was not prospectively registered.
Male and female patients, aged 18 to 65 years, who received a diagnosis of uncomplicated esophageal candidiasis within 5 days of the start of study treatment and who had one or more of the following symptoms were eligible for inclusion: dysphagia (higher than grade 0 but lower than grade 3), retrosternal pain and odynophagia (higher than grade 0), endoscopic confirmation of esophageal candidiasis (higher than grade 0), and confirmatory histology, cytology, or culture from esophageal brushings or biopsy samples (see Table S1 in the supplemental material). Uncomplicated esophageal candidiasis was defined as a single, mild to moderate episode of infection caused by Candida spp. Only postmenopausal or surgically sterile female patients were included, and all patients were included only if they had a life expectancy of at least 3 months with regard to their underlying condition, as judged by the study investigator.
Patients were excluded from the study if they were unable to swallow capsules due to dysphagia, if they had abnormalities that precluded endoscopy, or if they had reoccurrence of uncomplicated esophageal candidiasis after previous treatment with fluconazole within 3 months prior to the start of the study; more than two episodes of oral candidiasis or esophageal candidiasis within 12 months prior to the start of the study; another opportunistic fungal infection or therapy for another opportunistic fungal infection within 14 days prior to the start of the study; systemic antifungal therapy and treatment failure within 72 h prior to the start of the study; frank esophageal ulceration, or suspected other, or additional causes of esophagitis, e.g., viral, which may impact the clinical evaluation of the patient; hepatic dysfunction, including total bilirubin, alanine aminotransferase, or aspartate aminotransferase levels ≥5 times the upper limit of normal; moderate to severe renal dysfunction with a calculated creatinine clearance <50 ml/min or a history of oliguria (<20 ml/h) that was unresponsive to fluid challenge; or any concomitant medical condition that may have presented unacceptable risks to the patient, as judged by the study investigator.
Additional exclusion criteria included oral azole treatment within the 4 weeks prior to the first administration of study medication; concomitant use of rifampin, ritonavir, carbamazepine, long-acting barbiturates, ergot alkaloids, efavirenz, rifabutin, terfenadine, astemizole, cisapride, pimozide, quinidine, or neostigmine; and treatment with any investigational drug within 30 days prior to the first administration of study medication. Patients who received systemic antifungal therapy (systemic antifungal agent and/or oral, nonabsorbable, topical, antifungal agent) for at least 72 h prior to first administration of study medication and who had not clearly failed on this treatment (i.e., the patient's clinical condition had not worsened nor significantly improved) were also excluded from the study.
Study design.
The present study was designed as a phase 2, randomized, double-blind, parallel-group, noninferiority trial, to compare the safety and efficacy of three oral dosing regimens of isavuconazole with an oral fluconazole regimen in the treatment of uncomplicated esophageal candidiasis. It was conducted at eight clinical sites in South Africa from 30 December 2004 to 27 June 2005. In this report, isavuconazonium sulfate dosages are expressed in terms of the quantity of isavuconazole administered in milligram equivalents, e.g., isavuconazonium sulfate at 186.3 mg is equivalent to isavuconazole at 100 mg.
Patients were screened between days −5 and −1. Eligible patients who fulfilled the selection criteria were randomized 1:1:1:1 to four treatment arms according to a computer-generated randomization list. Patients in arm A received a single dose of oral isavuconazole at 200 mg on day 1 and then once-daily oral isavuconazole at 50 mg from day 2 to the end of therapy (EOT). Patients in arm B received a single dose of oral isavuconazole at 400 mg on day 1 and again on days 7, 14, and 21 (the day 21 dose was given only if day 14 was not the EOT). Patients in arm C received a single dose of oral isavuconazole at 400 mg on day 1 and then once-daily oral isavuconazole at 100 mg from day 2 to the EOT. Patients in arm D received a single dose of oral fluconazole at 200 mg on day 1 and then once-daily oral fluconazole at 100 mg from day 2 to the EOT.
Study drugs were administered as hard gelatin capsules, containing either isavuconazonium sulfate or fluconazole, with 200 ml of water. Oral isavuconazole at 400 mg was administered as four capsules, each containing isavuconazole 100 mg. Oral isavuconazole at 50 mg was administered as a single capsule, containing isavuconazole at 50 mg. There were no visible differences between the study drug capsules given to patients and all patients were given the same number of study drug capsules, with placebo given as necessary.
The dosing regimen used in arm A was chosen based on pharmacokinetics data obtained in phase 1 studies and on the in vitro MIC data obtained in studies of a number of Candida spp. However, since the efficacy of isavuconazole had not been evaluated in a clinical setting at the time of the study, a higher dosing regimen was also selected for arm C. The dosing regimen used in arm B was chosen based on the long terminal half-life of isavuconazole (i.e., 56 to 77 h when given orally) (14), which raised the possibility that high, weekly dosing may provide similar plasma isavuconazole levels as the dosing regimen in arm A. The dosing regimen used in arm D is the current U.S. Food and Drug Administration-approved regimen for fluconazole (24).
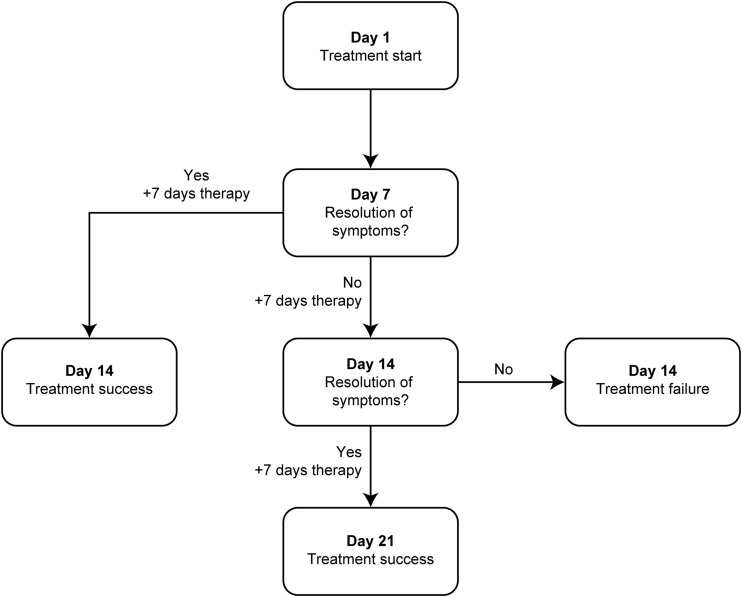
The duration of treatment was based on disease severity and clinical response and was consistent with previous clinical trials, current clinical practice, and guidelines for the treatment of esophageal candidiasis (3, 7, 25). The minimum and maximum treatment durations were 14 and 21 days, respectively. All patients received 7 days of treatment, followed by a clinical assessment on day 7. If resolution of clinical symptoms was observed at the day 7 assessment, the patient received an additional 7 days of treatment and was considered to be a treatment success (Fig. 1). If clinical symptoms were found to be ongoing on day 7, treatment was given for an additional 7 days, and another clinical assessment was conducted on day 14. If clinical symptoms had resolved by day 14, an additional 7 days of treatment were given (resulting in a treatment duration totaling a maximum of 21 days), and the patient was considered to be a treatment success. If clinical symptoms had not resolved on day 14, the patient was withdrawn from the study and classified as a treatment failure.
FIG 1.
Treatment and assessment schedule.
Patients considered to be a treatment success at the EOT completed follow-up assessments at 14 days (±2 days) and 28 days (±3 days) after the last dose of study drug. Patients considered to be a treatment failure at the EOT completed a follow-up assessment at 14 days (±2 days) after the last dose of study drug. Patients who did not attend follow-up assessments were considered treatment failures.
Endpoint assessments.
The primary efficacy endpoint was endoscopically confirmed clinical response at the EOT. Clinical response was categorized as cure (resolution of all clinical symptoms related to the infection, i.e., grade 0 in clinical and endoscopic evaluations; see Table S1 in the supplemental material), improvement (persistence of clinical symptoms but without worsening), failure (worsening of patient's condition or no response by day 3), and indeterminate (clinical evaluation not possible). Clinical success was defined as a clinical response of cure at the EOT, while clinical failure was defined as improvement or failure.
The secondary efficacy endpoints were overall therapeutic response at the EOT and microbiological response at the EOT. A positive therapeutic response was defined as resolution or improvement of clinical symptoms and endoscopic grades from baseline to the EOT. Improvement meant a reduction by ≥2 grades for each symptom and for the endoscopic evaluation.
During endoscopic evaluations, brushing for cytology and culture and a biopsy specimen for histology were taken. Microbiological response was categorized as either eradication (confirmation by histology or cytology that the original causative Candida spp. had been eradicated), persistence (confirmation by histology or cytology that the original causative Candida spp. was still present at the site of infection), residual colonization (patients had a positive culture for the causative Candida spp. but negative histology and an endoscopic mucosal evaluation of grade 0 at the EOT), or indeterminate (microbial evaluation not possible). Microbiological success was defined as endoscopically confirmed eradication, and failure was defined as persistence or residual colonization. Samples of positive culture growth were also analyzed for species identification and susceptibility testing was conducted according to Clinical and Laboratory Standards Institute reference methodology (M27-A3).
To gather data for endpoint assessments, clinical symptoms were evaluated at baseline, on days 3, 5, and 7, on day 14 or the EOT, and at the two follow-up visits. Endoscopy and microbiological sampling of the infected site were conducted at baseline, on day 14 or the EOT, and at the follow-up visits. Relapse was assessed at the follow-up visits for all patients who were considered to be a clinical success. Relapse was defined as a deterioration of clinical symptoms or the endoscopic mucosal evaluation (higher than grade 0) in patients who had demonstrated a resolution of both clinical symptoms and endoscopic grades at the EOT.
Safety assessments.
Safety was assessed in all patients during screening (days −5 to −1) and at regular intervals throughout the trial via monitoring of adverse events (AEs; number, nature, severity, and relationship to study drug), 12-lead electrocardiogram (ECG), physical examinations, vital-sign measurements (blood pressure, heart rate, respiration rate, body temperature, and weight), and clinical laboratory evaluations (hematology, biochemistry, and urinalysis). An AE was defined as any untoward medical occurrence in a patient given a study drug that did not necessarily have a causal relationship with the treatment. AEs were evaluated throughout the study.
Treatment-emergent AEs (TEAEs) were defined as those that started within the period from administration of the first dose of study drug through to the 28-day follow-up assessment. Drug-related TEAEs were TEAEs considered to be related (remotely, possibly, or probably) to the study drug that started in the dosing to follow-up period. Serious AEs (SAEs) were defined as any untoward medical occurrence that resulted in death, persistent or significant disability, or congenital abnormality, that was life-threatening, or that required hospitalization or prolongation of existing hospitalization.
Statistical analyses.
A sample size of 160 patients (to provide 40 patients per treatment arm) was chosen on the pragmatic basis of availability of patients within a reasonable time frame. No formal sample size calculation was performed. Patient demographics, baseline characteristics, and safety were evaluated in the intent-to-treat (ITT) population, which was defined as all patients who received at least one dose of study drug and who provided data for at least one post-baseline safety parameter. Variables were summarized using descriptive statistics.
Efficacy was evaluated in the per-protocol (PP) population, which was defined as all patients in the ITT population who had a valid, endoscopically confirmed clinical response at the EOT and sufficient treatment duration. The objective of the primary efficacy analysis was to show that each of the isavuconazole dosing regimens was no less effective (i.e., noninferior) than a once-daily fluconazole dosing regimen. Primary efficacy analysis was conducted as a success versus failure analysis in the PP population.
This study was designed as a noninferiority trial with a noninferiority margin of −15%. The calculated success rate of each of the three isavuconazole dosing regimens was compared to the calculated success rate of the fluconazole dosing regimen using a Cochran-Mantel-Haenszel weighted method (weighted by study center). If the lower limit of the calculated 95% confidence interval (CI) for the difference in success rates (isavuconazole − fluconazole) was >–15%, then isavuconazole was concluded to be noninferior to fluconazole.
Microbiological response was evaluated in all patients in the ITT population who had valid baseline microbiological data (MBE/ITT) and in all patients in the PP population who had valid baseline and EOT microbiological data (MBE/PP). Secondary efficacy analyses were conducted using methods similar to those used for the primary efficacy analysis.
RESULTS
Patients.
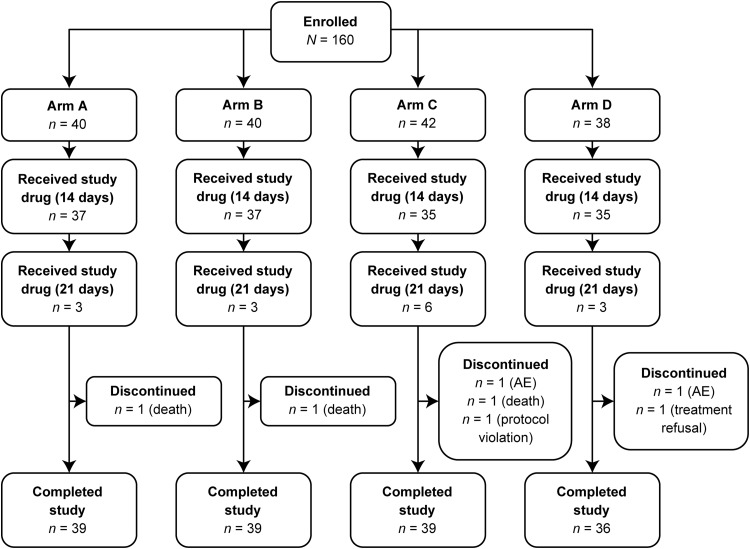
A total of 160 patients were enrolled in the study: 159, 153, 151, and 145 patients were included in the ITT, PP, MBE/ITT, and MBE/PP populations, respectively (Fig. 2). Seven patients discontinued the study prematurely due to AEs (n = 2), death (n = 3), treatment refusal (n = 1), and use of a prohibited concomitant medication (Rifafour for tuberculosis therapy; n = 1). Seven patients experienced one or more major protocol violations that excluded them from the PP, MBE/ITT, and MBE/PP populations. An additional eight patients were excluded from the MBE/ITT and MBE/PP populations due to insufficient microbiological evidence of Candida spp. at baseline.
FIG 2.
Study design. Arm A, oral isavuconazole at 200 mg on day 1; once-daily oral isavuconazole at 50 mg thereafter. Arm B, oral isavuconazole at 400 mg on days 1, 7, and 14 and then day 21, if required. Arm C, oral isavuconazole at 400 mg on day 1 and once-daily oral isavuconazole at 100 mg thereafter. Arm D, oral fluconazole at 200 mg on day 1 and then once-daily oral fluconazole at 100 mg thereafter. AE, adverse event.
Baseline patient demographics and characteristics were similar in all treatment arms in the ITT population (Table 1). Overall, 59 (37.1%) patients had HIV infection, 24 (15.1%) had pulmonary tuberculosis, 13 (8.2%) had oral candidiasis, six (3.8%) had Pneumocystis jirovecii pneumonia, five (3.1%) had tuberculosis, and four (2.5%) had AIDS. The majority of patients in the PP population had grade 1 dysphagia (n = 109, 71.2%), odynophagia (n = 101, 66.0%), and retrosternal pain (n = 87, 56.9%) (Table 2). Most patients were also characterized as grade 1 (n = 78, 51.0%) or grade 2 (n = 56, 36.6%) following endoscopic mucosal evaluation (Table 2). Grades of clinical and endoscopic evaluations were similar across treatment arms.
TABLE 1.
Baseline demographics and characteristicsa
| Parameter | Isavuconazoleb |
Fluconazolec |
|||
|---|---|---|---|---|---|
| Arm A | Arm B | Arm C | Arm D | Total | |
| Treatment regimen | 200/50 mg QD | 400 mg weekly | 400/100 mg QD | 200/100 mg QD | |
| Total no. of subjects | 40 | 40 | 41 | 38 | 159 |
| No. of subjects (%) | |||||
| Gender | |||||
| Male | 33 (82.5) | 32 (80.0) | 34 (82.9) | 33 (86.8) | 132 (83.0) |
| Female | 7 (17.5) | 8 (20.0) | 7 (17.1) | 5 (13.2) | 27 (17.0) |
| Race | |||||
| White | 1 (2.5) | 0 | 0 | 0 | 1 (0.6) |
| Black | 39 (97.5) | 38 (95.0) | 40 (97.6) | 36 (94.7) | 153 (96.2) |
| Other | 0 | 2 (5.0) | 1 (2.4) | 2 (5.3) | 5 (3.1) |
| Mean ± SD | |||||
| Age (yr) | 39.8 ± 8.2 | 40.0 ± 7.1 | 39.1 ± 8.4 | 40.2 ± 7.9 | 39.8 ± 7.9 |
| Wt (kg) | 55.5 ± 8.7 | 58.4 ± 10.7 | 57.7 ± 11.1 | 53.8 ± 7.7 | 56.4 ± 9.7 |
| BMI (kg/m2) | 19.5 ± 2.4 | 20.7 ± 4.7 | 20.4 ± 3.4 | 19.2 ± 2.6 | 20.0 ± 3.4 |
That is, in an intent-to-treat (ITT) population. BMI, body mass index; QD, once daily.
200/50 mg QD, 200 mg on day 1 and then 50 mg once daily; 400 mg weekly, 400 mg on day 1 and then 400 mg once weekly; 400/100 mg QD, 400 mg on day 1 and then 100 mg once daily.
200/100 mg QD, 200 mg on day 1 and then 100 mg once daily.
TABLE 2.
Per-protocol patient baseline clinical evaluation and endoscopic evaluation grades
| Condition and grade | No. of patients (%)a |
||||
|---|---|---|---|---|---|
| Isavuconazoleb |
Fluconazolec |
||||
| Arm A (200/50 mg QD, n = 38) | Arm B (400 mg weekly, n = 40) | Arm C (400/100 mg QD, n = 38) | Arm D (200/100 mg QD, n = 37) | Total (N = 153) | |
| Dysphagia | |||||
| Grade 0 | 0 | 0 | 1 (2.6) | 0 | 1 (0.7) |
| Grade 1 | 28 (73.7) | 26 (65.0) | 28 (73.7) | 27 (73.0) | 109 (71.2) |
| Grade 2 | 10 (26.3) | 14 (35.0) | 9 (23.7) | 10 (27.0) | 43 (28.1) |
| Grade 3 | 0 | 0 | 0 | 0 | 0 |
| Odynophagia | |||||
| Grade 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 1 | 23 (60.5) | 25 (62.5) | 29 (76.3) | 24 (64.9) | 101 (66.0) |
| Grade 2 | 15 (39.5) | 15 (37.5) | 9 (23.7) | 12 (32.4) | 51 (33.3) |
| Grade 3 | 0 | 0 | 0 | 1 (2.7) | 1 (0.7) |
| Retrosternal pain | |||||
| Grade 0 | 10 (26.3) | 5 (12.5) | 9 (23.7) | 6 (16.2) | 30 (19.6) |
| Grade 1 | 20 (52.6) | 22 (55.0) | 23 (60.5) | 22 (59.5) | 87 (56.9) |
| Grade 2 | 8 (21.1) | 13 (32.5) | 6 (15.8) | 8 (21.6) | 35 (22.9) |
| Grade 3 | 0 | 0 | 0 | 1 (2.7) | 1 (0.7) |
| Endoscopic mucosal evaluation | |||||
| Grade 1 | 22 (57.9) | 17 (42.5) | 22 (57.9) | 17 (45.9) | 78 (51.0) |
| Grade 2 | 11 (28.9) | 19 (47.5) | 13 (34.2) | 13 (35.1) | 56 (36.6) |
| Grade 3 | 3 (7.9) | 3 (7.5) | 3 (7.9) | 7 (18.9) | 16 (10.5) |
| Grade 4 | 2 (5.3) | 1 (2.5) | 0 | 0 | 3 (2.0) |
QD, once daily.
200/50 mg QD, 200 mg on day 1 and then 50 mg once daily; 400 mg weekly, 400 mg on day 1 and then 400 mg once weekly; 400/100 mg QD, 400 mg on day 1 and then 100 mg once daily.
200/100 mg QD, 200 mg on day 1 and then 100 mg once daily.
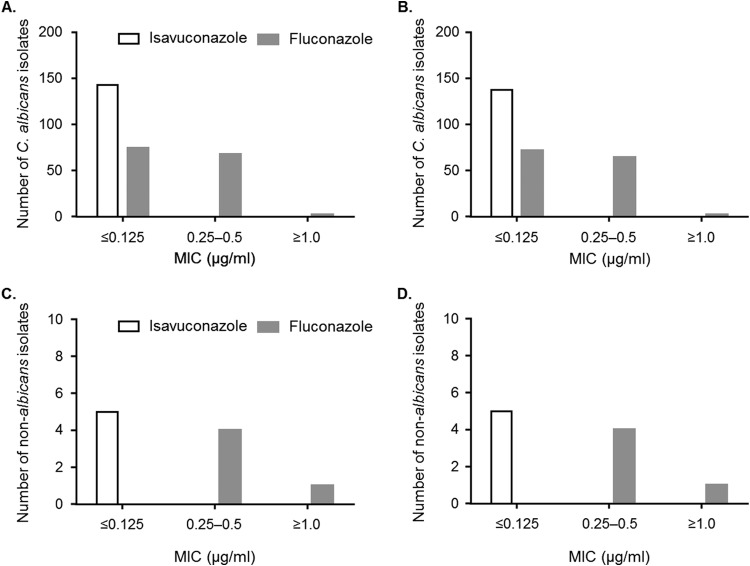
Baseline esophageal biopsy specimens showed that 75 (49.0%) patients had histological evidence of candidiasis and 146 (95.4%) patients had cytological evidence of candidiasis. In addition, 145 (94.8%) patients had a positive microbial culture, in which C. albicans was the most commonly identified species (n = 140, 96.6%), followed by C. glabrata (n = 4, 2.8%) and C. tropicalis (n = 1, 0.7%). Distribution of Candida spp. was similar across treatment arms. The baseline MIC values of Candida isolates obtained for isavuconazole were generally lower than those obtained for fluconazole (Fig. 3). The isavuconazole and fluconazole MIC ranges were comparable for C. albicans and non-albicans Candida spp.
FIG 3.
Baseline MICs of C. albicans and non-albicans Candida isolates in the MBE/ITT (A and C) and MBE/PP (B and D) populations. Non-albicans Candida spp. include C. glabrata (n = 4) and C. tropicalis (n = 1). MBE/ITT, microbiological evaluation/intent to treat population; MBE/PP; microbiological evaluation/per protocol population.
Study drug administration.
The majority (n = 144; 90.6%) of patients in the ITT population met the criteria for treatment duration of 14 days. The median (range) treatment duration was 14 (3 to 15) days. Fifteen patients met the criteria for treatment duration of 21 days. The median (range) treatment duration was 21 (14 to 21) days. Treatment duration was comparable between treatment arms.
Efficacy.
Overall, 146 (95.4%) patients in the PP population achieved endoscopically confirmed clinical success at the EOT. Statistical analysis demonstrated noninferiority between each of the isavuconazole arms A (−0.5%; 95% CI = −10.0 to 9.4), B (3.5%; 95% CI = −5.6 to 12.7), and C (−0.2%; 95% CI = −9.8 to 9.4), and arm D, i.e., the lower limits of the calculated 95% CIs for each comparison were >–15%.
Therapeutic response at the EOT in the PP population was positive in 147 (96.1%) patients. Positive therapeutic response rates were comparable between treatment arms, i.e., 97.4% (arm A), 97.5% (arm B), 94.7% (arm C), and 94.6% (arm D). Similar therapeutic response rates were reported for the MBE/PP population.
Microbiological response at the EOT was considered a success in 138 (95.2%) patients in the MBE/PP population. Statistical analysis showed noninferiority between arms A (−5.5%; 95% CI = −13.2 to 2.1) and B (−5.3%, −12.7 to 2.1), and arm D. However, the lower limit of the calculated 95% CI for isavuconazole treatment arm C was <–15% (−9.4%; 95% CI = −17.2 to −1.6). When the comparison of microbiological response was applied to the MBE/ITT population, only the isavuconazole treatment arm B was not inferior to arm D.
Three (2.0%) patients in the ITT population experienced a relapse by the 14-day follow-up evaluation, and eight (5.4%) patients experienced a relapse by the 28-day follow-up evaluation. No patients in arm A or arm D (versus two [5.1%] patients in arm B and one [2.7%] patient in arm C) were considered to have relapsed at the 14-day follow-up evaluation. No patients in arm A (versus three [7.7%] patients in arm B, two [5.4%] patients in arm C, and three patients in arm D [8.6%]) were considered to have relapsed at the 28-day follow-up evaluation.
Safety.
A total of 91 (57.2%) patients experienced at least one TEAE (Table 3). TEAEs were most common in patients in arm C (n = 29, 70.7%), while similar numbers of patients in arms A (n = 22, 55.0%), B (n = 18, 45.0%), and D (n = 22, 57.9%) experienced TEAEs. The most commonly reported TEAEs were influenza-like illness (n = 10, 6.3%), urinary tract infection (n = 8, 5.0%), hematuria (n = 7, 4.4%), and pulmonary tuberculosis (n = 7, 4.4%). Patients in arm C experienced a greater number of gastrointestinal disorders (n = 8, 19.5%; including diarrhea [n = 3, 7.3%], nausea [n = 2, 4.9%], vomiting [n = 2, 4.9%], and gastritis [n = 2, 4.9%]) and infections (n = 17, 41.5%; including urinary tract infection [n = 4, 9.8%] and gastroenteritis [n = 3, 7.3%]) than patients in arms A (gastrointestinal: n = 3, 7.5%; infections: n = 11, 27.5%), B (gastrointestinal: n = 2, 5.0%; infections: n = 7, 17.5%), and D (gastrointestinal: n = 5, 13.2%: infections: n = 6, 15.8%).
TABLE 3.
Summary of patients who experienced adverse eventsa
| Parameter | Isavuconazoleb |
Fluconazolec | ||
|---|---|---|---|---|
| Arm A | Arm B | Arm C | Arm D | |
| Treatment regimen | 200/50 mg QD | 400 mg weekly | 400/100 mg QD | 200/100 mg QD |
| Total no. of patients | 40 | 40 | 41 | 38 |
| No. of patients (%) with ≥1 TEAE | 22 (55.0) | 18 (45.0) | 29 (70.7) | 22 (57.9) |
| Mild | 12 (30.0) | 11 (27.5) | 14 (34.1) | 11 (28.9) |
| Moderate | 11 (27.5) | 9 (22.5) | 18 (43.9) | 13 (34.2) |
| Severe | 1 (2.5) | 1 (2.5) | 3 (7.3) | 5 (13.2) |
| Life-threatening | 1 (2.5) | 1 (2.5) | 1 (2.4) | 2 (5.3) |
| Drug related | 7 (17.5) | 4 (10.0) | 11 (26.8) | 7 (18.4) |
| Seriousd | 1 (2.5) | 2 (5.0) | 3 (7.3) | 4 (10.5) |
| TEAE leading to discontinuation | 1 (2.5) | 1 (2.5) | 2 (4.9) | 1 (2.6) |
That is, in an intent-to-treat (ITT) population. QD, once daily; TEAE, treatment-emergent adverse event.
200/50 mg QD, 200 mg on day 1 and then 50 mg once daily; 400 mg weekly, 400 mg on day 1 and then 400 mg once weekly; 400/100 mg QD, 400 mg on day 1 and then 100 mg once daily.
200/100 mg QD, 200 mg on day 1 and then 100 mg once daily.
Four serious TEAEs of pulmonary tuberculosis, pleural effusion, hepatic enzyme increased, and AIDS were experienced by one patient in arm D.
Twenty-nine (18.2%) patients experienced at least one drug-related TEAE (Table 3). The most commonly reported drug-related TEAEs were anemia (n = 5, 3.1%), diarrhea (n = 4, 2.5%), and nausea (n = 3, 1.9%). More patients in arm C (n = 11, 26.8%) experienced drug-related TEAEs, than in arms A (n = 7, 17.5%), B (n = 4, 10.0%), or D (n = 7, 18.4%). Gastrointestinal disorders (including diarrhea) were more common in arm C than in treatment arms A, B, and D, i.e., n = 5, 12.2% (C) versus n = 1, 2.5% (A), n = 2, 5.0% (B), and n = 2, 5.3% (D).
Ten (6.3%) patients experienced an SAE (Table 3). Three (1.9%) patients experienced SAEs that were considered drug related: two (4.9%; atrioventricular block and tuberculous pleurisy) in arm C and one (2.6%; moderate to severe increases in liver enzymes) in arm D.
Five (3.1%) patients experienced TEAEs that led to discontinuation of the study (Table 3). One patient in arm C with HIV infection and no other medical history experienced an SAE of mild second degree atrioventricular block on day 14, and isavuconazole treatment was discontinued. This patient had a pretreatment ECG rhythm that was considered abnormal with flat, smooth, and slightly bifid T-waves in the anteroseptal leads. On day 14, the bifid T-waves increased and a U-wave appeared; the corrected QT interval (QTc) was 458 ms, which was reported as no major QTc change from baseline. ECG rhythm analysis showed normal sinus rhythm with a morphology of second degree (Mobitz II) atrioventricular block with T-wave inversions. The SAE resolved on day 28 without treatment and was considered to be probably related to isavuconazole treatment.
One patient in arm C with active HIV infection and oral candidiasis experienced an SAE of tuberculous pleurisy on day 4. This patient was admitted to hospital on day 3 of treatment because of abdominal pain and dyspnea. Tuberculosis was suspected as the cause of ascites but was not confirmed, and the patient died the next day before planned antituberculosis treatment was initiated. The study investigator considered this SAE to be life-threatening in intensity and considered the death to be remotely related to isavuconazole treatment. All other AEs associated with study discontinuation were considered unrelated to either study drug.
There were five deaths during the study and follow-up period due to pulmonary tuberculosis (arm A), meningitis tuberculous (arm B), tuberculous pleurisy (arm C), diarrhea (arm D), and AIDS (arm D; n = 1 each). Most deaths were considered unrelated to study drug administration, except for the instance of tuberculous pleurisy, which was considered remotely related to isavuconazole treatment.
DISCUSSION
This trial compared the efficacy and safety of three different dosing regimens of oral isavuconazole with an approved once-daily oral fluconazole dosing regimen in patients with uncomplicated esophageal candidiasis. The majority of patients enrolled in this trial had grade 1 or 2 dysphagia, odynophagia, and retrosternal pain and were categorized as grade 1 or higher in endoscopic mucosal evaluations. The causative organism in almost all patients was C. albicans, which is consistent with earlier trials of triazole efficacy in esophageal candidiasis (3–6, 26, 27). The majority of Candida isolates displayed a greater susceptibility to isavuconazole than to fluconazole, which is consistent with previous in vitro studies (28, 29).
The results of this trial demonstrated that once-daily oral isavuconazole at 50 and 100 mg were not inferior to once-daily fluconazole at 100 mg for primary treatment of esophageal candidiasis. The endoscopic cure rate was ∼95% for each of these dosing regimens, and statistical analysis indicated that these rates were comparable between the isavuconazole and fluconazole treatment arms. These findings are in agreement with previous studies of fluconazole efficacy, which have shown that dose regimens of once-daily fluconazole at 100 and 200 mg are associated with endoscopic cure rates of ∼90% (3–6, 26, 27).
Once-weekly isavuconazole at 400 mg also demonstrated comparable efficacy with fluconazole. The endoscopic cure rate for this dose regimen was ∼98% and was not inferior to that of fluconazole. This finding suggests that weekly, high doses of isavuconazole may be a promising alternative to conventional once-daily dose regimens.
Overall, therapeutic and microbiological responses were also high in all treatment arms. The rates of positive therapeutic responses were ∼96% and the rates of eradication of Candida sp. infections were between 91 and 100%. Statistical testing revealed that the dose regimens used in arm A (isavuconazole at 50 mg once daily) and arm B (isavuconazole at 400 mg once weekly) were not inferior to the regimen used in arm D (fluconazole at 200 mg once daily) to eradicate Candida sp. infections.
Relapse rates in the current study were low and comparable with existing studies (4–6). At the 28-day follow-up assessment, no patients were considered to have relapsed in treatment arm A. The relapse rates were 7.7, 5.4, and 8.6% in arms B, C, and D, respectively; however, if patients missed follow-up visits, they were also classed as treatment failures, which may have affected these rates.
Each of the three isavuconazole dosing regimens was generally well tolerated in patients with esophageal candidiasis. The more common drug-related AEs were anemia, diarrhea, and nausea. Each of these AEs occurred in similar numbers across the treatment arms, and there was little difference between isavuconazole and fluconazole therapy.
Two patients discontinued the study due to AEs that were potentially related to isavuconazole treatment. Second-degree atrioventricular block in one patient was considered probably related to isavuconazole treatment, and tuberculosis on pleural tap in one patient was considered remotely related to isavuconazole treatment. Five patients died during the study and follow-up period. Four out of five deaths were unrelated to isavuconazole, and one death, due to tuberculosis on pleural tap, was considered remotely related to isavuconazole therapy.
The fluconazole dosing regimen used in the present study was lower than currently recommended by the Infectious Diseases Society of America (IDSA) for primary treatment of esophageal candidiasis. The 2009 IDSA guidelines recommend fluconazole at 200 to 400 mg daily for 14 to 21 days (7). However, IDSA guidelines at the time of the study recommended fluconazole at 100 to 200 mg daily for 14 to 21 days (30). In addition, the fluconazole dose regimen currently approved by the U.S. Food and Drug Administration is 200 mg on day 1 and then 100 mg daily thereafter; therefore, this regimen was used in the present study (24). No formal power calculation was conducted as part of this trial, which was also a limitation.
In conclusion, this randomized, double-blind clinical trial has demonstrated that the efficacy and safety of once-daily and once-weekly oral isavuconazole treatment regimens were comparable to once-daily oral fluconazole in the primary treatment of uncomplicated esophageal candidiasis.
Supplementary Material
ACKNOWLEDGMENTS
J.V. received grants from Astellas during the conduct of the study. N.A. is an employee of Astellas. A.-H.S.-H. is an employee of Basilea Pharmaceutica International, Ltd. M.G. received grants from Astellas during the conduct of the study, speaker fees from Astellas and Pfizer, Inc., and grants from the National Institutes of Health other than for the present study.
Isavuconazole is in codevelopment by Astellas and Basilea Pharmaceutica International, Ltd. Editorial assistance was provided by Neil M. Thomas, Envision Scientific Solutions, which is funded by Astellas.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04586-14.
REFERENCES
- 1.Lortholary O, Petrikkos G, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Groll AH, Herbrecht R, Hope WW, Jensen HE, Kullberg BJ, Lass-Florl C, Meersseman W, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect 18(Suppl 7):68–77. doi: 10.1111/1469-0691.12042. [DOI] [PubMed] [Google Scholar]
- 2.Shiboski C. High accuracy of common HIV-related oral disease diagnoses by non-oral health specialists in the AIDS Clinical Trial Group. AIDS Care, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ally R, Schurmann D, Kreisel W, Carosi G, Aguirrebengoa K, Dupont B, Hodges M, Troke P, Romero AJ. 2001. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin Infect Dis 33:1447–1454. doi: 10.1086/322653. [DOI] [PubMed] [Google Scholar]
- 4.de Wet N, Llanos-Cuentas A, Suleiman J, Baraldi E, Krantz EF, Della Negra M, Diekmann-Berndt H. 2004. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis 39:842–849. doi: 10.1086/423377. [DOI] [PubMed] [Google Scholar]
- 5.de Wet N, Bester AJ, Viljoen J, Filho F, Suleiman JM, Ticona E, Llanos EA, Fisco C, Lau W, Buell D. 2005. A randomized, double blind, comparative trial of micafungin (FK463) versus fluconazole for the treatment of oesophageal candidiasis. Aliment Pharmacol Ther 21:899–907. doi: 10.1111/j.1365-2036.2005.02427.x. [DOI] [PubMed] [Google Scholar]
- 6.Krause DS, Simjee AE, van Rensburg C, Viljoen J, Walsh TJ, Goldstein BP, Wible M, Henkel T. 2004. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin Infect Dis 39:770–775. doi: 10.1086/423378. [DOI] [PubMed] [Google Scholar]
- 7.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fichtenbaum CJ, Koletar S, Yiannoutsos C, Holland F, Pottage J, Cohn SE, Walawander A, Frame P, Feinberg J, Saag M, Van der Horst C, Powderly WG. 2000. Refractory mucosal candidiasis in advanced human immunodeficiency virus infection. Clin Infect Dis 30:749–756. doi: 10.1086/313765. [DOI] [PubMed] [Google Scholar]
- 9.Sangeorzan JA, Bradley SF, He X, Zarins LT, Ridenour GL, Tiballi RN, Kauffman CA. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med 97:339–346. doi: 10.1016/0002-9343(94)90300-X. [DOI] [PubMed] [Google Scholar]
- 10.Maenza JR, Keruly JC, Moore RD, Chaisson RE, Merz WG, Gallant JE. 1996. Risk factors for fluconazole-resistant candidiasis in human immunodeficiency virus-infected patients. J Infect Dis 173:219–225. doi: 10.1093/infdis/173.1.219. [DOI] [PubMed] [Google Scholar]
- 11.Maenza JR, Merz WG, Romagnoli MJ, Keruly JC, Moore RD, Gallant JE. 1997. Infection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiology. Clin Infect Dis 24:28–34. doi: 10.1093/clinids/24.1.28. [DOI] [PubMed] [Google Scholar]
- 12.Pagani JL, Chave JP, Casjka C, Glauser MP, Bille J. 2002. Efficacy, tolerability and development of resistance in HIV-positive patients treated with fluconazole for secondary prevention of oropharyngeal candidiasis: a randomized, double-blind, placebo-controlled trial. J Antimicrob Chemother 50:231–240. doi: 10.1093/jac/dkf101. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Jones RN, Castanheira M. 2014. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006-2011. Mycoses 57:602–611. doi: 10.1111/myc.12206. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warn PA, Sharp A, Parmar A, Majithiya J, Denning DW, Hope WW. 2009. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob Agents Chemother 53:3453–3461. doi: 10.1128/AAC.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother 52:1396–1400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illnait-Zaragozi MT, Martinez GF, Curfs-Breuker I, Fernández CM, Boekhout T, Meis JF. 2008. In vitro activity of the new azole isavuconazole (BAL4815) compared with six other antifungal agents against 162 Cryptococcus neoformans isolates from Cuba. Antimicrob Agents Chemother 52:1580–1582. doi: 10.1128/AAC.01384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin de la Escalera C, Aller AI, López-Oviedo E, Romero A, Martos AI, Cantón E, Peman J, Garcia Martos P, Martin-Mazuelos E. 2008. Activity of BAL 4815 against filamentous fungi. J Antimicrob Chemother 61:1083–1086. doi: 10.1093/jac/dkn076. [DOI] [PubMed] [Google Scholar]
- 19.Verweij PE, González GM, Wiedrhold NP, Lass-Flörl C, Warn P, Heep M, Ghannoum MA, Guinea J. 2009. In vitro antifungal activity of isavuconazole against 345 mucorales isolates collected at study centers in eight countries. J Chemother 21:272–281. doi: 10.1179/joc.2009.21.3.272. [DOI] [PubMed] [Google Scholar]
- 20.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and cyp51 mutant isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 57:6284–6289. doi: 10.1128/AAC.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepak AJ, Marchillo K, VanHecker J, Diekema D, Andes DR. 2013. Isavuconazole pharmacodynamic target determination for Candida species in an in vivo murine disseminated candidiasis model. Antimicrob Agents Chemother 57:5642–5648. doi: 10.1128/AAC.01354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo G, Gebremariam T, Lee H, Edwards JE Jr, Kovanda L, Ibrahim AS. 2014. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob Agents Chemother 58:2450–2453. doi: 10.1128/AAC.02301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA. 2009. Efficacy of isavuconazole, voriconazole, and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J Antimicrob Chemother 63:161–166. doi: 10.1093/jac/dkn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfizer, Inc. 2011. Diflucan (fluconazole tablets) (fluconazole injection for intravenous infusion only) (fluconazole for oral suspension). Pfizer, Inc., New York, NY: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019949s051lbl.pdf. [Google Scholar]
- 25.Barbaro G, Barbarini G, Calderon W, Grisorio B, Alcini P, Di Lorenzo G. 1996. Fluconazole versus itraconazole for candida esophagitis in acquired immunodeficiency syndrome. Candida Esophagitis Gastroenterol 111:1169–1177. [DOI] [PubMed] [Google Scholar]
- 26.Laine L, Dretler RH, Conteas CN, Tuazon C, Koster FM, Sattler F, Squires K, Islam MZ. 1992. Fluconazole compared with ketoconazole for the treatment of Candida esophagitis in AIDS: a randomized trial. Ann Int Med 117:655–660. doi: 10.7326/0003-4819-117-8-655. [DOI] [PubMed] [Google Scholar]
- 27.Villanueva A, Gotuzzo E, Arathoon EG, Noriega LM, Kartsonis NA, Lupinacci RJ, Smietana JM, DiNubile MJ, Sable CA. 2002. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med 113:294–299. doi: 10.1016/S0002-9343(02)01191-9. [DOI] [PubMed] [Google Scholar]
- 28.Pfaller MA, Castanheira M, Messer SA, Rhomberg PR, Jones RN. 2014. Comparison of EUCAST and CLSI broth microdilution methods for the susceptibility testing of 10 systemically active antifungal agents when tested against Candida spp. Diagn Microbiol Infect Dis 79:198–204. doi: 10.1016/j.diagmicrobio.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2013. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J Clin Microbiol 51:2608–2616. doi: 10.1128/JCM.00863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE, Infectious Diseases Society of America. 2004. Guidelines for treatment of candidiasis. Clin Infect Dis 38:161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- 31.Cornely OA, Böhme A, Schmitt-Hoffmann A, Ullmann AJ. 26 January 2015. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother doi: 10.1128/AAC.04569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.